Abstract
Biodegradable sodium alginate/gum Arabic (SG) films were obtained. The influence of Syzygium cumini seeds extract (SCSE) incorporation in SG films on morphological structure, polymer interaction, thermal behavior, antioxidant activity and physical characteristics were investigated. Moreover, the fabricated films were tested as wrapping materials to extend the shelf life of sunflower oil. Scanning electron microscopy micrographs showed heterogeneous and rough surface after incorporation by SCSE. Possible cross-linked interaction between alginate and gum Arabic and physical interaction between SCSE and SG films were assessed by FT-IR. Although, the addition of SCSE into SG films declined the thermal stability, elongation at break (EB), tensile strength (TS) and moisture content, films with better opacity, solubility and water vapor permeability were obtained. The SG-SCSE films showed obvious obstructing effects on the oil oxidation process during storage days. According to these results, it can be emphasized that edible films with the highest concentrations of added SCSE can serve as a good source of antioxidant compounds and physicochemical properties. Certainly, these properties can be usefully incorporated into the wrapped food commodity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Newly, the attention has been greatly changed to utilize of natural polysaccharides as biopolymers to form biodegradable plastic films. This interest was due to the great benefits of these related to their renewable origin, safety, low cost and their potential role for increasing the shelf life and maintaining quality of many food products [1]. There are a lot of carbohydrates polymers characterized by their linear or branched structure polymer backbone and unique colloidal properties, exhibiting the ability to form flexible and tough biodegradable transparent films. Sodium alginate (SA) is the alginic acid salt and it takes the shape of linear polysaccharides organized with various proportions and distributions in the chain with shape of blocks [2]. Alginate is used on a large scale as a thickening, emulsifying agent, form gelling case, stabilizing enzyme or drug carrier [3, 4]. Furthermore, linear alginate shape could arrange a strong fibrous template with high likely for edible and biodegradable films fabrication [5]. Gum Arabic (GA) obtained from Acacia species is a safe salt of arabinogalactan-type of polysaccharide containing calcium, potassium, magnesium, ions and about 2% protein [6, 7]. Additionally, its carbohydrate part has a highly branched shape composed by arabinofuranose, rhamnopyranose arabinopyranose, 1,6-galactopyranose residues, glucuronic acid, galactose, glucuronic acid and 4-O-methyl [8]. In food applications, it is widely used in the industrial sector as emulsification agent and encapsulation purposes because of its well-known amphiphilic properties [9, 10]. It was revealed that films fabricated by combining biopolymers showed better characteristics than the ones based in one biopolymer [11, 12]. Thus, the combination between two or more biopolymers and the incorporation with components were performed to raise the shelf life or maintain the quality of food products by obstructing microbial spoilage, oxidative rancidity and moisture loss. Additionally, the grafting of biopolymers with natural polyphenols as antioxidants agents is a favorable choice that could produce active packaging films to enhance the nutritional value in food application [13]. The incorporation of Azolla pinnata leaves extracts inside sodium alginate/maltodextrins films enhanced physicochemical, antioxidant and antibacterial properties of films [14]. Additionally, Allium tuberosum root extract contained promising natural components that improved antioxidant and antibacterial properties of chitosan-based biodegradable films [15]. It has been reported that Syzygium cumini extracts possess several antioxidant compounds such as polyphenols, carbohydrates, oils and alkaloids which provide multiple biological actions [16, 17]. Thus, the target of this study was to produce suitable biodegradable films by using two specific biopolymers incorporated with functional polyphenols that work as an oil storage packaging substance for food application purposes.
Materials and Methods
Reagents and Chemicals
The fruits of Syzygium cumini in the ripening stage were collected and obtained from the trees growing in Egypt. Sodium alginate (SA), gum Arabic (GA), D101 resin, 1,1-diphenyl-2-picryl hydrazyl (DPPH) and [2,2-azinobis-(3-ethyl-benzothiazolin-6-sulfonic acid)] diammonium salt (ABTS) were obtained from Sigma Aldrich Co., Ltd. (St. Louis, MO, USA).
Preparation of Syzygium cumini Seeds Extract
The syzygium cumini fruits were exposed to sun for 48 h to dry then the dried fruits were treated by a microwave for 30 s to get seeds. The seeds were dried at 50 °C for 12 h and ground to a fine powder. The extraction method was performed based on the optimization conditions reported by our previous method [18] to afford the target extract.
Preparation of SA/GA Films
The films were prepared by dissolving of 1.5 and 0.8 g of SA and GA, respectively, in 100 mL of distilled water. After stirring at 900 rpm and 70 °C for 1.5 h, the glycerol solution (35% w/w, based on SA and GA content) as plasticizer was added to the mixture and admitted to distribute well by stirring at 900 rpm and 70 °C for 30 min. Different concentrations from SCSE (0.4, 0.8, 1.2 and 1.6% based on SA and GA content) were added to the mixture to obtain groups of (SG-SCSE1, SG-SCSE2, SG-SCSE3 and SCSE4), respectively. The mixture without SCSE was considered control (SG). All groups were stirred at 900 rpm and 45 °C for 1 h to afford good blend. After that, an aliquot of 50 mL of each group was poured carefully into 150 mm petri dishes plates and oven-dried at 40 °C for 8 h. The films were removed by peeling from plates and were stored in a desiccator at 25 °C for further analysis.
Characterization of Films
Scanning Electron Microscopy (SEM) Photographs
SEM (SU8010, Hitachi, Japan) was applied to investigate the cursory of produced films at 10 kV. Films pieces (10 × 10 mm) dimensions were prepared and fixed on aluminum stubs by using carbon tape and shell out-covered with gold. And the imagination was set as 3000×.
FT-IR Characteristics
FT-IR analysis was performed by (Nicolet iS-50, Thermo, USA) machine. The utilized spectra were operated in the range between 4000 and 400 cm−1 according to Riaz et al. [15].
DSC Properties
DSC properties was performed by a DSC-60 plus SHIMADZU machine according to Akhtar et al. [19]. A standard aluminum pan was rolled and compressed on 3 to 5 mg of small film pieces and 10 °C/min was considered stable rate to heat from 0 to 250 °C.
Crystallization Characteristics
The crystallization properties of produced films were investigated by XRD analysis using Bruker D8 Advance, USA X-ray diffractometer and the machine was adjusted at a voltage of 40 kV and 100 mA. The scattered radiation was performed in regular range 2θ = 5–80°.
Thickness and Mechanical Characteristics Analysis
The thickness was estimated by a digital hand-held micrometer. Each sample was gauged ten times at different appropriate regions and the average value was measured.
Concerning the mechanical properties, the texture analyzer (TA.XT Plus, Stable Micro Systems Ltd., Surrey, UK) was used to determine the (TS) and (EB) of the films at room temperature. Strips of films (10 × 80 mm) position on grips (50 mm), the cross speed and tensile power were set at 50 mm/min and 5 kN, respectively. In accordance with the values of resistance to extension (N) and extensibility (mm), the values of TS and EB were estimated by the following equation:
where F is resistance to extension (N), X is the film thickness (mm) and W is the width of the film (mm). L is the film extensibility (mm) and L0 is the preliminary grip film length (mm).
Measurement of Moisture, Solubility and Swelling Ratio
The pieces of films were cut into 20 × 20 mm then weighed as wet weight (M0) and dried at 105 °C till stable weight to calculate the primary dry mass value (M1). The dried pieces were transferred to 100 mL beakers filled with distilled water (50 mL) enclosed with plastic wraps and preserved at 25 °C for 1 day. Then, the films were dehydrated metaphorically with filter papers and weight to get (M2). The saturated-hydrate films were dehydrated again to constant weight at 105 °C to get final dry mass (M3). The moisture content, swelling ratio and solubility were calculated using the subsequent equations;
where M0 is wet weight of the film, M1 is as initial dry mass, M2 represents the weight of film after drenching in water for 24 h and M3 describes the final dry mass.
Films Water Vapor Permeability (WVP) Analysis
The WVP values were estimated according to the described process [20] with suitable modifications. A glass cup with a diameter of 4 cm was stuffed with 5 g of anhydrous CaCl2 and the film (5 × 5 cm) was settled with elastics on the top of a weighing cup. Then the cups were transferred to a desiccator preserved at 75% RH with sodium chloride as a saturation solution. The weight of the cup was investigated at one-hour intervals over a 7-h period. Firstly, the rate of water vapor conveyance (WVTR, g/sm2) was calculated by the slope produced to define K (g/s) and calculate WVTR from the next equation:
Then from the first equation; WVP was measured according to the subsequent equation.
where A is the zone of the exposed surface (m), D is the thickness of sample average (m), and ∆P is the variance of pressure of water vapor among the sides of the tested films.
Determination of Color Properties and Opacity
The color properties of SG-SCSE films were estimated using CR-400 colorimeter (Konica Minolta, Japan). The color indices were described as following; ∆E (total color difference), a* (red/green), b* (yellow/blue) and L* (lightness). The ∆E values were calculated depending on the Eq. (8)
The color properties results were the mean of triplicate samples. where Lo refers to initial values and L1 is the final values.
The opacity of fabricated films was determined according to Atef et al. [21]. The SG-SCSE films were sheared into rectangle shapes (2.5 × 1 cm), and the absorbance was determined at 660 nm using a UV-1200 spectrophotometer (Mapada, Shanghai, China). The cuvette without the film was used as blank.
The opacity was calculated by using the next equation:
where X constitutes the film thickness.
Antioxidant Activity of Films
The samples (25 mg) of films were soaked in volume of 3 mL of distilled water to release the extract for subsequent assays;
For assay of scavenging activity on DPPH free radical, the determination was performed as described by the modified method of Siripatrawan and Harte [22]. Briefly, 3.0 mL of the extract was mixed with 1.0 mL of 0.1 mM DPPH in methanol solution and the blend was preserved in the dark for 30 min. The absorbance was recorded at 517 nm and the discoloration percentage was noticed according to the subsequent equation;
For assay of ABTS scavenging activity, the determination was performed depending on the procedure of Kim et al. [23]. Firstly, the work solution was produced by mixing of 7 mM ABTS with 145 mM of potassium persulfate to make oxidation. Then, the mixture was preserved in the dark for 12 h. After that, PBS buffer (0.2 M, pH 7.4) was prepared to dilute the ABTS solution affording work solution with appropriate absorbance, not more than (0.8 ± 0.02). An amount of 20 µL from film extract was mixed with 1980 µL of work solution. After preservation in the dark at 27 °C for 6 min, the absorbance was read at a wavelength of 734 nm. The scavenging activity of ABTS radical was estimated according to the equation of Božič et al. [24].
where A0 represents the absorbance of the preliminary ABTS free radicals, A1 is the absorbance of sample, and A2 is absorbance of a standard arranged as A1 whereas substituting ABTS radicals with PBS.
Determination of Films Oil Impedance
The oil impedance ability of the films was conducted by the method of Wang et al. [25] with minor modifications. A dimension of (4 cm × cm) from film samples were stabilized with wires on the top of glass test tubes filled with 5 mL of sunflower oil and were overturned on the dried filter paper (6 cm diameter) for 2 days. After that, the weight of filter paper was recorded and rate of oil absorption (OAR) was measured by the subsequent equation:
where w is the weight of filter paper after 2 days and w0 is the weight of dried filter paper.
Calculation of Peroxide Value (POV)
The films were fixed on the top of glass beakers filled with sunflower oil and kept at 50 °C for 28 days. A volume of 5 mL from oil was taken out from each beaker on interval days to determine the POV values depending on reported method of Firestone [26].
Statistical Analysis
All statistical analysis was done by software of SPSS version 19.0 (Chicago, IL, USA). Tukey’s honest (HSD) test was done to calculate the variances in relative abundance.
Results and Discussions
The obtained SCSE was analyzed by HPLC and the results were recorded as our previous report [18]. The main phenolic constituents were epicatechin, kaempferol, catechin, gallic, 5-caffeoylquinic, caffeic and ferulic acids. Gallic acid was the main compound that recorded (11.21 ± 0.31 mg/g) followed by 5-caffeoylquinic acid (8.64 ± 0.25 mg/g).
Characterization of Films Properties
Observation of Film Microstructure by SEM
SEM micrographs of the surface of fabricated films incorporated with and without SCSE are shown in Fig. 1. The surface of the SG films was smooth, homogeneous and continuous without any pores, cracks or irregularities (Fig. 1A). After incorporation with SCSE, the micrographs of the films displayed heterogeneous and rough surface. Additionally, some white spots continuously dispersed on the surface of films. At moderate concentration (0.8 and 1.2%), most of SCSE was dispersed uniformly in the SG matrix without obvious aggregation (Fig. 1C and D). The noticed surface characteristics were nearly from the same obtained by Sun et al. [27] who stated heterogeneous surface with the addition of polyphenols into chitosan films. Additionally, Norajit et al. [28] observed heterogeneous surface of alginate films after incorporation by ginseng extract.
Observation by FT-IR
To detect the intermolecular differences between SA, GA and SG added SCSE films, FT-IR analysis was conducted and the results are shown in Fig. 2. The spectral bands 1413.31 and 1603.21 cm−1 for GA corresponded to the symmetrical and asymmetric stretching vibration of –COO−, respectively [29]. Additionally, the spectral band 3287.12 cm−1 for GA represented O–H stretching, characteristic of a glucosidic ring, while 2953.42 cm−1 indicated the C-H stretching [30]. As shown in Fig. 2, the FT-IR spectrum of SA indicated principal points in wavenumber length around 3251.30, 1409.91, and 1025.06 cm−1, representing O–H, COO– (symmetric) and C–O–C stretching, respectively [31, 32]. Moreover, stretching vibrations of aliphatic C–H was detected at 2931.77 cm−1 [33]. The addition of GA to SA to form SG films caused shifting in wavenumbers as compared with SA and GA powder from 3287.28 to 3251.64 cm−1 to create a tough absorption band at 3266.49 cm−1 for O–H stretching. Furthermore, a new peak at 2889.28 cm−1 was formed, which did not appear in spectrum of SA or GA, indicating that the cross-linked reaction might have occurred between SA and GA. Additionally, the spectral bands between 800 and 1000 cm−1 revealed major shifting and combination and it might attribute to the interaction between SA and GA. The positive charges in the proteinaceous part of GA could form an electrostatic interaction between proteins in the second and the third fractions of GA with negatively charged carboxylate groups in SA [34]. With regard to the addition of SCSE concentrations to SG films, the resulted spectral analysis proved that there was no covalent interaction between SG polymers and SCSE and the nature of interaction was physically type.
The Mechanical Characteristics of Films
TS and EB for the SG films with different levels of SCSE are indicated in Fig. 3. The incorporation of SCSE resulted a significant reduction (p < 0.05) in TS compared with that of the SG film. The distribution of SCSE between SA and GA molecules might reduce the internal interactions and make differences in cross-linking between two polymers and as a result caused weakness in some films regions. On the other hand, the highest concentration of SCSE inside films caused lower percentage of EB. The minimum values for TS and EB (30.68 MPa and 47.32%), respectively, were displayed by using a concentration of 1.6% of SCSE inside films. The same manners of decreasing of TS and EB by consequent addition of polyphenols were reported by Riaz et al. [35]. Additionally, the enhancement of alginate films by black chokeberry extract promoted declining of the TS property [23]. The addition of epigallocatechin gallate into alginate/carboxy methylcellulose films caused the decreasing of EB [36].
DSC Analysis
DSC curves of SG films are displayed in Fig. 4. All the films exhibited three exclusive peaks corresponding to exothermic and endothermic events occurred during the analysis. The endothermic regions reflected the absorption of heat by all the films types and resulted in loss of water, the control SG films illustrated exothermic region in the range of 209.68 °C corresponding to the thermal degradation degree of polysaccharides [37]. In case of SG films, the Tg1 and Tg2 were 93.30 and 157.98 °C, respectively. The Tg1 and Tg2 for SG-SCSE4 were 86.02 and 154.42 °C, respectively. Thus, the addition of SCSE to SG films caused declining trend of peak temperature and as a result displayed low thermal stability. The addition of SCSE into SG films could change the structure arrangement between SA and GA polymers. The same trend was reported by Pankaj et al. [38].
XRD Properties
The crystaline strucures of SG-SCSE films and control were observed through Fig. 5. It is cleared from the spectrum that after addition of SCSE inside SG films, no changes on crystaline structure were ocured until the third concentration (SG-SCSE3) with single beak appearance at the 2θ with angle 33°. However, by the continous addition of SCSE polyphenols inside films, the single beak was divided into two parts in 2θ with angle 34.7°. The changes of XRD spectrum after addition of polyphenols inside alginate films were in the same properties of those obtained by Eltabakh et al. [14]. The randum attendance of SCSE between molecules of SA and GA polymers could cause disturbance in molecules arrangement and this might lead to differences in crystilization shape.
Antioxidant Properties of Prepared Films
The DPPH and ABTS radical scavenging activities of films are shown in Fig. 6. The results revealed that the DPPH and ABTS radical scavenging values increased significantly (p < 0.05) with the increase of SCSE concentrations inside films in a dose-dependent manner. Comparatively, the SCSE concentration 1.6% displayed the strongest antioxidant activity. It reduced 88.15% and 67.59% of stable DPPH and ABTS radicals, respectively. The strongest effect to scavenge the tested free radicals mainly attributed to the presence of polyphenols inside films. The SCSE mainly possessed epicatechin, catechin, kaempferol, 5-caffeoylquinic, caffeic, gallic and ferulic acids and these polyphenols might be the main reason for scavenging of DPPH and ABTS radicals [18]. However, the control films without SCSE showed ability to reduce the tested stable radicals and this might be explained by the reason that alginate polymer has antioxidant effect and this mainly increases by breaking the polymer chain [39, 40]. Furthermore, GA is a suitable dried viscous polymer and it has strong antioxidant activity [41].
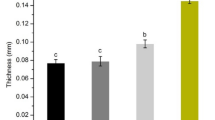
Film Thickness
The incorporation of active agents like SCSE can impact on the physical manners of the films. Hence, this work assessed these manners and make comprehension with the properties of pure SG films. As shown in Table 1, the presence of SCSE increased significantly (p < 0.05) the film thickness to reach its maximum value of 0.184 mm by using 1.6% of SCSE in comparison with SG film. This increase was probably due to an increase in the solid content after SCSE incorporation. The same manners were indicated with different coating materials by the previous reports of Peng et al. [42] and Tan et al. [43]. Through this manner, SCSE polyphenols would possibly act as a bridge, binding between SA and GA molecules and between molecules inside the same polymer due to the subsistence of poly hydroxyl groups in the structures; consequently, the distance between SA and GA molecules became narrower, encouraging the film structure more consolidated and for this reason might increase the film thickness.
Contact of Water Properties
The water absorption properties were investigated through four determinations the swelling degree, the solubility, the moisture content and the WVP. Interestingly, the swelling degree of film samples was decreased significantly (p < 0.05) by the increasing of SCSE concentration inside films (Table 1). This was probably due to structural, thickness and glycerol content between these samples [28, 44].
Concerning the solubility of the film, it was declined moderately with the maximum addition of 1.6% of SCSE. The films with little solubility are typically utilized for food packaging [45].
Notably, the moisture content of fabricated films was decreased significantly (p < 0.05) by increasing of SCSE concentration. The SG films without SCSE showed the highest content of moisture 14.65% and this might be owing to the interaction between hydrophilic groups of SA and GA with water molecules. The incorporation of SCSE polyphenols changed the mechanism of water molecules binding through potential hydrogen bonding. It might cause a competitive binding impact between SA, GA and polyphenols and as a result, limiting the interaction between hydrophilic groups of SA and GA with water molecules. The same phenomena were explained by using different polymers [19, 46, 47].
With regard to WVP, the principal purpose of packaging film is to protect the food products from the surrounded atmosphere. The movement of moisture from the surrounding atmosphere to the food product is one of the major causes of product degradation. Thus, it is better to use films for packaging products to retard this phenomenon and increase shelf life. WVP is one of the most determinations that can describe this function for films. As indicated in Table 1, the present results showed that the addition of SCSE to SG films caused significant decreasing (p < 0.05) effect on WVP of film samples except of the first concentration of 0.4%. WVP of the films decreased from 3.357 to 1.125 (×10−10 g H2O/m s p.a.) with the increase of SCSE concentration. The increasing of film thickness probably led to retard of water vapor molecule to permeate from the film [48]. Furthermore, the good distribution of SCSE hydrophilic groups could fill the interspaces between SA and GA molecules and as a result delaying the penetration of moisture through films. Moreover, the water vapor transmission mainly related to the hydrophobic-hydrophilic ratio of among the film components [28]. The obtained results followed the same manners with those obtained previously [28, 49, 50].
Color Properties
The values of the color indices, L*, a* b* and ∆E are indicated in Table 2, with the addition of SCSE to the SG films, L* (lightness/darkness) values decreased whereas a*, b* and ∆E values increased. The empty films (control) were lighter and indicated higher L* value of 81.51. The results indicated that the incorporation of SCSE imparted its yellow color to the films, resulting in a significant decrease (p < 0.05) of L* values and by contrary a significant increase (p < 0.05) of b* values which consider indicator for the high tendency towards yellowness. Similarity, with the incorporation of polyphenols into films, a significant decrease (p < 0.05) of L* and an increase in b* values were obtained [22].
Notably, SG films were more limpid (little opacity value) than those combined with SCSE (Table 1). The opacity values of the fabricated films significantly increased (p < 0.05) with increasing of SCSE concentration. The obtained results for opacity were in the consistent of Gómez-Estaca et al. [51] and Siripatrawan and Harte [22] who observed an increasing of films opacity by consequence addition of the antioxidants. The fabricated colored films in this study are edible for the applications where the visibility of products is not needed or in case of sensitivity to light products. And this function was attributed to the color variation and opacity by incorporation of SCSE inside films.
Oil Impedance Ability of Films
The oil impedance characteristics of SG-SCSE films was investigated. The results revealed that SG-SCSE4 film displayed lower OAR in comparison to the control film (Table 3). There was a decrease in OAR values in SG-SCSE1, SG-SCSE2,3 and SG-SCSE4 films. The possible cause that could explain this phenomenon, is the attendance of hydrophilic hydroxyl groups in the content of SG and the SCSE that caused obtaining thicker films as mentioned before and as a result, might obstruct oil molecules to penetrate the films. Thus, the low values of OAR indicated a higher oil impedance manner, which is a favorable manner for films with purposes of wrapping oily food products to prevent the release of oil.
Peroxide Value (POV)
POV is a suitable and most extensively utilized test to study the oxidation process of oils. The results of the POV of sunflower oil preserved for 28 days are mentioned in Table Winter. The SG-SCSE films indicated a significant decrease in the oil oxidation. All samples showed an increased POV negligent of the time. However, this growth of POV was with slower rate by SG-SCSE films as compared to open samples and SG film. After 28 days of storage, open samples recorded the maximum value of POV 53.81 ± 0.98 (mEq/kg) while for SG-SCSE4 it was 21.23 ± 0.88 (mEq/kg).
The attendance of polyphenols inside SG films provided a compact structure that could reduce oxidation process by obstructing penetration of oxygen through films [52]. Additionally, the antioxidant activity of the film was enhanced by incorporation of SCSE in the film, which also admitted with obstructing of oil oxidation during storage.
Conclusions
New active bio-composite films from SA and GA incorporated with SCSE were prepared in the present study. The presence of SCSE led to decrease of WVP that suggested maintaining moisture around wrapped products. The prepared films were functionalized to preserve oils from oxidation. The incorporation by SCSE up to 1.2 and 1.6% for the SG-SCSE3 and SG-SCSE4, respectively delayed the oxidation process during storage days. Generally, the results of the current study introduce useful biodegradable films to increase the shelf life and retard the oxidation rate of oils.
References
Martucci JF, Accareddu A, Ruseckaite RA (2012) Preparation and characterization of plasticized gelatin films cross-linked with low concentrations of Glutaraldehyde. J Mater Sci 47(7):3282–3292
Gomaa M, Fawzy MA, Hifney AF, Abdel-Gawad KM (2018) Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll 82:64–72
Brownlee I, Allen A, Pearson J, Dettmar P, Havler M, Atherton M, Onsøyen E (2005) Alginate as a source of dietary fiber. Crit Rev Food Sci Nutr 45(6):497–510
Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M (2016) Development of edible films and coatings from alginates and carrageenans. Carbohydr Polym 137:360–374
Blanco-Pascual N, Montero M, Gómez-Guillén M (2014) Antioxidant film development from unrefined extracts of brown seaweeds Laminaria digitata and Ascophyllum nodosum. Food Hydrocoll 37:100–110
Erben M, Pérez AA, Osella CA, Alvarez VA, Santiago LG (2019) Impact of gum arabic and sodium alginate and their interactions with whey protein aggregates on bio-based films characteristics. Int J Biol Macromol 125:999–1007
Prakash A, Joseph M, Mangino M (1990) The effects of added proteins on the functionality of gum arabic in soft drink emulsion systems. Food Hydrocoll 4(3):177–184
Idris O, Williams P, Phillips G (1998) Characterisation of gum from Acacia senegal trees of different age and location using multidetection gel permeation chromatography. Food Hydrocoll 12(4):379–388
Elmanan M, Al-Assaf S, Phillips GO, Williams PA (2008) Studies on Acacia exudate gums: Part VI. Interfacial rheology of Acacia senegal and Acacia seyal. Food Hydrocoll 22(4):682–689
Wyasu G, Okereke N (2012) Improving the film forming ability of gum Arabic. J Nat Prod Plant Resour 2(2):314–317
Basiak E, Lenart A, Debeaufort F (2017) Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch–whey protein blend edible films. J Sci Food Agric 97(3):858–867
Silva K, Mauro M, Gonçalves M, Rocha C (2016) Synergistic interactions of locust bean gum with whey proteins: effect on physicochemical and microstructural properties of whey protein-based films. Food Hydrocoll 54:179–188
Talón E, Trifkovic KT, Vargas M, Chiralt A, González-Martínez C (2017) Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr Polym 175:122–130
Eltabakh M, Kassab H, Badawy W, Abdin M, Abdelhady S (2021) Active bio-composite sodium alginate/maltodextrin packaging films for food containing Azolla pinnata leaves extract as natural antioxidant. J Polym Environ. https://doi.org/10.1007/s10924-021-02287-z
Riaz A, Lagnika C, Luo H, Dai Z, Nie M, Hashim MM, Liu C, Song J, Li D (2020) Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int J Biol Macromol 150:595–604
Mohamed AA, Ali SI, El-Baz FK (2013) Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS ONE 8(4):e60269
Prabhakaran S, Gothandam K, Sivashanmugam K (2011) Phytochemical and antimicrobial properties of Syzygium cumini an ethanomedicinal plant of Javadhu hills. Res Pharm 1(1):22–32
Abdin M, Hamed YS, Akhtar HMS, Chen D, Mukhtar S, Wan P, Riaz A, Zeng X (2019) Extraction optimisation, antioxidant activity and inhibition on α-amylase and pancreatic lipase of polyphenols from the seeds of Syzygium cumini. Int J Food Sci Technol 54(6):2084–2093
Akhtar HMS, Riaz A, Hamed YS, Abdin M, Chen G, Wan P, Zeng X (2018) Production and characterization of CMC-based antioxidant and antimicrobial films enriched with chickpea hull polysaccharides. Int J Biol Macromol 118:469–477
Zhang P, Zhao Y, Shi Q (2016) Characterization of a novel edible film based on gum ghatti: effect of plasticizer type and concentration. Carbohydr Polym 153:345–355
Atef M, Rezaei M, Behrooz R (2014) Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int J Biol Macromol 70:537–544
Siripatrawan U, Harte BR (2010) Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll 24(8):770–775
Kim S, Baek S-K, Song KB (2018) Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Packag Shelf Life 18:157–163
Božič M, Gorgieva S, Kokol V (2012) Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr Polym 87(4):2388–2398
Wang X, Yong H, Gao L, Li L, Jin M, Liu J (2019) Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll 89:56–66
Firestone D (1998) Association of Coaching Supervisors. Official and Recommended Practices of the American Oil Chemists’ Society, Official Methods and Recommended Practices. AOAC Press
Sun X, Wang Z, Kadouh H, Zhou K (2014) The antimicrobial, mechanical, physical and structural properties of chitosan–gallic acid films. LWT Food Sci Technol 57(1):83–89
Norajit K, Kim KM, Ryu GH (2010) Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J Food Eng 98(3):377–384
Kanikireddy V, Kanny K, Padma Y, Velchuri R, Ravi G, Jagan Mohan Reddy B, Vithal M (2019) Development of alginate-gum acacia‐Ag0 nanocomposites via green process for inactivation of foodborne bacteria and impact on shelf life of black grapes (Vitis vinifera). J Appl Polym Sci 136(15):47331
Ibekwe CA, Oyatogun GM, Esan TA, Oluwasegun KM (2017) Synthesis and characterization of chitosan/gum arabic nanoparticles for bone regeneration. Am J Mater Sci Eng 5(1):28–36
Pongjanyakul T, Puttipipatkhachorn S (2007) Xanthan–alginate composite gel beads: molecular interaction and in vitro characterization. Int J Pharm 331(1):61–71
Pongjanyakul T, Rongthong T (2010) Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohydr Polym 81(2):409–419
Nagpal M, Singh SK, Mishra D (2013) Synthesis characterization and in vitro drug release from acrylamide and sodium alginate based superporous hydrogel devices. Int J Pharm Invest 3(3):131
Sabet S, Rashidinejad A, Melton LD, Zujovic Z, Akbarinejad A, Nieuwoudt M, Seal CK, McGillivray DJ (2021) The interactions between the two negatively charged polysaccharides: Gum Arabic and alginate. Food Hydrocoll 112:106343
Riaz A, Lei S, Akhtar HMS, Wan P, Chen D, Jabbar S, Abid M, Hashim MM, Zeng X (2018) Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int J Biol Macromol 114:547–555
Ruan C, Zhang Y, Wang J, Sun Y, Gao X, Xiong G, Liang J (2019) Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int J Biol Macromol 134:1038–1044
Martins JT, Cerqueira MA, Vicente AA (2012) Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll 27(1):220–227
Pankaj SK, Bueno-Ferrer C, Misra N, O’Neill L, Tiwari B, Bourke P, Cullen P (2014) Physicochemical characterization of plasma-treated sodium caseinate film. Food Res Int 66:438–444
Falkeborg M, Cheong L-Z, Gianfico C, Sztukiel KM, Kristensen K, Glasius M, Xu X, Guo Z (2014) Alginate oligosaccharides: enzymatic preparation and antioxidant property evaluation. Food Chem 164:185–194
Şen M, Atik H (2012) The antioxidant properties of oligo sodium alginates prepared by radiation-induced degradation in aqueous and hydrogen peroxide solutions. Radiat Phys Chem 81(7):816–822
Al-Majed AA, Abd‐Allah AR, Al‐Rikabi AC, Al‐Shabanah OA, Mostafa AM (2003) Effect of oral administration of arabic gum on cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol 17(3):146–153
Peng Y, Wu Y, Li Y (2013) Development of tea extracts and chitosan composite films for active packaging materials. Int J Biol Macromol 59:282–289
Tan Y, Lim S, Tay B, Lee M, Thian E (2015) Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater Res Bull 69:142–146
da Silva MA, Bierhalz ACK, Kieckbusch TG (2009) Alginate and pectin composite films crosslinked with Ca2+ ions: effect of the plasticizer concentration. Carbohydr Polym 77(4):736–742
Campos CA, Gerschenson LN, Flores SK (2011) Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol 4(6):849–875
Hafsa J, ali Smach M, Khedher MRB, Charfeddine B, Limem K, Majdoub H, Rouatbi S (2016) Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci Technol 68:356–364
Leceta I, Guerrero P, De La Caba K (2013) Functional properties of chitosan-based films. Carbohydr Polym 93(1):339–346
Mali S, Sakanaka LS, Yamashita F, Grossmann M (2005) Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr Polym 60(3):283–289
Hosseini SF, Rezaei M, Zandi M, Ghavi FF (2013) Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem 136(3-4):1490–1495
Su J-F, Huang Z, Yuan X-Y, Wang X-Y, Li M (2010) Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr Polym 79(1):145–153
Gómez-Estaca J, Giménez B, Montero P, Gómez-Guillén M (2009) Incorporation of antioxidant borage extract into edible films based on sole skin gelatin or a commercial fish gelatin. J Food Eng 92(1):78–85
Mariniello L, Giosafatto C, Di Pierro P, Sorrentino A, Porta R (2010) Swelling, mechanical, and barrier properties of albedo-based films prepared in the presence of phaseolin cross-linked or not by transglutaminase. Biomacromolecules 11(9):2394–2398
Acknowledgements
The study was enhanced by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdin, M., El-Beltagy, A.E., El-sayed, M.E. et al. Production and Characterization of Sodium Alginate/Gum Arabic Based Films Enriched with Syzygium cumini Seeds Extracts for Food Application. J Polym Environ 30, 1615–1626 (2022). https://doi.org/10.1007/s10924-021-02306-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02306-z