Abstract
The recycling of used, post-industrial and post-consumer PLA is crucial to reduce both the consumption of renewable resources for the monomer synthesis and the environmental impact related to its production and disposal. Several processes are actually available: among these, there is a particular interest on the chemical recycling of PLA with production of its monomer. The aim of this work is to analyse the PLA dissolution behaviour in different organic solvents (acetone and Ethyl lactate) at different water concentrations in order to optimize the chemical depolymerisation process of PLA. New experimental data are presented and a kinetic model is provided for a first analysis. Preliminary results suggest that acetone based solvents (i.e., acetone water mixtures at various concentrations) are more effective to solubilize the PLA rather than the Ethyl-lactate based solvent. Anyway, an increase of water concentration in the solvent phase, determines both a reduction of the solvent power and a reduction of mass transport coefficient for the two solvents tested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the family of biodegradable polymers, nowadays the polylactic acid (PLA) is perhaps the most frequently used polyester, due to its many favourable properties, such as availability, relative good strength, biocompatibility and biodegradability [1–6].
The chemical recycling of PLA to its monomer is crucial to reduce both the consumption of renewable resources for the monomer synthesis and the environmental impact related to its production and disposal. The production of PLA from recycling also allows large energy savings, with respect to the traditional processes based on the use of virgin raw materials [7].
The PLA is a polyester derived from the lactic acid by a polycondensation reaction with water elimination. The elimination of each water molecule leads to the formation of an ester group (esterification reaction), starting from a hydroxyl and a carboxyl group (the latter provided by two distinct molecules of lactic acid). Placing the polymer in contact with water may cause the cleavage of ester linkages along the polymer chains.
The direct polycondensation is the simpler and less expensive method, but produces polymer with low molecular weight and therefore with poor mechanical properties. The low molecular weight is due to the unfavourable equilibrium constant of the reaction of the species lactic acid, polymer and water [22]. The complete removal of water is required in order to obtain an high degree of polymerization, so vacuum conditions and high temperature (T > Tmelting) are used. Furthermore, during the progress of the reaction, the concentration of reactive functional groups decreases, whereas secondary reactions become competitive.
The ring-opening polymerization (ROP), is the most used method in industry in order to produce PLA at high molecular weight. The production plant of PLA in Nebraska (NatureWorks LLC) [23] employs this method.
An alternative method for the chemical recycling of PLA to lactic acid is the thermal depolymerisation which reduces the polymer in the cyclic dimer of the lactic acid, the lactide. This method consists in a intramolecular transesterification of the polymer, which represents the reverse reaction of the ring-opening polymerization (ROP) [8].
The thermal depolymerization in lactide must be carried out at high temperature (200–250 °C) and under vacuum (4–5 mmHg) conditions, in the presence of suitable catalysts (such as organic compounds of zinc, tin, aluminium, titanium, zirconium) [9]. This process is quite complex and expensive, not only for the severe operating conditions but also for the need of several purification steps of the final product.
The hydrolysis, unlike the thermal degradation, is a rather simple depolymerization process, from a technical point of view, and it is not expensive: in fact it does not require severe operating conditions and it does not either need catalysts. The PLA hydrolysis rate in an aqueous phase is essentially influenced by the pH, the temperature and the crystallinity of the PLA [10–12]. From a general point of view, the hydrolytic degradation of a polymer matrix occurs through two processes in series: water diffusion into polymer mass and hydrolysis reaction. When the water diffusion rate is slow compared with the kinetic of hydrolysis reaction (i.e., when the diffusion is the controlling step), the process occurs through a mechanism of surface erosion (heterogeneous erosion), and the hydrolysis takes place predominantly on the polymer surface. On the other hand, when the water diffusion rate is fast compared with the kinetic of hydrolysis reaction (i.e., when the reaction is the controlling step), the process occurs through a mechanism of bulk erosion (homogeneous erosion), in which the hydrolysis takes place in the whole mass (volume) of the polymer.
The hydrolysis has been demonstrated to be an autocatalytic reaction [10, 12]: indeed, the reaction rate increases by the addition of carboxyl groups, formed by the breakdown of the ester bonds during the depolymerization reaction. The main effect of the carboxyl groups is to decrease the pH, by favouring the hydrolytic acid catalysis.
Some works [13, 14] deal with the PLA hydrolysis in aqueous phase at high temperatures, that result in high yields of lactic acid in a fairly short time, without the use of catalysts. These studies were carried out at temperatures ranging between 120 and 350 °C and it was observed that the hydrolysis proceeds by a mechanism of bulk erosion in both the solid and the molten PLA.
It is also seen that the reaction times decrease with increasing temperatures. However excessively high temperatures (above 250 °C) are not recommended because they induce racemization and decomposition of the lactic acid formed. On the contrary, temperatures lower than the melting one are favourable as for the energy consumption, but they result into much longer times to get high yields, mainly because of the crystalline residues that remain after degradation of the amorphous regions.
The first patents about the PLA depolymerization by hydrolysis (or by solvolysis with alcohol) date back to the early 90s and belong to DuPont [15, 16]. These patents report a very simple and economic process, in which the PLA is placed in aqueous solution (50–70 % by weight of PLA) and heated up to temperatures of 100–200 °C, in a pressurized reactor (autogenous pressure), as long as to achieve conversions of 70–100 %.
A process of particular interest for the chemical recycling of PLA by alcoholisys has recently been patented by Galactic (LOOPLA process): it uses a solvent to bring the PLA in solution and it is designed precisely for chemical recycling of used, post-industrial and post-consumer PLA [17].
Xiuyan et al. [18] report an interesting work on PLA hydrolysis into calcium lactate using ionic liquid [Bmim][OAc] to optimize the PLA chemical recycling process, while Plichta et al. [19] studied the chemical recycling of PLA via controlled degradation with protic (macro)molecules. The work of Sanchez et al. [20] on the selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions, is also interesting.
The aim of this work is to analyse the PLA dissolution behaviour in different organic solvents, acetone and Ethyl lactate, at different water concentrations, at 50 and 80 °C, in order provide some guidelines to optimize the chemical depolymerisation process of PLA. Ethyl lactate was chosen as PLA solvent, because of its use in the PLA solubilization process patented by Coszach et al. [17]. On the contrary, acetone was chosen as a solvent since it is very cheap, has a high vapour pressure, shows a high solvent power and is completely miscible with water. These characteristics are very useful in the lactic acid production process from PLA depolymerization.
Materials and Methods
Experimental Procedure
The kinetic tests have been carried out in a batch reactor where the solvent phase (mixtures of water and Acetone or Ethyl-lactate, ranging from 11 to 30 ml) and solid PLA (ranging from 1 to 6 g) are brought into contact. Pure solvents have been purchased from Sigma Aldrich. For each solvent the experimental runs have been performed at different water concentrations and at fixed pressure (1 bar) and temperature. Preliminary tests (not reported here) have been suggested to perform solubility tests at 50 and 80 °C for Acetone and Ethyl-lactate based solvents, respectively. The batch reactor was equipped with an heating jacket to set the reaction temperature and a vertical vapour condenser to recover solvent and water vapours. The experimental apparatus was placed on a magnetic stirrer to guarantee the right agitation inside the reactor (see Fig. 1).
The fragments of solid PLA were obtained by grinding shells of PLA (IngeoTM biopolymer 2003D) provided by Nature-Works. At the beginning of each run, the reactor was dried and filled with weighed amounts of solvent mixture and PLA.
At fixed time, the liquid solution within the reactor was removed and centrifuged to separate any the solid particles in suspension. Then, the liquid phase was filtered by a 25 micron pore filter to separate smaller solid particles present in the liquid phase. Subsequently, a weighed amount of the solution was taken and put in a stove under vacuum (10 kPa) at 50° C. Evaporation of the solvent take place so the amount of precipitated PLA can be determined gravimetrically. The weight fraction of PLA in solution is straightforwardly determined from the knowledge of initial mass of solution and of mass of PLA dissolved. The solid amount of PLA not dissolved was also evaluated gravimetrically after dehydration in order to check the experimental data consistency.
Mathematical Modelling
The PLA dissolution in the solvent phase has been modelled with a first order differential equation derived from the PLA mass balance in the liquid phase
where X PLA and X PLA,S are the PLA compositions (by weight) in the liquid phase and at saturation conditions, respectively; k L is the mass transport coefficient between the PLA solid phase and liquid phase, a is the specific surface, k is hydrolysis kinetic reaction rate and X 0 W is the water concentration in the liquid phase, assumed constant during time.
X PLA,s can be calculated from experimental kinetic curves when saturation conditions are reached (plateau for each curve).
It is possible to rewrite the mass balance in a dimensionless form with respect to PLA concentration, by assuming \(\tilde{X}_{PLA} = X_{PLA} /X_{PLA,s}\)
where β = kX 0 W /ak L is a dimensionless number derived from the ratio of the dissolution and reaction characteristic times.
Integration of Eq. (2) gives the PLA concentration in the liquid phase as function of time
It is worth noting that when β → 0 the hydrolysis reaction is very slow compared to PLA dissolution in the liquid phase, therefore Eq. (3) assume the simplified form
while, when β → ∞ all the PLA dissolved in the liquid phase is “consumed” by hydrolysis, therefore in this extreme condition PLA concentration in the liquid phase reaches the zero value. Of course, if β > 1 the PLA concentration reaches a plateau which range around X PLA,s /β.
Results
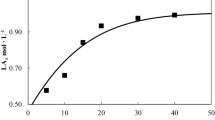
The PLA dissolution process has been tested over two different organic solvents, Acetone and Ethyl-Lactate, at different water concentrations and fixed pressure and temperature. In particular, Fig. 2 shows the PLA dissolution behaviour in Acetone–Water solvent at different water concentration over time for a fixed temperature (50 °C). As it can be seen, an increase of water concentration determines a reduction of PLA weight fraction % in the solvent phase, up to a near zero value after 420 min for a water concentration near to 35 % by weight.
Trend of PLA concentration in water + acetone mixtures versus time at 50 °C. a Filled black square 100 % acetone, white square 95 % acetone, filled black triangle 90 % acetone; b white dimand 85 % acetone, filled black circle 65 % acetone. Solid lines represent fitted curves with Eq. (3)
Figure 3 reports the PLA dissolution curves for the Ethyl Lactate-Water system at different concentrations of water vs time at 80 °C. Also in this case it is possible to observe a reduction of PLA weight fraction % in the solvent phase at increasing water concentration. In particular, a water concentration in the solvent phase near to 20 % by weight, determines an almost zero PLA dissolution during a tested run of 420 min.
Trend of PLA concentration in water + ethyl lactate mixtures versus time at 80 °C. a Filled black square 100 % ethyl lactate, white square 95 % ethyl lactate; b filled black triangle 90 % ethyl lactate, white circle 80 % ethyl lactate. Solid lines represent fitted curves with Eq. (3)
From the comparison of the two figures, it is clear that the acetone based solvent is more reliable to dissolve the PLA rather than the Ethyl-lactate solvent. The experimental runs with acetone water mixtures were carried out at a lower temperature because of the high volatility of acetone: in this way the loss of solvent was reduced.
Discussion
The obtained experimental results have been analysed by using Eq. (3) reported in “Materials and Methods” section. In this preliminary study it has been assumed that experimental conditions would lead to an hydrolysis reaction minimization, that is β → 0. In fact, data relating to the hydrolysis kinetics of PLA oligomers of high molecular weight in aqueous solutions, are generally scarce and only data on low molecular weight oligomers are reported by Codari et al. [21]. On the other hand, the presence of organic solvents such as ethyl lactate and acetone, promotes the solubilisation of high molecular weight oligomers then, in presence of water, data about hydrolysis kinetic of such oligomers should be necessary. Anyway Codari et al. [21] have demonstrated that the kinetic of hydrolysis is strongly influenced by temperature and by water concentration. As for temperature dependence, the experimental tests have been performed in the temperature range from 40 to 120 °C, showing values of kinetic constants at 40 and 120 °C that differ of two orders of magnitude: therefore at low temperature hydrolysis the reaction is very slow. Furthermore low ratios between water and PLA, as the ones utilized in this paper, further reduce the reaction rate. This assumption is also supported from experimental data collected on undissolved amount of PLA (PLA in solid phase). Furthermore it has also been assumed that particle size used in this work does not affect PLA dissolution behaviour. Therefore, it has been considered just ak L as an adjustable parameter obtainable from fitting of the experimental data. The experimental data collected have been fitted following a least square criterion.
Figures 2 and 3 shows the comparison between the experimental and calculated kinetic curves for the two selected solvents, while Tables 1 reports the obtained fitted parameters at different water concentration in the solvent phase, as well as statistical information about fitting reliability. The figures clearly show the good agreement between the experimental and calculated curves.
The analysed kinetic curves highlight a strong influence of water concentration on PLA dissolution behaviour. In order to find a correlation between the mass transport coefficient and solvent phase composition a linear regression on the obtained k values as function of the water weight fraction in the solvent phase has been carried out, as reported in Fig. 4 for the two analysed system. It is clear from the figure the linear dependence of k over water weight fraction. Therefore an increase of water concentration in the solvent phase determines both a reduction of solvent power (thermodynamic condition) and of mass transport coefficient (kinetic condition). Anyway, it is worth noting that for high water concentration, hydrolysis reaction could take place, therefore model hypothesis (β → 0) may be not still reliable. In this case, the strongly reduction on PLA dissolution behaviour at high water concentration in the liquid phase could be due to PLA hydrolysis that “consume” PLA in the solution and observed plateau is not yet indicative of thermodymanic equilibrium conditions. In order to deeply analyse this aspect independent experiments must be carried out to evaluate saturation condition in absence of hydrolysis reaction at high water concentration. This aspects will be objectives of further works.
Conclusions
In this paper new preliminary experimental data about the PLA dissolution in a mixtures of water and organic solvents were presented. Some interesting considerations can be derived:
-
1.
The Acetone based solvent is more effective to solubilise PLA rather than Ethyl-lactate based solvent;
-
2.
An increase of water concentration in the solvent phase determines a reduction of solvent dissolution capacity for both solvents tested;
-
3.
An increase of water concentration in the solvent phase determines a reduction of mass transport coefficient for both solvents tested.
To sum up, the findings reported in this study can be useful to carry out preliminary considerations on the dissolution process of PLA finalized to the optimization of hydrolytic depolymerization of PLA. In particular optimization of water weight fraction in the solvent mixture is of utmost importance because water is required to promote the hydrolysis reaction; on the contrary, the solvent power of the mixture organic compound—water is negatively affected by water weight fraction. For the sake of the clarity, it must be point out that this is a preliminary analysis and further works will be carried out in order to deeper analyse the effect of hydrolysis reaction and PLA particle size on dissolution behaviour.
References
Hakkarainen M (2002) Aliphatic polyesters: abiotic and biotic degradation and degradation products. Adv Polym Sci 157:113
Piemonte V, Gironi F (2011) Bioplastics and petroleum-based plastics: strengths and weaknesses. Energy Source Part A Energy Recover Environ Eff 33:1949–1959
Piemonte V, Gironi F (2012) Bioplastics and GHGs saving: the land use change (LUC) emissions issue. Energy Source Part A Energy Recover Environ Eff 34(21):1995–2003
Piemonte V, Gironi F (2011) Land use change emissions: how green are the bioplastics? Environ Prog Sustain Energy 30(4):685–691
Piemonte V (2011) Bioplastic wastes: the best final disposition for energy saving. J Polym Environ 19:988–994
Gironi F, Piemonte V (2011) Life cycle assessment of PET and PLA bottles for drinking water. Environ Prog Sustain Energy 30(3):459–468
Piemonte V, Sabatini S, Gironi F (2013) Chemical recycling of PLA: a great opportunity towards the sustainable development? J Polym Environ 21(3):640–647
Liang Z, Zhang M, Ni X, Li X, Shen Z (2013) Ring-openingpolymerization of cyclic esters initiated by lithium aggregatecontaining bis(phenolate) and enolate mixed ligands. Inorg Chem Commun 29:145–147
Noda M, Okuyama H (1999) Thermal catalytic depolymerisationof poly(L-lactic acid) oligomer into LL-lactide: effects of Al, Ti, Zn and Zr compounds as catalysts. Chem Pharm Bull 47:467–471
Henton DE, Gruber P, Lunt J, Randall J (2005) Polylactic acidtechnology. In: Mohanty K, Misra M, Drzal LT (eds) Naturalfibers. CRC Press, Boca Raton
Proikakis CS, Mamouzelos NJ, Tarantili PA, Andreopulos AG (2006) Swelling and hydrolytic degradation of poly(d, l-lacticacid) in aqueous solutions. Polym Degrad Stab 91:614–619
Inkinen S (2011) Structural modification of poly(lactic acid) bystep-growth polymerization and Stereocomplexation, Ph.D Thesis, Chemical Engineering Department, Abo Akademi University
Tsuji H, Daimon H, Fujie K (2003) A new strategy for recyclingand preparation of poly(l-lacticacid): hydrolysis in the melt. Biomacromolecules 4:835–840
Tsuji H, Saeki T, Tsukegi T, Daimon H, Fujie K (2008) Comparativestudy on hydrolytic degradation and monomer recoveryof poly(l-lactic acid) in the solid and in the melt. Polym Degrad Stab 93:1956–1963
Brake LD, Subramanian NS (1993) US Patent 5,229,5281993
Brake LD (1993) Recovery of polyhydroxy acids. US Patent 526,461,4
Coszach P, Bogaert JC, Willocq J Chemical recycling of PLA byalcoholysis. WO Patent 2010/118955 A
Xiuyan S, Hui W, Xuequn Y, Fusheng L, Shitao Y, Shiwei L (2014) Hydrolysis of poly(lactic acid) into calcium lactate using ionic liquid [Bmim][OAc] for chemical recycling. Polym Degrad Stab 110:65–70
Plichta A, Lisowska P, Kundys A, Zychewicz A, Debowski M, Florjanczyk Z (2014) Chemical recycling of poly(lactic acid) via controlled degradation with protic (macro)molecules. Polym Degrad Stab 108:288–296
Carne Sanchez A, Collinson SR (2011) The selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions. Eur Polym J 47(10):1970–1976
Codari F, Lazzari S, Soos M, Storti G, Morbidelli M, Moscatelli D (2012) Kinetics of the hydrolytic degradation of poly (lactic acid). Polym Degrad Stab 97:2460e–2466e
Chen GX, Kim HS, Kim ES, Yoon JS (2006) Synthesis of high-molecular-weight poly(l-lactic acid) through the direct condensation polymerization of l-lactic acid in bulk state. Eur Eur 42:468–472
Gruber PR, Hall ES, Kolstad JJ, Iwen ML, Benson RD, Borchardt RL (1992) Continuous process for manufacture of lactide polymers with controlled optical purity. US Patent 514,202,3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gironi, F., Frattari, S. & Piemonte, V. PLA Chemical Recycling Process Optimization: PLA Solubilization in Organic Solvents. J Polym Environ 24, 328–333 (2016). https://doi.org/10.1007/s10924-016-0777-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0777-4