Abstract
In order to improve nutrient uptake by plants and at the same time decrease losses by leaching, polymeric compounds based on biodegradable poly(3-hydroxybutyrate) (PHB) and montmorillonite clay (MMt) were melt processed with KNO3 and NPK fertilizers (Fert). We present a comprehensive analysis of releasing profile of the potassium (K+), nitrogen (NH4+ and NO3−) and phosphorous (PO43−) from PHB/MMt/fertilizer at 90/0/10, 50/0/50, 80/10/10, 40/10/50, 25/25/50 formulation. The amount and type of the fertilizer and the presence of clay delineated the release of the cations and anions from the matrix. Cations released faster than anions through MMt composites due to cations–MMt interactions and its diffusion through MMt phase. The nutrients delivery from composites was lower than pure fertilizer. Composites formulations with smaller amount of PHB and MMt (25 wt%) were enough to encapsulate the fertilizer and delay the ions releasing. Further, the simple method of preparation also attains the sustainability of the whole process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Slow/controlled release fertilizer system is a way to delivery essential nutrient, such as potassium, nitrogen and phosphorus for plants, according to absorption curve of the culture [1, 2]. Polymer-based fertilizer releasing system is an area of increased interest seeking to protect the fertilizers availability from adverse environmental conditions by increasing their shelf-life and assure their health-promoting properties [3, 4]. Clays, silica, lignin and polymeric particles are used as the holder to fertilizer and other agrochemical products [5]. Clay minerals are hydrated layer silicates able to exchange cations and intercalate species between the interlayer regions. Pereira et al. [6]. prepared slow release fertilizer nanocomposite based on urea intercalation into montmorillonite clay by an extrusion process and observed a slow urea release even for composites with 20 wt% of MMt. Biodegradable materials are specially suitable as carrier for fertilizers and other agrochemical products due to their low toxicity, high degradability in soil and controlled releasing capacity of active ingredient [7, 8]. Poly(hydroxybutyrate) (PHB) is a polymer produced by different types of microorganisms from sugarcane and molasses as intracellular storage materials [9]. PHB is fully biodegradable polyester with hydrophobic properties, low O2, CO2 and H2O permeability and high melting temperature and crystallinity [10,11,12]. The development of composites by incorporating low percentages of clay into a PHB matrix is known to improve properties such as ion exchange capacity, water absorption speed and mechanical resistance [1]. The higher surface area created for polymer/clay interaction compared with conventional composites enhances the thermal and oxidative barrier [13]. In a previous work Souza et al. [14] prepared composites based on PHB, starch, glycerol and montmorillonite clay (MMt) as carrier for KNO3 and NPK. They observed PHB/starch compatibility and homogeneity increased in presence of MMt, KNO3 and NPK. MMt–NPK particles were dispersed in the homogeneous and compatible polymeric phase while MMt–KNO3 was set preferentially on the PHB phase.
In this way, the present study investigates the polymeric composite based on biodegradable polymers (PHB) and natural clay (montmorillonite) to control the NPK and KNO3 release. The hydrophobicity and high crystallinity of PHB make it insoluble in water. Although, this polymer may be suitable and employed in nutrients delivery when it is processed with MMt, which may facilitate the water permeability and the host nutrient release. The effect of clay amount on nutrients release time will be discussed in the present work. In addition, the nutrient amount was also evaluated to understand the relationship between the structure and the nutrient ions release.
Experimental
Chemicals
Poly(3-hydroxybutyrate) powder (Tm = 160–175 °C; Tdecomp. = 230 °C; Biocycle, Brazil), sodium montmorillonite clay (MMtNa+, Brasgel Aço A, Bentonit União, Brazil), KNO3 (Salpetre Krista, K) and commercial granular NPK 4-14-8 (NH4+, P2O5, K2O) from Yara Brazil Fertilizantes S.A. were used without purification. Table 1 shows the percentage of each nutrient in fertilizers.
Preparation of MMt–KNO3 and MMt–NPK
Firstly, a mixture of montmorillonite and fertilizers was prepared by the mechanochemical method [14], which is a manual grinding in the agate mortar of the MMt (7 g) with distilled water (couple of droplets) and 7 g of fertilizer (NPK or KNO3) until a homogeneous material were achieved. The compound was dried in the oven at 60 °C and crushed to obtain a homogeneous powder.
Preparation of Composites
The composites based on PHB, MMt and fertilizers (KNO3 and NPK (4-14-8), Fert) were processed in an internal mixer chamber of a Haake torque rheometer with cam roller motors at 160 °C, 50 rpm for 6 min. The composites PHB/Fert 90/10 and 50/50 (wt%) and PHB/MMt/Fert 80/10/10, 25/25/50 and 40/10/50 (wt%) were hot pressed at 150 °C, 14 MPa for 3 min and the composites (0.5 × 0.5 × 0.1 cm3) were used to accomplish the ion release analysis and characterizations.
Characterization of Composites
X-ray diffraction (XRD) were recorded using a Shimadzu LAB 600 diffractometer in a diffraction range 2°–90° with a CuKα incident beam (λ = 0.1546 nm). The scanning speed was 2° min−1 and the voltage and current of the X-ray tubes were 30 kV and 30 mA, respectively.
Spectroscopic analyses in the infrared region of the polymers and composites were analyzed using reflectance measurement in the range of 4000–500 cm−1. The FTIR spectra data was obtained using Perkin Elmer Spectrum One instrument.
The morphology of the samples was analyzed by scanning electron microscopy (SEM) (FEI Magellan microscope 400L) equipped with an energy dispersive analysis system of X-ray spectrometer-EDX. The samples were fractured for cryo-SEM by immersing in liquid nitrogen. Cross-sections of fractured samples were mounted with the cross-section positioned upward on the stubs and dispersed over carbon tape pasted on the surface of a metallic disk (stub). The disk was then coated with gold and palladium in an ionization chamber and analyzed.
Thermogravimetric analysis (TGA) was performed on SDT 2960-TA Instruments, from 40 to 900 °C under synthetic air flow of 100 cm3 min−1 and heating rate of 10 °C min−1, using 10 mg of the sample. DSC was carried on DSC-60 Shimadzu under 5 mg of the sample, nitrogen air flow of 50 mL min−1 and heating rate of 10 °C min−1 on sealed aluminum pans, from 40 to 350 °C.
Release Rate of Nutrients in Water
Potassium, nitrogen and phosphorous releasing in ultrapure water (Milli-q) was quantified by flame photometer (Digimed DM-62, Brazil), total organic carbon analysis (TOC-L with TNM, Shimadzu) and colorimetry by UV–Visible DR5000 (Hach), respectively. The cumulative concentrations were determined as a function of time using a calibration curve of the fertilizers (KNO3 and NPK) (80–800 mg L−1). The composite [0.50 g ± 0.01 g (w/w) with 0.5 × 0.5 × 0.1 cm3 size] was immersed in 50 mL of ultrapure water at 25 °C and the ions released were determined after 1 h and until 25 h (three replicates). The final amount of nutrient released from the composites was expressed as a percentage based on the initial amount of nutrients in the materials.
Results and Discussions
Structural, Morphological and Thermal Properties
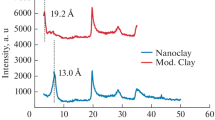
The diffraction patterns at 2θ = 13°, 17°, 19°, 21°, 22°, 25° and 27° (Fig. 1a) are according to the orthorhombic structure of PHB [15, 16]. Montmorillonite clay (Fig. 1b) shows d001 basal spacing at 2θ = 5.46° corresponding to a 1.63 nm [17]. The PHB–KNO3 90/10 (Fig. 1e) and PHB–NPK 90/10 (Fig. 2e) composites retain the main reflection peak of PHB. The clay interlamellar distance after fertilizers or PHB mixture can be monitored by the displacement of the diffraction angle d001 (Figs. 1 and 2 detailed). MMt peak at 5.46° displaced to 6.11° after PHB incorporation and to 6.75° for KNO3-containing PHB–MMt–KNO3 25/25/50 composite (Fig. 1C, I—detailed). Similar behavior was observed for NPK-based composite (Fig. 2). The MMt diffraction peak displaced from 5.46° to 6.58° for PHB–MMt–NPK 80/10/10 and 6.71° for PHB–MMt–NPK 25/25/50 (Fig. 2F–I). These displacements are related to the decrease of the interlayer space (1.63 nm for MMt to ~ 1.30 nm for PHB–MMt–fert) and an indicative of ions exchange (Fig. 2). NPK and KNO3 fertilizers comprise cations such as ammonium and potassium and anions from phosphate and nitrate. Then, both positive ions will be able to exchange for sodium ions in the interlamellar region. However, NH4+ and K+ have smaller hydration sphere than Na+, which explains the decrease of the basal space for MMt–fert materials [18].
From infrared spectroscopy analyses results (Supplementary Fig. S1), the incorporation of MMt and/or fertilizers into PHB did not change the PHB spectrum since there were no significant interactions among the components. Characteristic absorption bands of PHB are attributed to CH3 (2978, 1454, 1379 cm−1), CH2 (2930 cm−1), C=O stretching (1727 cm−1), and C–O–C stretching (1182 cm−1) [19,20,21]. The MMt main band is related to Si–O vibrations at 900 cm−1. The FTIR spectrum of KNO3 shows a band at 1385 cm−1 assigned to the NO3− symmetric stretching, at 821 cm−1 due to NO group bending and at 690–763 cm−1 assigned to NO2 deformation [22]. However, the NO3– band is suppressed by CH3 band observed in the PHB samples. FTIR spectrum of NPK shows bands at 1631 cm−1 assigned to the N–H angular deformation and 3421 and 3211 cm−1 of the asymmetric and symmetric axial deformation of N–H, respectively. Bands at 1154 cm−1 of P–O axial deformation (P2O5) and between 597 and 675 cm−1 are characteristic of PO43−. The overlaying around 1018 cm−1 is due to the axial deformation of P–O (NPK) and the Si–O group of MMt [23].
Figure 3 shows the morphological aspects of the PHB and its MMt and KNO3 containing composites. MMt or KNO3 were dispersed on PHB, for binary composites, PHB–MMt (Fig. 3a) generated a heterogeneous interface due to MMt weak interactions with PHB matrix. For PHB–KNO3 (Fig. 3b, d), the interactions between the nutrient and matrix depends on the amount of nutrient. The KNO3 dispersion on PHB-matrix was higher for PHB–KNO3 50/50 than 90/10 since nitrate acts as plasticizer [14, 24] (as it can be corroborated by DSC analysis) that promotes the percolation of nutrient into PHB matrix. For ternary composites (PHB–MMt–KNO3) the fertilizer was associated with both polymeric matrix and MMt, as evidenced by EDS analysis (Fig. 3c, e, f). Also, PHB–MMt–KNO3, 40/10/50 (Fig. 3e) and 25/25/50 (Fig. 3f) showed great dispersion for higher fertilizer amount assigned to the plasticizing effect as well. Panayotidou et al. [25] observed high clay dispersion due to the chain diffusion through the clay gallerias for PHB/MMt with low amount of clay (1–5 wt%). In our work, besides the high amount of clay, the presence of fertilizer precludes the intercalation of the polymer into clay galleries.
NPK-contained composites (Fig. 4a, c), showed heterogeneous surface independently of NPK amount. The nutrient (NH4+, PO43−, K+) are associated with clay (Fig. 4b) for composites with small amount of NPK (10%) and dispersed on the polymer/clay matrix for higher NPK content (50%) (Fig. 4d, e). EDS analyses (Fig. 4) realized on selected points presented clay and fertilizers trace dispersed in the polymeric matrix. Differently from KNO3-containing composites, NPK did not act as plasticizer as can also be observed on DSC analysis.
TG/DTG curves of PHB/Fert and PHB/MMt/Fert composites are given in Fig. 5 and Table S1 (Supplementary Material) shows the onset temperature. Two main stages of mass loss for KNO3-containing composites were observed (Fig. 5 and Table S1, Supplementary Material). The first, 250–300 °C, attributed to PHB and the second one, 600–850 °C, assigned to inorganic components degradation. The addition of KNO3 decreased the PHB–MMt thermal stability from 261 to under 234 °C, Table S1. This behavior was associated with the polymer chains intercalation in clay, which promotes polymer thermal stability. However, the thermal stability of PHB/MMt/KNO3 decreased because the K+ into the clay lamellas preventing the entry of the polymer which leaves the polymer more susceptible to thermal degradation.
In a recent study Harmaen et al. [26] showed that residues from NPK reaction accelerated the thermal degradation of PLA. However, the presence of NPK on PHB composites changed the Tonset of PHB to higher values, which corroborate with DSC thermal degradation results (Table S2, Supplementary Material).
Additionally, K2O is the residue from composites containing KNO3, which is taken to calculate the total KNO3 in the material, resulting in residue of 45% [17]. NPK residues of thermal degradation are related to N2O, N2, NO2, H2O, Cl, NH4Cl, etc. [26]. NPK residue is referenced in 70% [14]. The residue value of the MMt and fertilizers calculated are similar from theoretical value, Table S1. The small difference was related to the losses during the sample thermal processing.
DSC curves and data are shown in Fig. 6 and Table S2, respectively. MMt affected the PHB nucleation and decreased its crystallinity (reported around 70% for neat PHB) [27]. According to Teixeira [2], MMt reduces the crystallinity of PHB, since MMt prevents the organization of PHB molecules (breaking the chains). Consequently, lower crystallinity of PHB was observed when MMt clay is present.
The addition of 10 wt% of KNO3 (PHB–MMt–KNO3 80/10/10) reduced the Tm from 174 to 170 °C and degradation temperature (Tdegrad) from 286 to 210 °C comparing to the PHB–MMt 90/10. Although, the crystallinity kept higher than other samples due to the high PHB percentage. On the other hand, higher amount of KNO3 (PHB–MMt–KNO3 25/25/50 and 40/10/50) reduced even more the crystallinity degree and the degradation temperature. These effects are caused by the plasticizing effect of nitrate in PHB [14]. It agreed with the lower values of Tonset on TG curves and the greater dispersion of the clay/nutrient in the polymer matrix observed on SEM analysis (Fig. 3) and XRD results.
NPK-containing composites behaved differently than KNO3 ones. PHB/MMt/NPK presented two melting peaks. The first Tm peak is characteristic of less ordered crystal fractions, and the second one to more ordered crystals, which melt at a higher temperature (Table S2). In this case, NPK did not act as a plasticizer for PHB and degradation temperature arose between 290 and 300 °C for all NPK-containing composites. However, higher values of NPK fertilizer, also, decreased significantly the PHB crystallinity.
Effect of Clay on Nutrient Release in Water
Nitrogen, Phosphorus and Potassium Delivery from NPK Fertilizer
Nutrients release from PHB–NPK and PHB–MMt–NPK are shown in Fig. 7. The amount of the nutrients affected the release profile, since nutrients delivery for nitrogen and potassium are dependent of the concentration but not for P. For example, for both PHB/NPK 90/10 and 50/50 the phosphorous delivery did not reach 50% of the total amount. The presence of clay slows down the phosphorous released for PHB–MMt–NPK 25/25/50 and 40/10/50. Phosphorus interacts with MMt and its release is inhibited once it is adsorbed by colloidal complex (clay) which characterize a promising composition for phosphorus fertilizer in NPK-containing material.
In general, the nutrient releases were directly associated to MMt in the matrix. The higher the amount of clay, the greater the nutrient release due to the hydrophilicity of the MMt-composite, which increases the solubility of the nutrients, following the sequence: potassium, nitrogen and phosphorus. Once MMt has high affinity with nutrients and water, and also, the nutrients are less protected by PHB.
This observation leads us to conclude that as K+ interacts physically with MMt it is not easily leached and may be replaced by other cations. Whereas anions such as nitrate and sulfate tend to be repelled by the negative charge of the clay and remain dissolved in the solution. For this reason, they tend to interact with the polymer matrix while the cations are dispersed in the composite clay. Thus, the presence of clay in the composite is important to control the release of cationic nutrients while accelerating the release of anionic ones.
Nitrogen and Potassium Delivery from KNO3 Fertilizer
The delivery of KNO3 in PHB matrix is presented in Fig. 8. The release of potassium and nitrogen was increased when the amount of KNO3 was up to 50% (PHB–KNO3 90:10 to PHB–KNO3 50:50). However, the presence of MMt on composites performed a distinct effect on potassium and nitrogen delivery. Comparing the composites PHB–MMt–KNO3 80:10:10 and PHB–MMt–KNO3 40:10:50, the same rate of nitrogen release (40%) was observed when clay amount was increased by only 10% and the amount of PHB was decreased.
Once nitrate acts a plasticizing for PHB, the nitrogen interacts with the polymer and protected it while the potassium mixes with MMt. This behavior increased the potassium delivery due to its high hydrophilicity. (Figure 8) For PHB–KNO3 and PHB–MMt–KNO3 the ions release order was potassium > nitrogen. Thus, the presence of clay in the composite is important to control the release of anionic nutrients while accelerating the release of cationic ones.
Release Kinetic
In the present study the release kinetic was evaluated using a semi-empirical model, known as the power law or the Korsmeyer–Peppas model [28, 29]. The kinetic parameters for studies of drugs or nutrients release were obtained using the Eq. 1:
where Ct/C is the fraction of nutrients release at any time t, K is a constant depending on the kinetic features and experimental conditions, and n is a diffusion exponent related to the release mechanism. The K and n values are obtained from log Ct/C versus log t [30] by the Eq. 2:
The release mechanism was investigated fitting data according to Eq. 1. Plots of log (Ct/C) versus log t, Eq. 2, provide the release exponent, n, the release factor K, and the correlation coefficient R2, listed in the Table 2. Potassium and nitrogen release for KNO3-contained composites, and potassium, nitrogen and phosphorus release for PHB–MMT–NPK 80/10/10 and PHB–NPK 90/10 and 50/50 show release exponent n ≤ 0.5, this indicate a quasi-Fickian diffusion mechanism [31, 32], in which nutrients diffuses partially though a swollen matrix and water filled pores in the composites, similarly observed for potassium release based on chitosan hydrogels [30]. However, the n values were 0.5 for phosphorus from PHB–MMt–NPK 25/25/50 and 40/10/50 suggesting the release was controlled by diffusion based, due the higher size of ion PO43− in high concentration.
Conclusions
The fertilizer interacts physically with PHB and PHB/MMt, and the MMt had the influence to increase the rate of the fertilizer release in water. This behavior is according with the morphology, due to the clay and fertilizer agglomerates in a heterogeneous structure. The water was efficiently diffusion due to the more affinity with MMt as demonstrated by the kinetic model. The release of fertilizers in composites was efficient even for MMt-containing composites (release about 30% in 9 h) enable them to be used as controlled release fertilizers. Composites made with higher concentration of fertilizer and clay and lower PHB amount are potential for industrial application with cost reduction. PHB was considered as an expensive material however its cost has been reduced to €1.5/kg in 2010 since new processes and production optimization have been achieved. This scenario is expected to be more rentable for 2020 [33] since its use for fertilizer encapsulation drives interesting attributes such as eco-friendly composites, easily formulation and processing.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
Bortolin A, Aouada FA, Mattoso LHC, Ribeiro C (2013) Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: evidence of synergistic effects for the slow release of fertilizers. J Agric Food Chem 61:7431–7439. https://doi.org/10.1021/jf401273n
Teixeira Chagas WF, Guelfi DR, Emrich EB et al (2016) Agronomic efficiency of polymer-coated triple superphosphate in onion cultivated in contrasting texture soils1. Rev Cienc Agron 47:439–446. https://doi.org/10.5935/1806-6690.20160053
Azeem B, Kushaari K, Man ZB et al (2014) Review on materials & methods to produce controlled release coated urea fertilizer. J Control Release 181:11–21. https://doi.org/10.1016/j.jconrel.2014.02.020
Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803. https://doi.org/10.1016/j.biotechadv.2011.06.007
Chen J, Lü S, Zhang Z et al (2018) Science of the total environment environmentally friendly fertilizers: a review of materials used and their effects on the environment. Sci Total Environ 613–614:829–839. https://doi.org/10.1016/j.scitotenv.2017.09.186
Pereira EI, Minussi FB, Cruz CCT et al (2012) Urea−montmorillonite-extruded nanocomposites: a novel slow-release material. J Agric Food Chem 60:5267–5272. https://doi.org/10.1021/jf3001229
Grillo R, Zocal N, Maruyama CR et al (2012) Poly(ɛ-caprolactone)nanocapsules as carrier systems for herbicides: physico-chemical characterization and genotoxicity evaluation. J Hazard Mater 231–232:1–9. https://doi.org/10.1016/j.jhazmat.2012.06.019
Volova TG, Prudnikova SV, Boyandin AN (2016) Biodegradable poly-3-hydroxybutyrate as a fertiliser carrier. J Sci Food Agric 96:4183–4193. https://doi.org/10.1002/jsfa.7621
Chandra R, Rustgi R (1998) Pergamon biodegradable polymers. Prog Polym Sci 23:1273–1335. https://doi.org/10.1016/S0079-6700(97)00039-7
Amico DAD, Manfredi LB, Cyras VP (2011) Relationship between thermal properties, morphology, and crystallinity of nanocomposites based on polyhydroxybutyrate. J Appl Polym Sci 123:200–208. https://doi.org/10.1002/app.34457
Maiti P, Batt CA, Giannelis EP (2007) New biodegradable polyhydroxybutyrate/layered silicate nanocomposites. Biomacromolecules 8:3393–3400
Wee CY, Liow SS, Li Z et al (2017) New Poly[(R)-3-hydroxybutyrate- co-4-hydroxybutyrate] (P3HB4HB)-based thermogels. Macromol Chem Phys 218:1700196. https://doi.org/10.1002/macp.201700196
Bruzaud S, Bourmaud A (2007) Thermal degradation and (nano) mechanical behavior of layered silicate reinforced poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. Polym Test 26:652–659. https://doi.org/10.1016/j.polymertesting.2007.04.001
Souza JDL, Chiaregato CG, Faez R (2017) Green composite based on PHB and montmorillonite for KNO3 and NPK delivery system. J Polym Environ 26:1–10. https://doi.org/10.1007/s10924-017-0979-4
Barud HS, Souza JL, Santos DB et al (2011) Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr Polym 83:1279–1284. https://doi.org/10.1016/j.carbpol.2010.09.049
Thiré RMSM, Ribeiro TAA, Andrade CT (2006) Effect of starch addition on compression-molded poly(3-hydroxybutyrate)/starch blends. J Appl Polym Sci 100:4338–4347. https://doi.org/10.1002/app.23215
Dos Santos BR, Bacalhau FB, Pereira TDS et al (2015) Chitosan-montmorillonite microspheres: a sustainable fertilizer delivery system. Carbohydr Polym 127:340–346. https://doi.org/10.1016/j.carbpol.2015.03.064
França D, Medina ÂF, Messa LL et al (2018) Chitosan spray-dried microcapsule and microsphere as fertilizer host for swellable—controlled release materials. Carbohydr Polym 196:47–55. https://doi.org/10.1016/j.carbpol.2018.05.014
Vogel C, Wessel E, Siesler HW (2008) FT-IR imaging spectroscopy of phase separation in blends of poly(3-hydroxybutyrate) with poly(L-lactic acid) and poly(E-caprolactone). Biomacromolecules 9:523–527
Lai S, Sun W, Don T (2015) Preparation and characterization of biodegradable polymer blends from poly (3-hydroxybutyrate)/poly (vinyl acetate)—modified corn starch. Polym Eng Sci 55:1321–1329. https://doi.org/10.1002/pen
Xu J, Guo BH, Yang R et al (2002) In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 43:6893–6899. https://doi.org/10.1016/S0032-3861(02)00615-8
Yang Y, Tong Z, Geng Y et al (2013) Biobased polymer composites derived from corn stover and feather meals as double-coating materials for controlled-release and water-retention urea fertilizers. J Agric Food Chem 61:8166–8174. https://doi.org/10.1021/jf402519t
Tănase EE, Popa ME, Râpă M, Popa O (2015) PHB/cellulose fibers based materials: physical, mechanical and barrier properties. Agric Agric Sci Procedia 6:608–615. https://doi.org/10.1016/j.aaspro.2015.08.099
Correa AC, Brait V, Alexandre J et al (2017) Biodegradable blends of urea plasticized thermoplastic starch (UTPS) and poly (ε-caprolactone) (PCL): morphological, rheological, thermal and mechanical properties. Carbohydr Polym 167:177–184. https://doi.org/10.1016/j.carbpol.2017.03.051
Panayotidou E, Kroustalli A, Baklavaridis A et al (2015) Biopolyester-based nanocomposites: structural, thermo-mechanical and biocompatibility characteristics of poly (3-hydroxybutyrate)/montmorillonite clay nanohybrids. J Appl Polym Sci 132:1–11. https://doi.org/10.1002/app.41628
Harmaen AS, Khalina A, Azowa I et al (2015) Thermal and biodegradation properties of poly(lactic acid)/fertilizer/oil palm fibers blends biocomposites. Polym Compos 36:576–583. https://doi.org/10.1002/pc.22974
Souza JL, Santos AF, Crespi MS, Ribeiro CA (2011) Thermal characterization of the interaction of poly(3-hydroxybutyrate) with maleic anhydride. J Therm Anal Calorim 106:453–458. https://doi.org/10.1007/s10973-011-1706-3
Ritger PL, Peppas NA (1987) A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 5:23–36. https://doi.org/10.1016/0168-3659(87)90034-4
Siepmann J, Peppas NA (2001) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 48:139–157. https://doi.org/10.1016/S0169-409X(01)00112-0
Jamnongkan T, Kaewpirom S (2010) Potassium release kinetics and water retention of controlled-release fertilizers based on chitosan hydrogels. J Polym Environ 18:413–421. https://doi.org/10.1007/s10924-010-0228-6
Sankar C, Mishra B (2003) Development and in vitro evaluations of gelatin A microspheres of ketorolac tromethamine for intranasal administration. Acta Pharm 53:101–110
Pradhan R, Budhathoki U, Thapa P (2008) Formulation of once a day controlled release tablet of indomethacin based on HPMC-mannitol. Kathamandu Univ J Sci Eng Technol 4(1):55–67
Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110:621–632. https://doi.org/10.1016/j.jbiosc.2010.07.014
Acknowledgements
The authors are grateful to FAPESP-Brazil (Process Number 2014/06566-9) for financial support, Bentonit União for supplying the clay, and Biocycle/PHB Industrial for supplying PHB, LCE-Structural Characterization Laboratory (UFSCAR) for SEM analysis. R. Faez is CNPq researcher.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Souza, J.L., de Campos, A., França, D. et al. PHB and Montmorillonite Clay Composites as KNO3 and NPK Support for a Controlled Release. J Polym Environ 27, 2089–2097 (2019). https://doi.org/10.1007/s10924-019-01498-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01498-9