Abstract
The proximal femoral morphology in rodents of different body sizes, locomotor modes, and from the three main rodent lineages (Sciuromorpha, Myomorpha, and Hystricomorpha) exhibit a separated condition of the femoral head and greater trochanter. We assessed the femoral ossification of eight species of all six genera of a subterranean lineage of mammals, the African mole-rats (Bathyergidae), including the naked mole-rat (Heterocephalus glaber). Here we report a surprising level of intraspecific variation in the ossification of the proximal femur of H. glaber, which presents both separated and coalesced conditions, regardless of sex and reproductive status. The other bathyergids, including chisel-tooth and scratch-diggers exhibit a separated condition, similar to the typical rodent condition. Because the coalesced condition is uncommon among rodents, our data suggests that the presence of two femoral morphologies in H. glaber represent developmental plasticity in this species. Such a dual condition may result from a constricted femoral head and greater trochanter morphology and slow skeletal growth rates, which could be also influenced by differential loading histories, such as magnitude and orientation of forces acting on the limb during ontogeny. This is the best documented case of intraspecific variation for this trait amongst non-human vertebrates, and its investigation is important to understanding the mechanisms of skeletal development and phenotypic plasticity in mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The naked mole-rat (Heterocephalus glaber) is a specialized subterranean rodent that excavates extensive burrow systems in the hot and dry tropical regions of northeast Africa (Sherman et al. 1991; Bennett and Faulkes 2000). They are the smallest member (∼35 g) of the highly specialized subterranean African mole-rats (Bathyergidae) and form the largest cooperative social systems within the family, consisting of up to 300 individuals per colony, with a mean colony size of 75 (Jarvis et al. 1994). Despite their relatively small body size, they show an impressive extended longevity of more than 30 years, thus constituting the longest lifespan recorded amongst the family and among rodents in general (O’Connor et al. 2002; Sherman and Jarvis 2002; Dammann and Burda 2007; Buffenstein 2008). Because of these and other peculiarities of their unusual biology, naked mole-rats (NMRs) and other members of the family are the focus of extensive biomedical and ecophysiological research, particularly as models for ageing (e.g., Buffenstein 2008; Bennett 2009; Edrey et al. 2011; Buffenstein et al. 2012; Tian et al. 2013; Park et al. 2017; Skulachev et al. 2017). However, their skeletal development and morphology have received far less attention.
Recent studies on the structural and geometric properties of their long bones and bone remodeling dynamics have revealed that their bone integrity is maintained over most of their lifespan, presenting thick cortical bone walls with scarce bone resorption (minimal osteopenia) and scarce formation of Haversian systems (Pinto et al. 2010; Edrey et al. 2011; Carmeli-Ligati et al. 2019; Montoya-Sanhueza et al. 2021a, 2021b). The analysis of their bone histology has also shown a predominance of slowly deposited bone tissues, altogether suggesting slow skeletal growth during ontogeny and low overall bone turnover (Montoya-Sanhueza et al. 2021a). One of the most striking features of NMRs that differentiates them from other bathyergids is their unique limb bone morphology lacking both a projected deltoid tuberosity in the humerus and distal tibio-fibular fusion, which suggests the presence of a different morphogenetic variant among bathyergids (Montoya-Sanhueza et al. In Review). These findings are aligned to previous investigations reporting phenotypic differences between NMRs and other bathyergids. The lack of fur, reduced number of molars, and extremely reduced claws in the manus and pes represent the most conspicuous phenotypic differences among these taxa (Table 1). Such findings suggest a considerable degree of morphological variation between naked mole-rats and the rest of the family, which is quite surprising for a subterranean group of mammals that often exhibit similar morphologies because of their functional requirements associated with digging behaviour (Hildebrand 1985; Nevo 1999; Stein 2000). Although some authors have placed Heterocephalus into a distinct family (i.e., Heterocephalidae), we consider such classification premature (see detailed discussion in Montoya-Sanhueza 2020; Braude et al. 2021; Buffenstein et al. 2022). In the present study, we follow the traditional taxonomic classification of African mole-rats as a single family that includes the naked mole-rats (Bryja et al. 2018; Visser et al. 2019).
Altogether, the unique morphology of NMRs emphasizes the importance of further advancing our understanding of the skeletal biology of NMRs and African mole-rats in general, especially considering the recent renaissance of investigations elucidating the developmental mechanisms of non-model animals with unique phenotypes (see Franz-Odendaal and Hockman 2019; Hockman and Franz-Odendaal 2019). Because of their wide spectrum of social organizations and underground lifestyle involving different digging modes (chisel-tooth and scratch-digging), African mole-rats also represent an ideal family for assessing physiological processes (Bennett 2009) and its interplay with skeletal adaptation (Buffenstein et al. 2012; Montoya-Sanhueza 2020). Such research is essential to understand the myriad of processes and factors modulating the morphogenesis and complex adaptations of skeletal phenotypes (Atchley and Hall 1991).
Due to its clinical implications, particularly in humans, the femoro-pelvic articulation is an extensively investigated aspect of the appendicular system of mammals (e.g., Edgren 1965; Serrat et al. 2007; Miyamoto et al. 2008; Struijs et al. 2011; Cole et al. 2013). This articulation is formed by the femoral head, which articulates with the acetabulum of the pelvis, and the trochanters which serve for the insertion of several muscles involved with the flexion and extension of the thigh/hip (Polly 2007). Because the femoral head unites the hind limb with the axial skeleton, this system is fundamental for the whole musculoskeletal functionality of the hind limb, and most importantly it represents the main propulsion system of quadrupeds (Howell 1965). Among mammals, the shape and size of the femoral neck and trochanters exhibit a substantial amount of variation, thus reflecting the wide repertoire of locomotory adaptations of this lineage (Samuels and Van Valkenburgh 2008; Wilson and Geiger 2015). Among subterranean mammals, which are generally small-sized animals with elongated cylindrical bodies and short limbs that move in narrow spaces (Hildebrand 1985; Nevo 1999; Stein 2000), the effects of bone morphology and the development of the proximal femoral epiphysis on their locomotion are not completely known. However, behavioral experiments have shown important modifications in their locomotor pattern due to postural challenges (Eilam et al. 1995; Horner et al. 2016). Many subterranean mammals, including all bathyergids, have implemented backward locomotion as a recurrent behavior to principally remove and extrude soil out of the burrow and onto the surface (Bennett and Faulkes 2000; Stein 2000). Thus, locomotion and soil transport are closely linked activities that can be considered as a distinct locomotor pattern in a dense medium (McNab 2002), and may have important consequences on the configuration of the proximal morphology of the femur.
Serrat et al. (2007) comprehensively assessed the femoral morphology of the proximal epiphysis of a phylogenetically diverse sample of mammalian species. They found that mammals exhibit two main conditions, where the femoral head and the greater trochanter are coalesced or separated. The coalesced condition is the most broadly distributed among different mammalian lineages including marsupials, artiodactyls, perissodactyls, bats, carnivores, and several primates, although this ossification type is almost non-existent in rodents, which rather have a separated condition, like some carnivores, primates, treeshrews and elephant shrews (Serrat et al. 2007). To determine the ossification process of the proximal epiphysis of mole-rats with different digging modes, we document the variation in femoral morphology of all the six bathyergid genera by categorizing each individual in the sample according to the presence of a “separate” or “coalesced” proximal epiphysis. A second goal of the present study is to assess the effects of sex, body size and femoral morphology on the ossification type of NMRs. This study represents an important contribution to our understanding of the mechanisms of skeletal development and phenotypic plasticity of African mole-rats.
Materials and Methods
Eight species of African mole-rats including all genera within the family Bathyergidae were studied (Table 2; Online Resource 1): naked mole-rats (Heterocephalus glaber), silvery mole-rat (Heliophobius argenteocinereus), Cape dune mole-rat (Bathyergus suillus), Namaqua dune mole-rat (Bathyergus janetta), Cape mole-rat (Georychus capensis), common mole-rat (Cryptomys hottentotus), Damaraland mole-rat (Fukomys damarensis) and Mechow's mole-rat (Fukomys mechowii). For most species, relatively complete ontogenetic series were collected (Table 2). Most specimens were wild-caught, although some individuals of F. damarensis and all individuals of H. glaber come from captive colonies. The latter species are from colonies living in tunnels made of glass without substrate to dig (see details in Montoya-Sanhueza et al. 2021a). Because of the rarity of B. janetta in museums, only a few specimens from this taxon were obtained. A total of 303 femora from either the right or left side were analyzed; most specimens were dissected and cleaned of flesh and connective tissue so that the femoral head (FH) and greater trochanter (GT) were clearly visible with the naked eye (Fig. 1). In order to determine the first stages of epiphyseal ossification, and therefore to deduce their main morphological changes during ontogeny we also analyzed pups cleared and stained with Alizarin red and Alcian blue for identification of bone matrix and cartilaginous tissue, respectively. The details of the preparation of diaphonized specimens are presented in a complementary study on the development of the appendicular system of bathyergids (Montoya-Sanhueza et al. In Review).
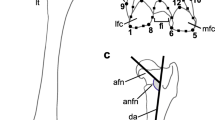
Morphology of the proximal epiphysis of the femur of African mole-rats (Bathyergidae) and their phylogenetic relationships including their closest non-subterranean relatives. Most bathyergids exhibit a separated condition (SC) with distinct femoral head (FH) and greater trochanter (GT), whereas mature specimens of Heterocephalus glaber develop both a SC and a coalesced condition (CC). The FH is separated from the GT by a U-shape bridge (Br), which is less defined in H. glaber due to the clustered nature of such structures in this species. Note the more ellipsoidal shape of the FH in H. glaber. The three outgroups Thryonomys swinderianus (Thryonomyidae), Petromus typicus (Petromuridae) and Hystrix africaeaustralis (Hystricidae) also exhibit a SC. See the text for details on T. swinderianus. Femora are not to scale. Abbreviations: LT, lesser trochanter. Rodent silhouettes not to scale
The proximal epiphysis of each femur was classified according to the presence of a “separate” or a “coalesced” condition. The “separated” condition occurs when the femoral head and greater trochanter remain functionally separated throughout ontogeny, while the “coalesced” condition occurs when the capital and trochanteric ossification centers coalesce into a single osseous epiphysis (Serrat et al. 2007). This classification involves the evaluation of ontogenetic processes and assumes that the origin of the proximal epiphysis in mammals occurs from a single cartilaginous proliferation (chondroepiphysis) with the subsequent formation of two independent secondary centers of ossification (Serrat et al. 2007). However, it has been reported that a secondary center of ossification never develops in the proximal femur of mice, and the epiphysis rather undergoes only direct endochondral ossification (Cole et al. 2013). This suggests that the formation of two secondary centers of ossification in rodents may not represent a generalized process in the group. The study of Serrat et al (2007) was based on subadult individuals (not skeletally mature), whereby the final stages of growth were not assessed, which is problematic for the determination of the coalesced condition that may occur in more advanced ontogenetic stages (Serrat et al. 2007). Considering this, our study focuses primarily on the analysis of adult specimens. In our study, the separated condition (SC) occurs when the femoral head and greater trochanter represent clearly separated units forming a two-element proximal epiphysis, whereas a coalesced condition (CC) occurs when both the femoral head and greater trochanter are fused forming a unique single proximal epiphysis (Fig. 1; Online Resource 1). We recorded such stages for each individual in our sample and include a third condition for pups; presence of a single chondroepiphyses (CH) that is continuous with the resting cartilage of the diaphyseal growth plate, and where the femoral head and greater trochanter are yet not ossified or distinguishable (Online Resource 1). Our data set corresponds to a cross-sectional study, so that only one observation per individual is obtained, and no data for the progression of the ossification is available for the individual. Nevertheless, to have a reliable determination of the ossification process for each species, as well as a better representation of their “ontogenetic trend”, we include a representative number of individuals of multiple ages per species, except for some of them where only a few specimens were available (e.g., B. janetta) (Table 2).
To determine the pattern of ossification of the closest relatives of the bathyergids, we also assessed an ontogenetic series (n = 18) of the Cape porcupine Hystrix africaeaustralis (Hystricidae), which is the sister taxon of Caviomorpha + Phiomorpha (Online Resource 1). Unfortunately, no ontogenetic series were available for the other closest non-subterranean relatives of bathyergids, the greater cane rat Thryonomys swinderianus (Thryonomyidae) and the dassie rat Petromus typicus (Petromuridae). Therefore, we only assessed the adult phenotype of such taxa based on one individual per species (Online Resource 1). Specimens of P. typicus and H. africaeaustralis were obtained from the Iziko SA Museum (Cape Town, South Africa). The adult skeletal phenotype of T. swinderianus was obtained from previous anatomical descriptions and illustrations reported by Onwuama et al. (2018), study which is based on descriptions of 12 individuals.
Statistical Analysis
To assess the effects of morphology (body size and femoral size) and sex on the ossification pattern of NMRs, femoral length (FL) and body mass (BM) were obtained for most of the specimens (Online Resource 2). FL corresponds to the maximum distance (in mm) from the proximal articular surface to the distal articular surface of the bone (Online Resource 2). BM (g) of specimens #506–511 were obtained immediately following death, while the BM of the rest of the specimens was obtained after defrosting them, so these data could be underestimated and therefore should be interpreted with caution (Online Resource 2). A Mitutoyo digital caliper (0.01 mm) was used to measure FL, while a standard electronic balance (0.01 g) was used to quantify BM. Comparisons for ossification groups and sexes were performed independently with parametric analysis (Student's t-test), due to the missing data for sex and BM for some individuals. A 95% CI was used in all statistical analyses. Normality was assessed with Shapiro–Wilk tests. Statistical analyses and graphs were performed in PAST version 4.40 (Hammer et al. 2001). Data are presented as mean and standard deviation (m ± s.d.).
Results
Our results show that all bathyergid species analyzed have a separated epiphyseal condition, i.e., separated femoral head and greater trochanter (Fig. 1; Online Resource 1). The femoral head and greater trochanter are distinctive in adulthood (and attain similar height), and a conspicuous U-shape bridge develops between these structures. The lesser trochanter develops distal to the femoral head and greater trochanter (Fig. 1). The juveniles of all specimens analyzed also showed separated structures (Online Resource 1). However, NMRs show a dramatic difference in the ossification pattern as compared to other bathyergids; H. glaber exhibits both a separated and a coalesced condition of the femoral head and greater trochanter; 44.93% of the individuals having a defined proximal epiphysis showed a separated condition and 55.07% a coalesced one (Figs. 1 and 2; Online Resource 1). Another difference between H. glaber and the rest of the bathyergids is the shape of the femoral head, which is circular in most bathyergids with a well-developed femoral neck, so that the femoral head and trochanters are clearly differentiated and separated (Fig. 1). In contrast, the femoral head of H. glaber has a tear-drop shape and the femoral neck appears comparatively shorter to other bathyergids (Fig. 2a). In this species, the sharpest part of the femoral head is orientated laterally pointing to the greater trochanter (Fig. 2b-e). Furthermore, the U-shape bridge between the femoral head and greater trochanter of H. glaber is less defined when compared to other species (Figs. 1 and 2). The femoral head of the outgroup taxa H. africaeaustralis, T. swinderianus, and P. typicus is also circular, although the greater trochanter surpasses the length of the femoral head, especially in H. africaeaustralis and T. swinderianus, taxa which also show an extremely developed U-shape bridge between such structures (Fig. 1).
Detail of the coalesced ossification of the femoral head (FH) and greater trochanter (GT) in Heterocephalus glaber. a. Anterior view of the proximal epiphysis of the femur showing the bone tissue (red arrow) connecting the greater trochanter (GT) with the femoral head (FH) in an individual with unfused proximal epiphyses; b. FH of H. glaber in ventral view (left) and of Bathyergus suillus in dorsal view (right). Note the tear-drop shape (*) in H. glaber, which orientates laterally and connects with the GT over the bridge (Br) formed by the projection of the short femoral neck (FN); c. Dorsal view of the same bone with epiphyses (FH and GT) removed, showing the growth plate zone (GPZ) with an evident trabecular (Tr) arrangement; d. Anterior (left) and posterior (right) views of another specimen showing the thin ossified connection (red arrows) between FH and GT; e. Anterior view of an individual with fused proximal epiphyses showing the complete fusion of FH and GT (red arrow). Abbreviations: LT, lesser trochanter
The Proximal Epiphysis during Early Ontogeny
The analysis of early ontogenetic stages of H. glaber, H. argenteocinereus, B. janetta, and Fukomys (Table 2; Online Resource 1) showed that most individuals did not develop secondary centers of ossification until several days after birth. The newborns and a few days old individuals of all these species, have a single globular chondroepiphysis that is continuous with the resting cartilage of the diaphyseal growth plate and includes both the femoral head and greater trochanter, although the lesser trochanter develops independently inferior to the femoral head. Some individuals of F. damarensis have a lesser trochanter that is in close proximity with the femoral head and greater trochanter. The third trochanter, also cartilaginous, grows apart in the proximal region of the diaphysis with no connection with the proximal epiphysis. In all these species, the femoral head is considerably larger than the greater trochanter and always globular, while the greater trochanter has a reduced size and an elongated shape and is of a smaller size. In B. janetta, these cartilaginous structures are already separated in individuals of two days old, although no secondary centers are yet observed at this age. In H. argenteocinereus, the first secondary center of ossification to develop is the one in the FH, which is observed in individuals of 14 days (Online Resource 1). In H. glaber, the first secondary center to develop also appears in the femoral head and is observed in individuals of 76 days, although this was not observed in individuals of 63 days. At 63 days old, the chondroepiphysis of H. glaber begins to differentiate into two separate cartilaginous entities, although they remain united at the base of the growth plate. Altogether, this information suggests that the femoral head and greater trochanter are developed by independent secondary centers, which precedes the cartilaginous separation of such structures, and that the femoral head develops first in these bathyergids.
Comparison between Ossification Groups (CC vs SC) and Sexes
Femoral morphology (FL) and body mass (BM) were similar between individuals of both ossification groups (Fig. 3a). There was no significant effect of ossification pattern in FL, t(67) = 1.61, p = 0.111 (CC = 14.4 ± 1.06; SC = 14.0 ± 1.16), and BM t(54) = 0.84, p = 0.404 (CC = 32.14 ± 11.07; SC = 29.66 ± 10.27) (Fig. 3b). Similarly, there were no significant sex differences for FL in the CC group, t(33) = 2.01, p = 0.052 (FLmales = 14.86 ± 0.82; FLfemales = 14.21 ± 1.05) or the SC group t(23) = 0.37, p = 0.717 (FLmales = 14.40 ± 1.08; FLfemales = 14.22 ± 1.06) (Fig. 3c). A similar trend was detected for BM, where no significant differences were detected within the CC group, t(31) = 1.15, p = 0.257 (BM males = 34.83 ± 10.18; BM females = 30.36 ± 11.79) or SC group, t(16) = 0.50, p = 0.626 (BM males = 34.64 ± 14.52; BM females = 32.13 ± 7.04) (Fig. 3c). These data suggest that the type of epiphyseal ossification in H. glaber is poorly associated with femoral length or body mass.
Comparisons showing morphological similarities between males and females and ossification groups in Heterocephalus glaber. a. Dispersion graph showing the morphological similarity of femoral length (FL) and body mass (BM) between individuals exhibiting a coalesced condition (CC) and a separated condition (SC) of the proximal epiphysis; b. Bar charts showing mean (dot) and standard deviation (whiskers) of FL and BM for both ossification groups; c. Bar charts showing mean (dot) and standard deviation (whiskers) of males (♂) and females (♀) within each ossification group (CC and SC), for FL and BM. All comparisons are statistically non-significant (see details in main text)
Discussion
The proximal morphology of the femur of mammals is comprised of two main morphotypes, a separated or a coalesced condition of the femoral head and greater trochanter (Serrat et al. 2007). Rodents of different body sizes and locomotor modes, including species from the three main rodent clades, Myomorpha (house mouse, Mus musculus; black rat, Rattus rattus; brown rat, Rattus norvegicus; North American beaver, Castor canadensis), Sciuromorpha (eastern chipmunk, Tamias striatus; groundhog, Marmota monax) and Hystricomorpha (Cuban hutia, Capromys pilorides; coypu, Myocastor coypus; North American porcupine, Erethizon dorsatum; crested porcupine, Hystrix cristata; capybara, Hydrochoerus hydrochaeris; spotted paca, Cuniculus paca; Guinea pig, Cavia porcellus) exhibit a separated condition (Serrat et al. 2007; Araújo et al. 2013; Moncayo‐Donoso et al. 2018). This condition is also present in other mammalian lineages such as the Carnivora, Primates, Scandentia, and Macroscelida (Serrat et al. 2007). Based on the developmental reports of a few mammalian species, two distinct secondary ossification centers appear during postnatal skeletogenesis for the formation of the femoral head and greater trochanter (Riser 1973; Farnum 2007; Struijs et al. 2011; Xie et al. 2020). Therefore, it is assumed that in rodents and the other taxa exhibiting a separated condition, these secondary centers remain distinct throughout ontogeny, while the coalesced condition results from fusion of secondary centers of ossification (Serrat et al. 2007; but see Cole et al. 2013 for an exception in mice).
Our investigation assessing a comprehensive collection of African mole-rats of all genera demonstrates the ossification process of the proximal femur in an untraditional mammalian model. As in most other rodents previously studied, African mole-rats with different digging modes (chisel-tooth and scratch-digging) exhibit a separated condition of the femoral head and greater trochanter (Fig. 1), which is attained early during postnatal ontogeny (Online Resource 1 and 2). However, we report a considerable degree of intraspecific variation in the ossification process of NMRs, which contrasts with the main developmental pathway of other bathyergid genera. More than half of NMRs examined in the current study presented a coalesced condition, regardless of sex and reproductive status (Figs. 1, 2, and 3; Online Resource 1). Such a dual condition in NMRs is unconventional among rodents and mammals in general. Currently, based on the observation of 70 mammalian species, the only case of intraspecific variation for this trait has been reported in the arboreal North American porcupine (E. dorsatum), which also presented both a separated and a coalesced morphology (based on three specimens; Serrat et al. 2007). Our study represents the best documented case of intraspecific variation for this trait amongst the 70 vertebrate taxa currently examined. Because the development of a coalesced condition in rodents is uncommon, the reason why some NMRs exhibit such a phenotype is intriguing. Nevertheless, it is possible that such intraspecific variation may have been largely overlooked in other taxa since often only a few individuals per species are analyzed in comparative studies involving a wider taxonomic sample. If such, apparently, unique intraspecific variation among rodents is real, it is noteworthy that this lineage appears to be the only group showing such level of variation. Further studies focused on the assessment of skeletal variation at the population level in a diverse range of mammalian species would improve our understanding on the morphological diversity of the femoral epiphysis of this group.
The variation observed in NMRs is unlikely to be the result of developmental pathologies, i.e., morphogenetic abnormality. No evidence of malformations or abnormalities of the external bone morphology were found in the specimens with a coalesced condition. Neither were there any signs of pathological growth in histological sections of the mid-diaphysis of the same femora and other bones of individuals analyzed here (see Montoya-Sanhueza et al. 2021a). Furthermore, none of the other bones (humerus, ulna, tibia, and fibula) of the specimens with a coalesced condition showed alterations or variations of any kind in their epiphyses (see Montoya-Sanhueza et al. 2021a). Life in captivity is unlikely to have promoted such intraspecific variation. None of the captive individuals of F. damarensis analyzed here presented a coalesced condition (although it should be noted that they have been kept in captivity for much shorter period as compared to NMRs) (Online Resource 1). In this sense, no previous investigations have reported cases of abnormal growth or altered morphology associated with the proximal femur in captive or domestic mammals (O’Regan and Kitchener 2005). This information suggests that living in captivity has probably not caused the development of a coalesced condition in NMRs, and therefore this process may represent a developmental/growth variant amongst the family.
The morphology of the proximal femur of NMRs differs from that of other bathyergids by having a rather ellipsoidal femoral head, a shorter femoral neck, and a poorly defined bridge between the femoral head and greater trochanter (Figs. 1, 2, and 3). Such configuration, observed in all NMR specimens, suggests that such structures are generally in proximity and tend to grow towards each other, and therefore the normal separation of the ossification centers during postnatal ontogeny may be limited. The more elliptical shape of the femoral head, which is orientated towards the greater trochanter, even in the specimens with a separated condition (Fig. 2), also supports this idea. Considering this, it is probable that both a tendency of these structures to growth internally, as well as at a slow rate during early ontogeny may result in having a rather clustered (i.e., constricted) proximal morphology. This may lead to some specimens eventually fusing their ossification centers in early adulthood. In this respect, Serrat et al. (2007) mentioned that factors such as body size, phylogeny, and locomotion have no clear relationship with ossification type, and such ossification is most likely an artifact of femoral shape and neck length, i.e., the femoral head and greater trochanter remain separate in mammals with long and distinct femoral necks simply as a consequence of their increased spatial separation (Serrat et al. 2007). Additionally, some cases of atypical pathological contact of the femoral head and greater trochanter, along with a shortened femoral neck in humans (Edgren 1965; Struijs et al. 2011), result in morphologies similar to the normal coalesced pattern described in other mammals (Serrat et al. 2007; Struijs et al. 2011). Because such atypical growth observed in humans is like the growth pattern seen in other mammalian species, Struijs et al. (2011) suggested this growth not to be a developmental disturbance but a late manifestation of a congenital anomaly or even a genetically determined growth variant. In such developmental terms, it is possible to hypothesize that faster epiphyseal growth rates during early ontogeny in comparatively large mammals may lead to an early divergence of such structures and therefore to its quick separation during development, thus preventing its coalescence later in life. The slower somatic growth rates of young NMRs in comparison to other bathyergids and other surface-dwelling eutherians of similar size (O’Riain 1996; O'Riain and Jarvis 1998; Bennett and Faulkes 2000), as well as the slower growth rates reported for the long bones of NMRs within the family (Montoya-Sanhueza 2020), may give support to such developmental hypothesis with the subsequent formation of a coalesced morphology in this species.
From a biomechanical perspective, the presence of two femoral morphologies in NMRs may be influenced by their fluctuating loading history. Several studies have reported how the loading history (i.e., magnitude and orientation of tensile and compressive forces acting on the limb) and muscle activity during the lifetime of the individual influenced their proximal femoral morphology (Yadav et al. 2017, 2021). Three femoral abductors, the m. gluteus medius, m. gluteus profundus, and m. piriformis insert on the greater trochanter of mammals, including the bathyergids G. capensis and B. suilllus (Parsons 1896; Sahd et al. 2019; Böhmer et al. 2020). The main functions of these muscles are to extend the hip joint and abduct the femur, and the early development of such muscles, as well as the onset of locomotion and digging behavior may have an important effect on the development of the proximal epiphysis. Riser (1973) mentions that the development and contraction of the muscles of the pelvis stimulate the formation of the trochanteric fossa, and that the greater trochanter is extended by the dorsal and medial pull of the three pairs of gluteal muscles. Xie et al. (2020) also described an interesting relationship between mechanical demands and development of secondary centers of ossification in six bat species (Chiroptera) and two jerboa species (Dipodidae), where advanced ossification is observed in limbs subjected to mechanical activity early in life.
In this sense, it has been reported that the growth trends of NMRs are highly variable within and between colonies, with the first litters of a colony showing a more stable growth pattern, and subsequent litters exhibiting a higher variation in terms of asymptotic weight, inflection time and projected time to attain adult mass (Jarvis et al. 1991; O’Riain 1996). Considering this, and the fact that the pups of NMRs (and other social bathyergids) wander out of the nest earlier than solitary bathyergids (Bennett and Faulkes 2000), it is possible that their musculoskeletal system experiences earlier and variable mechanical strains soon after birth.
Later in life, NMRs form complex groups of cooperative diggers where colony members work together in relays forming chains, and in addition they have a peculiar way of disposing soil (termed “volcanoing”) (Jarvis and Sale 1971). It is unknown if this digging sequence involves a separation of distinct tasks among members of the colony, although it has been reported that NMRs have ontogenetic polyethism among subordinates (Lacey and Sherman 1991). Recently, Siegmann et al. (2021) reported that NMRs vary in their overall contribution to cooperative behaviors and that some of this variation may be explained by differences in age and body mass, so that age and size have an effect on the type of behavior performed. This would suggest fluctuating mechanical strains during the life of the individual and among members of the colony. It is important to consider that all NMRs studied here come from colonies with tunnels made of glass without substrate to dig, so it is probable that the biomechanical strains experienced by these individuals during their life was considerably lower than in natural conditions. However, despite the extended time that this colony has been inbred and have not been supplied with soil to dig, the members of the colony maintain a clear digging behavior including both chisel-tooth digging and scratch-digging, as well as soil removal, where despite the absence of soil the limb still works as if pushing it out of the tunnel using backward locomotion and potent synchronized kicks of both hind limbs (Montoya-Sanhueza, 2020).
Overall, the intraspecific variation found in NMRs is part of a wide range of variable traits reported for this species (Table 1). Subordinate NMRs exhibit a high intra-colony polymorphism in terms of body size, which has been associated with differential growth rates among individuals (Jarvis et al. 1991; O’Riain 1996; Montoya-Sanhueza et al. 2021a). O'Riain and Jarvis (1998) suggested that such plasticity in growth is the product of different ontogenetic histories (behaviors) of colony members that may serve as the basis for variation in size among adults. This was recently supported by ontogenetic analyses of the limb bone histology of NMRs showing high intraspecific variation in the formation of bone tissue matrices and bone vascularization among individuals (Montoya-Sanhueza et al. 2021a). Based on the latter study, which investigated the same specimens used in the current study, we suggest that the tissue-level mechanisms leading to these two ossification variants (epiphyseal fusion vs. non-fusion) in NMRs are the result of multiple factors, such as having a constrained epiphyseal morphology, probably associated with the generalized slow bone growth rates of this species, and fluctuating postnatal activity levels among members of the colony. Additional studies on the complete developmental sequence of these femoral structures in individuals throughout life are required to fully understand the specific ossification mechanisms in this species. Furthermore, experimental studies of small mammals are needed to assess the relationship between proximal femoral morphology, fluctuating loading histories and variation in locomotor modes.
Proximal Femoral Morphology: Functional Implications
The unique morphology of the proximal femur of NMRs among bathyergids (and among Phiomorpha) suggests a different functional anatomy and locomotory performance for this taxon. Because the greater trochanter functions as a primary lever for the extension of the hip, it is often long and robust in cursorial and fossorial species, thus providing a longer moment arm and consequently an increased mechanical advantage for high-speed movements, hip stabilization and body maneuverability (Polly 2007; Serrat et al. 2007; Wilson and Geiger 2015).
The fact that most cursorial species from divergent mammalian lineages, like artiodactyls and perissodactyls, present a coalesced condition (Serrat et al. 2007) suggests that the proximal morphology of the femur might be under biomechanical regulation in this ecomorphological guild. Recently, Etienne et al. (2021) found the morphology of the proximal femur of a wide range of bovid species to be affected by body mass and habitat: the greater trochanter is larger (medio-laterally and antero-posteriorly) in open habitat and larger species, thus permitting increased cursoriality, more robust articulations, and stronger insertion areas for the muscles that propel the limb. Because all cursorial species analyzed by Serrat et al. (2007) exhibited a coalesced condition (and limited hip mobility), they hypothesized that the species that require increased tridimensional femoral mobility and locomotor demands would present modifications in the neck length and therefore will show a separated morphology. Similar functional consequences have been reported in humans having apophyseal-epiphyseal coalescence (i.e., coxa valga), which results in relatively small lever arm (which is mechanically less efficient), smaller femoral offset (which reduces mobility), and a smaller head-neck offset (which may result in anterior femoro-acetabular impingement) (Struijs et al. 2011).
Although Wilson and Geiger (2015) reported that fossorial hystricognath rodents, including the bathyergids C. hottentotus and G. capensis, and the broad-headed spiny rat Clyomys laticeps (Echimyidae), have an enlarged (medio-laterally) relative width of the proximal femur (i.e., greater trochanter index) compared to other non-fossorial rodents, there is no apparent relationship with the ossification pattern of fossorial and semi-fossorial mammals. Based on the limited data available in the literature, three digger species including the crested porcupine (Hystrix cristata), woodchuck (Marmota monax), and chipmunk (Tamias striatus), have a separated condition, whereas two species, including the nine-banded armadillo (Dasypus novemcinctus) and tenrec (Tenrec ecaudatus), have a coalesced condition (Serrat et al. 2007). All these taxa build underground shelters by excavation with their fore- and hind limbs, although most of their activities are performed aboveground, so that they are not strictly subterranean species in the true sense. They rather exhibit a variable degree of terrestrial locomotion, ranging from ambulatory to cursorial. Therefore, no clear relationship exists between the size of the greater trochanter (i.e., length), degree of fossorial specialization, and the ossification type (i.e., separated or coalesced), and additional investigations are needed to understand the processes regulating epiphyseal morphology, ossification pattern and function/behavior. Thus, it is apparent that the relationship between femoral morphology and locomotion in small mammals is more complex than expected, and that the functional interpretations derived from basic anatomical observations may not be as evident as in larger and heavier cursorial mammals.
Among subterranean rodents, the appendicular skeleton represents a complex system that must allow good performance of digging and locomotion. These activities include certain functional requirements such as an efficient locomotor performance enabling bidirectional locomotion to move in narrow spaces and a dense medium (Eilam et al. 1995; Lin et al. 2019; Montoya-Sanhueza et al. 2019), as well as scratch-digging behavior including posterior anchoring with hind limbs and transporting soils throughout burrows. The non-subterranean mole-rat relatives H. africaeaustralis and P. typicus, which have an ambulatory and rock climbing locomotory mode, respectively, exhibit an enlarged greater trochanter, compatible with a more “cursorial phenotype” (for high-speed movements), although differing from large cursorial mammals by showing a separated –and less robust– condition of the proximal epiphysis. Compared to these taxa, all bathyergids analyzed (except NMRs) showed a reduced level of development of such structure, probably suggesting a lower cursorial ability in this family, but also indicating a gain in the stability of the femoro-pelvic articulation, suitable for digging activities. In this context, NMRs would exhibit a less specialized proximal morphology in the femur within the bathyergid lineage. It is possible that subterranean social species such as NMRs, which form large colonies and large burrow systems, may preclude them from developing certain functional adaptations and therefore develop a different femoral configuration, thus enabling colony members to exhibit variable degrees of appendicular flexibility to move in tunnels along with other members of the colony, especially when forming organized sequences of “cooperative” digging (Jarvis and Sale 1971). Activities such as soil disposal would require an increased individual dynamism and flexibility to move over other individuals pushing soil backwards over obstructed tunnels, rather than having a powerful femoro-pelvic articulation. The generally reduced greater trochanters of NMRs may suggest a negative effect on the strength of the pelvic girdle and hind limbs to anchor their bodies against the burrow walls during chisel-tooth digging. Similarly, NMRs having the typical rodent (separated) condition may also experience lower ranges of hip extension, thus resulting in slower speed capabilities as compared to other conspecifics. However, individuals having a coalesced condition (relatively more robust proximal epiphysis), more like larger cursorial mammals, would experience an increased area for the attachment of the gluteal muscles and therefore a comparatively better stabilization of the femoro-pelvic articulation and a stronger pelvic area allowing the anchorage of their hind limbs to burrow walls during digging. A better understanding of such processes would require close examination of the locomotor dynamics among members of the same colony of this species, as well as detailed descriptions of the morphological changes during ontogeny and among different litters.
Availability of data and material
All data are included in the text and as electronic supplementary material.
References
Araújo FA de, Sesoko NF, Rahal SC, Teixeira CR, Müller TR, Machado MR (2013) Bone morphology of the hind limbs in two caviomorph rodents. Anat Histol Embryol 42(2):114-23. https://doi.org/10.1111/j.1439-0264.2012.01172.x
Atchley WR, Hall BK (1991) A model for development and evolution of complex morphological structures. Biol Rev 66:101–157. https://doi.org/10.1111/j.1469-185X.1991.tb01138.x
Bennett NC (2009) African mole-rats (Family Bathyergidae): models for studies in animal physiology. Afr Zool 44:263–270. https://hdl.handle.net/10520/EJC18111
Bennett NC, Faulkes CG (2000) African Mole Rats: Ecology and Eusociality. Cambridge University Press, Cambridge.
Böhmer C, Theil J-C, Fabre A-C, Herrel A (2020) Atlas of Terrestrial Mammal Limbs. CRC Press, Boca Raton, Florida.
Braude S, Holtze S, Begall S, Brenmoehl J, Burda H, Dammann P, Del Marmol D, Gorshkova E, Henning Y, Hoeflich A et al (2021) Surprisingly long survival of premature conclusions about naked mole-rat biology. Biol Rev 96:376–393. https://doi.org/10.1111/brv.12660
Bryja J, Konvičková H, Bryjová A. et al. (2018) Differentiation underground: range-wide multilocus genetic structure of the silvery mole-rat does not support current taxonomy based on mitochondrial sequences. Mamm Biol 93:82–92. https://doi.org/10.1016/j.mambio.2018.08.006
Buffenstein R, Amoroso V, Andziak B, Avdieiev S, Azpurua J, Barker AJ, Bennett NC, Brieño-Enríquez MA, Bronner GN, Coen C, Delaney MA, Dengler-Crish CM, Edrey YH, Faulkes CG, Frankel D, Friedlander G, Gibney PA, Gorbunova V, Hine C, Holmes MM, Jarvis JUM, Kawamura Y, Kutsukake N, Kenyon C, Khaled WT, Kikusui T, Kissil J, Lagestee S, Larson J, Lauer A, Lavrenchenko LA, Lee A, Levitt JB, Lewin GR, Lewis Hardell KN, Lin TD, Mason MJ, McCloskey D, McMahon M, Miura K, Mogi K, Narayan V, O'Connor TP, Okanoya K, O'Riain MJ, Park TJ, Place NJ, Podshivalova K, Pamenter ME, Pyott SJ, Reznick J, Ruby JG, Salmon AB, Santos-Sacchi J, Sarko DK, Seluanov A, Shepard A, Smith M, Storey KB, Tian X, Vice EN, Viltard M, Watarai A, Wywial E, Yamakawa M, Zemlemerova ED, Zions M, Smith ESJ (2022) The naked truth: a comprehensive clarification and classification of current 'myths' in naked mole-rat biology. Biol Rev 97(1):115–140. https://doi.org/10.1111/brv.12791
Buffenstein R, Park T, Hanes M, Artwohl JE (2012) Naked mole rat. In: Suckow MA, Stevens KA, Wilson RP (eds) The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Elsevier, London, UK, pp. 1055-1074.
Buffenstein R (2008) Negligible senescence in the longest living rodent, the naked molerat: insights from a successfully aging species. J Comp Physiol B 178:439–445. https://doi.org/10.1007/s00360-007-0237-5
Carmeli-Ligati S, Shipova A, Dumont M, Holtz S, Hildebrandt T, Shahar R (2019) The structure, composition and mechanical properties of the skeleton of the naked mole-rat (Heterocephalus glaber). Bone 128-115035. https://doi.org/10.1016/j.bone.2019.115035
Cole HA, Yuasa M, Hawley G, Cates JM, Nyman JS, Schoenecker JG (2013) Differential development of the distal and proximal femoral epiphysis and physis in mice. Bone 52(1):337-46. https://doi.org/10.1016/j.bone.2012.10.011
Dammann P, Burda H (2007) Senescence patterns in African mole-rats (Bathyergidae, Rodentia). In: Begall S, Burda H, Schleich C (eds) Subterranean Rodents: News from Underground. Springer-Verlag, Heiderberg, Germany, pp. 251-263.
Dengler-Crish C, Catania KC (2009) Cessation of reproduction-related spine elongation after multiple breeding cycles in female naked mole-rats. Anat Rec (Hoboken) 292:131–137. https://doi.org/10.1002/ar.20793
Dengler-Crish CM, Catania KC (2007) Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J Exp Biol 210:4351–4358. https://doi.org/10.1242/jeb.009399
Doubell NS, Sahd L, Kotzé SH (2020) Comparative forelimb morphology of scratch‐digging and chisel-tooth digging African mole‐rat species. J Morphol 281:1029– 1046. https://doi.org/10.1002/jmor.21229
Edgren W (1965) Coxa plana: a clinical and radiological investigation with particular reference to the importance of the metaphyseal changes for the final shape of the proximal part of the femur. Acta Orthop Scand 84:1–129.
Edrey Y, Park J, Kang H, Biney A, Buffenstein R (2011) Endocrine function and neurobiology of the longest-living rodent, the naked molerat. Exp Gerontol 46:116–123. https://doi.org/10.1016/j.exger.2010.09.005
Eilam D, Adijes M, Vilensky J (1995) Uphill locomotion in mole rats: a possible advantage of backward locomotion. Physiol Behav 58:483–489. https://doi.org/10.1016/0031-9384(95)00076-U
Ellerman JR (1956) The subterranean mammals of the world. Trans R Soc South Africa 35:(1)11–20. https://doi.org/10.1080/00359195609519005
Ellerman JR (1940) The Families and Genera of Living Rodents, Vol. 1. Rodents other than Muridae. British Museum, Natural History, London.
Etienne C, Filippo A, Cornette R, Houssaye A (2021) Effect of mass and habitat on the shape of limb long bones: A morpho-functional investigation on Bovidae (Mammalia: Cetartiodactyla) J. Anat 238:886– 904. https://doi.org/10.1111/joa.13359
Farnum CE (2007) Postnatal growth of fins and limbs through endochondral ossification. In: Hall BK (ed) Fins into Limbs: Evolution, Development, and Transformation. University Chicago Press, Chicago (Illinois) and London, USA, pp. 118–151.
Franz-Odendaal T, Hockman D (2019) Non-model organisms and unique approaches are needed for the future of evo-devo. Dev Dyn https://doi.org/10.1002/dvdy.71
Gomes Rodrigues H, Šumbera R, Hautier L. (2016) Life in burrows channelled the morphological evolution of the skull in rodents: the case of African mole-rats (Bathyergidae, Rodentia). J Mamm Evol 23:175–189. https://doi.org/10.1007/s10914-015-9305-x
Gomes Rodrigues H, Marangoni P, Šumbera R, Tafforeau P, Wendelen W, Viriot L (2011) Continuous dental replacement in a hyper-chisel tooth digging rodent. PNAS 108:17355–17359. https://doi.org/10.1073/pnas.1109615108
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4:1–9.
Hamilton WJ Jr (1928) Heterocephalus, the remarkable African burrowing rodent. Mus Brooklyn Inst Arts Sci 3(5):173-191.
Henry EC, Dengler-Crish CM, Catania KC (2007) Growing out of a caste-reproduction and the making of the queen mole-rat. J Exp Biol 210:261–268. https://doi.org/10.1242/jeb.02631
Hildebrand M. (1985) Digging of quadrupeds. In: Hildebrand M, Bramble D, Liem K, Wake DB (eds) Functional Vertebrate Morphology. The Belknap Press of Harvard University Press, Massachusetts and London, USA, pp. 89–109.
Hill WC, Porterr A, Bloom T, Seago J, Southwick MD (1957) Field and laboratory studies on the naked mole-rat (Heterocephalus glaber). Proc Zool Soc Lond 128:455-513. https://doi.org/10.1111/j.1096-3642.1957.tb00272.x
Hockman D, Franz-Odendaal TA (2019) Evo-devo explores the endless forms most beautiful, from extreme traits to subtle diversities. Dev Dyn 248(11):1026-1027. https://doi.org/10.1002/dvdy.123
Horner AM, Hanna JB, Biknevicius AR (2016) Crouching to fit in: the energetic cost of locomotion in tunnels. J Exp Biol 219:3420–3427. https://doi.org/10.1242/jeb.132449
Howell B (1965) Speed in Mammals, their Specialization for Running and Leaping. Hafner Publishing Company, New York and London.
Jarvis JUM, Sherman PW (2002) Heterocephalus glaber. Mamm Species No. 706:1-9. https://doi.org/10.1644/0.706.1
Jarvis JUM, O’Riain MJ, Bennett NC, Sherman PW (1994) Mammalian eusociality: a family affair. Trends Ecol Evol 9:47–51. https://doi.org/10.1016/0169-5347(94)90267-4
Jarvis J, O’Riain MJ, McDaid E (1991) Growth and factors affecting body size in naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD (eds) The Biology of the naked Mole-rat. Princeton University Press, New Jersey, USA, pp. 258–383.
Jarvis J, Sale J. (1971) Burrowing and burrow patterns of East African mole-rats Tachyoryctes, Heliophobius and Heterocephalus. J Zool 163:451–479. https://doi.org/10.1111/j.1469-7998.1971.tb04544.x
Katandukila J (2020) Craniometrics analysis for ontogenetic physiognomy and sexual dimorphism in Emin’s silvery Mole-Rats (Heliophobius argenteocinereus emini: Bathyergidae) from Tanzania. Tanz J Sci 46 (3):647-660.
Lacey EA, Sherman PW (1991) Social organization of naked mole-rat colonies: evidence for divisions of labor. In: Sherman PW, Jarvis JU, Alexander RD (eds.) The Biology of the Naked Mole-Rat. Princeton University Press, New Jersey, USA, pp. 275–336.
Lin Y-F, Konow N, Dumont ER (2019) How moles walk; it’s all thumbs. Biol Lett 15:20190503. https://doi.org/10.1098/rsbl.2019.0503
McNab BK (2002) The Physiological Ecology of Vertebrates. Associates, Comstock Publishing, Ithaca and London.
Mason MJ, Cornwall HL, Smith ESJ (2016) Ear structures of the naked mole-rat, Heterocephalus glaber, and its relatives (Rodentia: Bathyergidae). Plos One 11(12):e0167079. https://doi.org/10.1371/journal.pone.0167079
Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD (2008) Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg 16(10):596–607. https://doi.org/10.5435/00124635-200810000-00005
Moncayo‐Donoso M, Guevara JM, Márquez-Flórez K, Fontanilla MR, Barrera LA, Garzón‐Alvarado DA (2018) Morphological changes of physeal cartilage and secondary ossification centres in the developing femur of the house mouse (Mus musculus): A micro‐CT based study. Anat Histol Embryol 1–8. https://doi.org/10.1111/ahe.12417
Montoya-Sanhueza G, Bennett NC, Oosthuizen MK, Dengler-Crish CM, Chinsamy A (2021a). Long bone histomorphogenesis of the naked mole-rat: histodiversity and intraspecific variation. J Anat 238: 1259–1283. https://doi.org/10.1111/joa.13381
Montoya-Sanhueza G, Bennett NC, Oosthuizen, MK, Dengler-Crish CM, Chinsamy A (2021b) Bone remodeling in the longest living rodent, the naked mole-rat: interelement variation and the effects of reproduction. J Anat 239:81-100. https://doi.org/10.1111/joa.13404
Montoya-Sanhueza G (2020) Functional Anatomy, Osteogenesis and Bone Microstructure of the Appendicular System of African Mole-Rats (Rodentia: Ctenohystrica: Bathyergidae). PhD Dissertation, University of Cape Town.
Montoya-Sanhueza G, Wilson LAB, Chinsamy A (2019) Postnatal development of the largest subterranean mammal (Bathyergus suillus): morphology, osteogenesis, and modularity of the appendicular skeleton. Dev Dyn 1-28. https://doi.org/10.1002/dvdy.81
Nevo E (1999) Mosaic Evolution of Subterranean Mammals. Regression, Progression and Global Convergence. Oxford University Press, Oxford.
O’Connor TP, Lee A, Jarvis J, Buffenstein R (2002) Prolonged longevity in naked molerats: age-related changes in metabolism, body composition and gastrointestinal function. Comp Biochem Physiol A 133(3):835–42. https://doi.org/10.1016/S1095-6433(02)00198-8
Onwuama KT, Ojo SA, Hambolu JO, Dzenda T, Zakari FO, Salami SO (2018) Macro-anatomical and morphometric studies of the hindlimb of grasscutter (Thryonomys swinderianus, Temminck-1827). Anat Histol Embryol 47(1):21-27. https://doi.org/10.1111/ahe.12319
O’Regan HJ, Kitchener AC (2005) The effects of captivity on the morphology of captive, domesticated and feral mammals. Mamm Rev 35:215–30. https://doi.org/10.1111/j.1365-2907.2005.00070.x
O’Riain MJ, Jarvis J, Alexander R, Buffenstein R, Peeters C (2000) Morphological castes in a vertebrate. PNAS 97:13194–7. https://doi.org/10.1073/pnas.97.24.13194
O’Riain MJ, Jarvis J (1998) The dynamics of growth in naked mole-rats: the effects of litter order and changes in social structure. J Zool 246:49–60. https://doi.org/10.1111/j.1469-7998.1998.tb00131.x
O’Riain MJ (1996) Pup Ontogeny and Factors Influencing Behavioural and Morphological Variation in Naked Mole-Rats, Heterocephalus glaber (Rodentia, Bathyergidae). PhD Dissertation, University of Cape Town.
Park TJ, Reznick J, Peterson BL, Blass G, Omerbasic D, Bennett NC, Kuich PHJL, Zasada C, Browe BM, Hamann W et al (2017). Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356:307–311. https://doi.org/10.1126/science.aab3896
Parsons FG (1896) Myology of rodents –part II. An account of the myology of the Myomorpha, together with a comparison of the muscles of the various suborders of rodents. J Zool 64:159–192. https://doi.org/10.1111/j.1096-3642.1896.tb03033.x
Pinto M, Jepsen KJ, Terranova CJ, Buffenstein R (2010) Lack of sexual dimorphism in femora of the eusocial and hypogonadic naked mole-rat: a novel animal model for the study of delayed puberty on the skeletal system. Bone 46:112–120. https://doi.org/10.1016/j.bone.2009.08.060
Polly D (2007) Limbs in mammalian evolution. In: Hall BK (ed) Fins into Limbs: Evolution, Development, and Transformation. University Chicago Press, Chicago (Illinois) and London, USA, pp. 245–268.
Riser WH (1973) Growth and development of the normal canine pelvis, hip joints and femurs from birth to maturity: a radiographic study. Vet Radiol 14:24-34. https://doi.org/10.1177/030098587501200404
Sahd L, Bennett NC, Kotzé SH (2019) Hind foot drumming: morphological adaptations of the muscles and bones of the hind limb in three African mole-rat species. J Anat 235(4):811–824. https://doi.org/10.1111/joa.13028
Samuels JX, Valkenburgh B Van (2008) Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol 269:1387–1411. https://doi.org/10.1002/jmor.10662
Serrat MA, Reno PL, McCollum MA, Meindl RS, Lovejoy CO. (2007) Variation in mammalian proximal femoral development: comparative analysis of two distinct ossification patterns. J Anat 210(3):249-58. https://doi.org/10.1111/j.1469-7580.2007.00694.x
Sherman PW, Jarvis JU, Alexander RD (1991) The Biology of the Naked Mole-Rat. Princeton University Press, New Jersey.
Sherman P, Jarvis J (2002) Extraordinary life spans of naked mole-rats (Heterocephalus glaber). J Zool 258(3):307-311. https://doi.org/10.1017/S0952836902001437
Siegmann S, Feitsch R, Hart DW, Bennett NC, Penn DJ, Zöttl M (2021) Naked mole-rats (Heterocephalus glaber) do not specialise in cooperative tasks. Ethol 127:850–864 https://doi.org/10.1111/eth.13160
Skulachev VP, Holtze S, Vyssokikh MY, Bakeeva LE, Skulachev MV, Markov AV, Hildebrandt TB, Sadovnichii VA (2017) Neoteny, prolongation of youth: from naked mole rats to “naked apes” (humans). Physiol Rev 97:699–720. https://doi.org/10.1152/physrev.00040.2015
Stein B (2000) Morphology of subterranean rodents. In: Lacey EA, Patton J, Cameron GN (eds) Life Underground: the Biology of Subterranean Rodents. The University of Chicago Press, Chicago, USA, pp. 19–61.
Struijs PAA, Oostra RJ, van Rijn RR, Besselaar PP (2011) Abnormal growth of the proximal femur due to apophyseal-epiphyseal coalescence resulting in coxa valga–a report of two cases in adolescents. Acta Ortho 82:507–509. https://doi.org/10.3109/17453674.2011.584210
Thorley J, Katlein N, Goddard K, Zottl M, Clutton-Brock T (2018) Reproduction triggers adaptive increases in body size in female mole-rats. Proc R Soc B 285:20180897. https://doi.org/10.1098/rspb.2018.0897
Tian J, Azpurua C, Hine A, Vaidya M, Myakishev-Rempel J, Ablaeva Z, Mao E, Nevo V, Gorbunova A, Seluanov (2013) High-molecular-mass hyaluronan mediates the Cancer resistance of the naked mole rat. Nature 499:346–349. https://doi.org/10.1038/nature12234
Tucker, R (1981) The digging behavior and skin differentiations in Heterocephalus glaber. J Morphol 168:51–71. https://doi.org/10.1002/jmor.1051680107
Visser JH, Bennett NC, Van Vuuren BJ (2019) Phylogeny and biogeography of the African Bathyergidae: a review of patterns and processes. PeerJ 7:e7730. https://doi.org/10.7717/peerj.7730
Wilson LAB, Geiger M (2015) Diversity and evolution of femoral variation in Ctenohystrica. In: Cox PG, Cox L (eds) Evolution of the Rodents - Advances in Phylogeny, Functional Morphology and Development. Cambridge University Press, Cambridge, UK, pp. 510–538.
Xie M, Gol'din P, Herdina AN, Estefa J, Medvedeva EV, Li L, Newton PT, Kotova S, Shavkuta B, Saxena A, Shumate LT, Metscher BD, Großschmidt K, Nishimori S, Akovantseva A, Usanova AP, Kurenkova AD, Kumar A, Arregui IL, Tafforeau P, Fried K, Carlström M, Simon A, Gasser C, Kronenberg HM, Bastepe M, Cooper KL, Timashev P, Sanchez S, Adameyko I, Eriksson A, Chagin AS (2020) Secondary ossification center induces and protects growth plate structure. Elife 9:e55212. https://doi.org/10.7554/eLife.55212
Yadav P, Peña Fernández M, Gutierrez-Farewik EM (2021) Influence of loading direction due to physical activity on proximal femoral growth tendency. Med Eng Phys 90:83-91. https://doi.org/10.1016/j.medengphy.2021.02.008
Yadav P, Shefelbine SJ, Pontén E, Gutierrez-Farewik EM (2017) Influence of muscle groups' activation on proximal femoral growth tendency. Biomech Model Mechanobiol 16(6):1869-1883. https://doi.org/10.1007/s10237-017-0925-3
Young AJ, Bennett NC (2010) Morphological divergence of breeders and helpers in wild Damaraland mole-rat societies. Evol 64(11):3190–3197. https://doi.org/10.1111/j.1558-5646.2010.01066.x
Acknowledgements
We are very grateful to Tim Clutton-Brock for providing mole-rat specimens and Dave Gaynor, Tanja van de Ven, Jacob Brown, Carlijn Brands and Philippe Vullioud for assisting with the access to specimens in the Kalahari Meerkat Project (Kuruman Station). We also thank Marcelo Sánchez-Villagra (Universität Zürich) and Sabine Begall (Universität Duisburg-Essen, Germany) for providing mole-rats specimens. Denise Hamerton (Curator), Jofred Opperman (Collections Manager) and Gabriel Lukoji (Assistant Collections Manager) are also acknowledged for kindly giving access to petromurid and hystricid specimens at the Iziko SA Museum (Cape Town, South Africa). We thank the editors, Darin Croft and Rachel H Dunn, as well as Alexandra Houssaye and an anonymous reviewer for their constructive comments on the manuscript, which significantly improved our study.
Funding
This project was supported by Becas Chile, Government of Chile (CONICYT, 72160463), National Research Foundation (NRF-117716), the SARChI chair of Mammalian Behavioural Ecology and Physiology (DST-NRF-64756), and the Czech Science Foundation (GAČR, 20-10222S).
Author information
Authors and Affiliations
Contributions
GMS and AC designed the study; AC supervised GMS’s doctoral thesis and supported the experimental procedures; NB and RS provided mole-rat specimens and supported the experimental procedures; GMS, analysed data, prepared the manuscript, acquired images, created figures; All authors read, edited and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest/Competing Interests
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montoya-Sanhueza, G., Šumbera, R., Bennett, N.C. et al. Developmental Plasticity in the Ossification of the Proximal Femur of Heterocephalus glaber (Bathyergidae, Rodentia). J Mammal Evol 29, 663–675 (2022). https://doi.org/10.1007/s10914-022-09602-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-022-09602-y