Abstract
To improve the interfacial interaction between poly(lactic acid) (PLA) and bamboo particles (BP), sodium hydroxide solution was employed to pretreat BP, and then maleic anhydride (MAH) was used to compatibilize PLA/BP biocomposites. The biocomposites were prepared through melt blending and hot-pressing. Effects of MAH concentration on the properties of BP and PLA/BP biocomposites were investigated using Fourier transform infrared spectroscopy, mechanical measurements, differential scanning calorimetric, melt flow rate (MFR) analysis and scanning electron microscope. Results showed that interfacial interaction between PLA and BP in the biocomposites was improved with MAH compatibilizer. Tensile strength and elongation at break of PLA/BP biocomposites, reached maximal values of 47.6 MPa and 6.22 %, respectively, when treated with 1.0 % MAH. Maximal flexural strength of 72.61 MPa and flexural modulus of 4.65 GPa were obtained with 0.5 % MAH treatment, and thermal properties were also improved at this concentration. MFR of the blends was enhanced with MAH compatibilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The desire of sustainable development as well as government and environmental policies toward the creation of eco-civilization and use of sustainable material are continuously driving research in green composites [1]. Utilization of natural particles and biobased polymers as alternatives to certain synthetic has been attracting more attentions in recent years [3–6]. Poly(lactic acid) (PLA) is one of the most produced biodegradable polymers. It is renewable and recyclable with good thermal behaviors and even better mechanical properties (i.e., tensile/flexural strength and modulus) than some commodity plastics, e.g., poly(propylene) [4]. It is hydrolysable aliphatic semi-crystalline polyester produced through direct condensation of its monomer, lactic acid, followed by a ring opening polymerization of the cyclic lactide dimmer. Lactic acid can be obtained from renewable resources such as polysaccharides [7]. However, PLA presents some drawbacks like high cost and relatively low heat resistance, which limit its broader applications [8].
Bamboo particles has been used as fillers mainly in polyolefins in the literature because of their abundance, renewability, nonabrasiveness, low density, and low price [9–11]. Using of bamboo particles in PLA composite reduces production costs of PLA and has the potential to improve its mechanical properties. Moreover, the reinforced composites are also biodegradable. But as reported, there are abundant polar groups like hydroxyl and phenolic hydroxyl groups contained in bamboo particles, presenting relatively strong chemical polarity and hydrophilicity, whereas PLA is hydrophobic [12–15]. This results in poor compatibility. The interfacial interaction between PLA matrix and BP is weak, leading to reduced mechanical properties.
Interfacial adhesion and dispersion of the particles in a polymer matrix can be improved through compatibilization. Several compatibilization techniques such as acylation, alkali treatment, etherification, isocynate treatment and use of coupling agents have been researched in the literature to improve particles/matrix interface bonding strength [16–18]. Kang and Kim [19] observed enhancement in the mechanical properties of PLA/BP composites treated by sodium chlorite and/or a silane coupling agent. Our previous article reported improvements in properties of PLA/BP composites prepared by a two-roll mill that were based on 0.30 N alkali surface treated BP [8].
Maleic anhydride (MAH) reaction is one of the most efficient approaches of compatibilization, and various MAH grafted polymers have been commercialized. Consequently, MAH grafted PLA was synthesized in laboratory scale through solution polymerization or reactive extrusion [20, 21]. Carlson et al. [22] reported that free-radical chemistry was involved in the maleation of PLA during reactive extrusion (in situ melt blending in an extruder). Petchwattana et al. [23] compatibilized polyethylene/rice hull composites with maleic anhydride and found that mechanical properties and interfacial adhesion were improved. Similar results were reported with different fillers and matrices such as sisal whisker, kenaf fibers, polypropylene, acrylonitrile–butadiene–styrene, poly(butylene adipate-co-terepthalate), etc. [24–28].
We have previously investigated the influences of different treatment times with low-concentration alkaline solution on PLA/BP composites [8]. Alkaline treatment indeed improved the compatibility of PLA/BP composites. Little attention was paid on detailed information about MAH further compatibilization of PLA/BP composites. The aim of this work was to investigate the effects of MAH as compatibilizer for the alkali treated BP reinforced composites using five different mass fractions of MAH. We expected the mechanical properties and thermal behavior could be further improved after MAH compatibilizing. The prepared composites were characterized using differential scanning calorimetry (DSC), scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FT-IR), melt flow rate (MFR) analysis and mechanical testing (i.e., tensile and flexural analysis).
Experimental
Materials
PLA pellets (ES701, melt index 10.8 g/10 min at 190 °C, T g 57.5 °C and T m 153.9 °C), obtained from Tongjieliang Biomaterials Co., Ltd. Shanghai, were used as matrix materials. They were grounded to about 600 μm and dried for 24 h at 50 °C. BP was obtained from a local bamboo processing factory located in Zhejiang Province. The BP was grounded into particles of 200–400 μm, and then dried in an oven at 105 ± 2 °C. Sodium hydroxide (NaOH), maleic anhydride (MAH) and acetone (C3H6O) were obtained from Sinopharm Chemical Reagent Co., Ltd.. Dicumyl peroxide ([C6H5C(CH3)2]2O2, DCP) was obtained from Aladdin Reagent Co., Ltd.
Alkali Treatment of Bamboo Particles
BP was immersed in 0.30 N NaOH solution with a ratio of 1:15 (wt/v) in 40.0 °C water batch for 3.0 h [8]. Then, BP was rinsed with distilled water and washed with acetic acid solution for neutralization. They were further rinsed with distilled water and filtered under vacuum and dried at 105 ± 2 °C for 24 h.
Preparation of MAH Compatibilized PLA/BP Composites
The amounts of MAH for different mass fractions viz., 0.5, 1.0, 1.5, 2.0, 2.5 % (based on 50 g of the blends) were weighed respectively. And they were dissolved in 25 mL of acetone with 0.05 g DCP. 15.0 g alkali-treated bamboo particles were soaked in the resulting solution, and the excess solution was drained off from BP surface and they were dried overnight in the fume hood at 50 °C.
About 50 g of the blends, with fixed PLA to BP ratio of 70:30 (wt %) were prepared in a co-rotating twin screw lab-scale compounder under 180 °C and 50 rpm for 10 min [8]. The compounded samples were placed in the desiccator for 24 h and then paved into a mold. Vulcanization molding machine (GT-7014-A50C, GTM Inc.) was adopted to preheat the samples for 10 min at 180 °C and then samples were hot pressed under the conditions of 180 °C and 2.0 MPa. The pressure was maintained for 5 min and then the sample was cooled with water circulation in the press platen. The sizes of samples for tensile and flexural tests were 165 × 13 × 4 mm3 and 127 × 10 × 4 mm3, respectively, on the basis of ASTM D638 and ASTM D790. All samples were kept in desiccator for further characterization.
Surface Morphology Observation
Surface morphology of BP was observed using launch scanning electron microscope (S-4800, Hitachi, Japan). The samples were coated with gold before observing. The launching voltage of electron microscope was 10.0 kV.
Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR (Nicolet-380, Thermofisher Scientific Inc.) was adopted for infrared representation with the scanning range of 4,000–400 cm−1. The spectra were obtained with the KBr pellet technique. BP, PLA and 1 % MAH treated PLA/BP composite were ground into powder, then mixed and compressed with KBr powder into thin discs. 16 scans were co-added.
Composites Mechanical Testing
Tensile and flexural tests of PLA/BP composites were carried out using universal testing machine (CMT4503, MTS Inc.) according to ASTM D638 and ASTM D790. The gauge length and the crosshead speed for tensile test were set at 50 mm and 5 mm/min, respectively. The support span and the crosshead speed for flexural test were set at 64 mm and 5 mm/min, respectively. Five specimens were measured for each test.
Melt Flow Rate (MFR) Analysis
About 5.0 g of PLA/BP composites were dried and tested on a MFR testing machine (ZRZ-1452, Skyan Inc.) with the temperature of 190 °C and loading weight of 2,160 g.
Thermal Analysis
Differential scanning calorimeter (200F3, Netzsch) was adopted to study the thermal properties of untreated PLA/BP, 1 and 2.5 % MAH treated PLA/BP composites. About 10.0 mg of sample was accurately weighed and sealed. The sample was kept at a constant temperature of 0 °C for 5 min and then heated at a rate of 10 °C/min to 200 °C. Nitrogen was used as purging gas at a rate of 50 mL/min. Crystallinity (X c) was estimated according to the following equation,
where, ΔH c refers to the crystallization enthalpy of PLA/BP composites, ΔH 0 refers to the enthalpy value during 100 % crystallization of PLA, which is 93.6 J/g [29], X PLA refers to the weight ratio of PLA in PLA/BP composites.
Results and Discussion
Micromorphology Analysis
Figure 1a, b shows the SEM micrographs of BP after alkali and MAH treatment, respectively. The alkaline treatment eliminated the inorganic impurities on the particles surface. Obvious grooves were also presented as a result of mercerization due to the partly decomposition of hemicellulose. After MAH grafting, the grooves became unclear. The surface of BP became smooth as well and was covered with MAH, which indicates that MAH was cross-linked with part of the polar functional groups on the BP and grafted onto the BP surface. Figure 1c, d show the SEM micrographs of fracture surface of the composites after tensile test. Cavities resulted from pulling out of fibers were clearly seen in the uncompatibilized particles reinforced composites at the interface between PLA and particles, indicating a poor interfacial bonding. Conversely, the fracture surface of 1 % MAH compatibilized BP reinforced PLA composites was relative smooth and almost no cavity was observed, indicating a better interfacial adhesion between the two substance.
FT-IR Spectroscopy
The changes of surface functional groups were monitored by FTIR analysis. In Fig. 2, FT-IR spectra of neat PLA, alkali treated particles and 1.0 % MAH treated blends are reported. In Fig. 2b, only the spectral region of 2,000–500 cm−1 was shown to highlight the main differences between neat PLA and compatibilized PLA/BP composites. The spectra was enhanced with respect to the absorption band centered at 3,426 cm−1 (–OH stretching). The enhanced intensity was probably caused by the existing hydroxyl of BP or the slight degradation of PLA through oxidation of revulsant DCP. As it can be observed in Fig. 2b, new absorption bands at about 1,508 cm−1 (–C=C– stretching), the intensity of which was a function of MAH amount, appeared in the spectra of compatibilized composites. The absorption bands at 1,758 and 1,046 cm−1 were strengthened, which could be attributed to the asymmetric stretching of the MAH carbonyl group and to the polysaccharide acetal group of the BP involved into the grafting reaction, thus confirming that the MAH grafting between PLA and BP [30]. The peaks in three places, i.e., 956, 869, 756 cm−1 were weakened. These bands could be attributed to the C–H wagging of the methyl group, the C–C stretching and the C–H stretching on the benzene ring, respectively. The followings are possible chemical reactions occurred during preparing process of the composites [2]:

Mechanical Properties
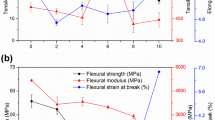
The effects of compatibilizer (MAH) content on the tensile strength and elongation at break of PLA/BP blends are illustrated in Fig. 3. With the increase of MAH content, the tensile strength of composites first increased due to the improvement of interfacial bonding and then sharply decreased due to the oxidation with excess MAH. The tensile strength of alkali treated composites was 44.21 MPa, whereas the maximal tensile strength of 47.60 MPa was obtained for 1.0 % MAH compatibilized composites. These results suggested that the stress generated by stretching had been transferred from matrix PLA to BP through interface, and MAH improved the interfacial compatibility of PLA/BP composites. When MAH content was less than 1.0 %, elongation at break of composites slightly changed. After that, with the increasing of MAH content, it decreased sharply. These results meant that resistance to deformation of the composites decreased attributed to uneven distribution of BP when molding and the molecular chain rigidity increased.
The influences of MAH contents on the flexural strength and flexural modulus of PLA/BP composites are given in Fig. 4. Maximal flexural modulus of 4.65 GPa were found with 0.5 % MAH treated composites. With further increasing of MAH content, the flexural modulus decreased. Appropriate amount of MAH could graft PLA macromolecular chain with BP, improving the interfacial compatibility between the hydrophobic PLA and hydrophilic BP. However, when excess MAH was added in the blends, it excessively inserted between the polymer molecular chain, and weakened the stress between them. Which leaded to increase of the molecular chain mobility and decrease of crystallinity, resulting in reduced modulus. The flexural strength was also decreased as the increasing of MAH content.
MFR Analysis
The influences of MAH compatibilizer on the melt flow rate of PLA/BP composites are shown in Fig. 5. With the increasing of MAH content, the MFR of PLA/BP blends increased. The trend was fitted using three order polynomial primary function. Due to the symmetrical structure of maleic anhydride molecular, the inductive or conjugation effect could not be easily generated. As a result, the esterification reaction with PLA molecules depended on the probability of macromolecular radicals contacting with MAH molecules. Nevertheless, the excessive MAH could not react with the insufficient macromolecular radicals, when MAH and macromolecular radicals reacted with equilibrium. The impacted probability between MAH and primary radical increased, which leaded to cage effect and other side reaction resulting in increased MFR [31].
DSC of PLA/BP Composites
PLA is a semi-crystalline polymer [30, 32]. During heating or cooling, cold crystallization can be observed in some order areas in the melt. The strength and modulus of the material are greatly influenced by the degree of crystallinity, X c. DSC thermograms of alkaline treated, 1.0 and 2.5 % MAH treated BP reinforced PLA composites are given in Fig. 6 and the corresponding transition temperatures and enthalpies are summarized in Table 1. The thermal behavior and crystallization characteristics were influenced with the addition of MAH in the composites. The alkali treated BP/PLA composites was characterized by a glass transition temperature (T g) at 55.6 °C, a cold crystallization peak (T c) at 120.3 °C, and finally a melting peak (T m) at 149.5 °C. T g and T c of the blends increased by about 2.1 and 5.2 °C with 0.5 % MAH treatment, which was explained by the restricted chain mobility, due to the esterification reaction at the fiber/polymer interphase. After that, as the increasing of MAH content, the T g and T c decreased to 50.7 and 117.2 °C, respectively. These observations suggested that the excessive MAH acted as plasticizer, leading to reduced polymer viscosity because of plasticization.
Degree of crystallinity of PLA in composites decreased from 14.35 to 8.33 % after 0.5 % MAH treatment, because part of BP had been grafted onto PLA hindered form of crystal nucleus and crystal growth. Moreover, the degree of crystallinity increased to 31.5 % because of the nucleation of the excess MAH. Melting enthalpy (ΔH m) of the blends first decreased and then increased after MAH treatment. It is worth noting that there was a double melting peak in the PLA/BP composites with 2.5 % MAH treatment. The former melting peak was part of the initial crystal melting while the latter caused by melted crystalline recrystallization melting.
Conclusion
The interfacial interaction of PLA/BP composites was improved with sodium hydroxide solution pretreated BP and MAH compatibilizing. Tensile strength and elongation at break of PLA/BP composites reached maximal values of 47.6 MPa and 6.22 %, respectively, when treated with 1.0 % MAH. Maximal flexural strength and flexural modulus of 72.61 MPa and 4.65 GPa were obtained with 0.5 % MAH treatment. With further increasing of MAH content, both tensile and flexural performances decreased. MFR of the blends was enhanced with MAH compatibilization. T g was improved, whereas X c and ΔH m were decreased with 0.5 % MAH treatment. When excessive MAH was used, T g decreased and X c increased significantly.
References
Graupner N, Herrmann AS, Müssig J (2009) Compos A Appl Sci 40:810
Mwaikambo LY, Ansell MP (2002) J Appl Polym Sci 84:2222
Gupta A, Kumar V (2007) Eur Polym J 43:4053
Nyambo C, Mohanty AK, Misra M (2011) Macromol Mater Eng 296:710
Ma L, He H, Jiang C, Zhou L, Luo Y, Jia D (2012) J Macromol Sci B 51:2232
Zhou C, Shi Q, Guo W, Terrell L, Qureshi AT, Hayes DJ, Wu Q (2013) ACS Appl Mater Interfaces 5:3847
Gregorova A, Hrabalova M, Wimmer R, Saake B, Altaner CJ (2009) Appl Polym Sci 114:2616
Qian S, Mao H, Sheng K, Lu J, Luo Y, Hou C (2013) Appl Polym Sci 130:1667
Mukherjee T, Kao N (2011) J Polym Environ 19:714
Baek BS, Park JW, Lee B, Kim HJ (2013) J Polym Environ 21:702
Cai G, Wang J, Nie Y, Tian X, Zhu X, Zhou X (2011) Polym Compos 32:1945
Baheti V, Militky J, Marsalkova M (2013) Polym Compos 34:2133
Wollerdorfer M, Bader H (1998) Ind Crops Prod 8:105
Zou H, Wang L, Gan H, Yi C (2012) Polym Compos 33:1659
Tokoro R, Vu DM, Okubo K, Tanaka T, Fujii T, Fujiura T (2008) J Mater Sci 43:775
Kumar S, Rath T, Mahaling R, Das C, Srivastava R, Yadaw S (2009) Polym Compos 30:655
Chattopadhyay SK, Khandal RK, Uppaluri R, Ghoshal AK (2011) J Appl Polym Sci 119:1619
Quero F, Eichhorn SJ, Nogi M, Yano H, Lee KY, Bismarck A (2012) J Polym Environ 20:916
Kang JT, Kim SH (2011) Macromol Res 19:789
Raquez JM, Nabar Y, Srinivasan M, Shin BY, Narayan R, Dubois P (2008) Carbohydr Polym 74:159
Mani R, Bhattacharya M, Tang J (1999) J Polym Sci Pol Chem 37:1693
Carlson D, Nie L, Narayan R, Dubois P (1999) J Appl Polym Sci 72:477
Petchwattana N, Covavisaruch S, Chanakul SJ (2012) Polym Res 19:9921
Ahmad EEM, Luyt AS (2012) Polym Compos 33:1025
Delgado PS, Lana SLB, Ayres E, Patrício POS, Oréfice RL (2012) Mater Res 15:639
Fuentes CA, Tran LQN, Van Hellemont M, Janssens V, Dupont-Gillain C, Van Vuure AW, Verpoest I (2013) Colloids Surf A 418:7
Kushwaha PK, Kumar R (2010) J Reinf Plast Compos 30:73
Teamsinsungvon A, Ruksakulpiwat Y, Jarukumjorn K (2013) Polym Plast Technol Eng 52:1362
Li Y, Chen C, Li J, Sun XS (2012) J Appl Polym Sci 124:2968
Avella M, Bogoeva-Gaceva G, Bužarovska A, Errico ME, Gentile G, Grozdanov A (2008) J Appl Polym Sci 108:3542
van Dijk M, Smit TH, Sugihara S, Burger EH, Wuisman PI (2002) Spine 27:682
Auras R, Harte B, Selke S (2004) Macromol Biosci 4:835
Acknowledgments
The authors are grateful to the Special Fund for Agro-scientific Research in the Public Interest of China (No. 201003063).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, S., Mao, H., Zarei, E. et al. Preparation and Characterization of Maleic Anhydride Compatibilized Poly(lactic acid)/Bamboo Particles Biocomposites. J Polym Environ 23, 341–347 (2015). https://doi.org/10.1007/s10924-015-0715-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-015-0715-x