Abstract
The inhomogeneous phase of a smart meta-superconductor has a great effect on its superconductivity. In this paper, the effect of concentration, dimensions, electroluminescence (EL) intensity, and distribution of the inhomogeneous phase on the superconducting critical temperature \((T_{C})\) has been systematically investigated. An ex situ solid sintering was utilized to prepare smart meta-superconducting \(\hbox {MgB}_{2}\) doped with six kinds of electroluminescent materials, such as \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) and \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\) flakes. Elemental mappings through energy dispersive spectroscopy (EDS) show that the inhomogeneous phase is comparatively uniformly dispersed around the \(\hbox {MgB}_{2}\) particles; thus V, Y, and Eu were accumulated at a small area. The measurement results show that the optimum doping concentration of the meta-superconducting \(\hbox {MgB}_{2}\) is 2.0 wt%. The offset temperature (\(T_{C}^{{ off}}\)) of the sample doped with 2.0 wt% dopant A is 1.6 K higher than that of pure \(\hbox {MgB}_{2}\). The improvement in \(T_{C}^{{ off}}\) is likely related to the sizes, thickness, and EL intensity of the inhomogeneous phase of \(\hbox {MgB}_{2}\) smart meta-superconductor. This experiment provides a novel approach to enhance \(T_{C}\).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The superconductivity of \(\hbox {MgB}_{2}\) was first discovered in 2001 [1]. \(\hbox {MgB}_{2}\) is a promising material with large-scale applications because of its simple crystal structure, high \(T_{C}\) (39.0 K), large coherence lengths, and high critical current densities and fields [2]. Various methods have been proposed to improve \(T_{C}\) of \(\hbox {MgB}_{2}\). One of the most important methods is chemical doping. Al and C are considered useful elements for effective substitutional doping in \(\hbox {MgB}_{2}\). However, partially substituting Mg with Al or B with C decreases \(T_{C}\) of \(\hbox {MgB}_{2}\) [3,4,5,6,7]. A high doping content even causes the loss of superconductivity. It is generally considered that the decrease in \(T_{C}\) is caused by the large electron concentration [8, 9]. Thus, researchers have tried to improve the \(T_{C}\) by increasing the density of hole carriers by substituting Mg with Li. However, the results of this method are unsatisfactory [10, 11]. The crystal lattice of \(\hbox {MgB}_{2}\) is distorted during the substitutional doping process. In non-substitutional doping, a dopant, which acts as an impurity phase, is present in the grain boundary of \(\hbox {MgB}_{2}\). This condition decreases grain connectivity and increases impurity scattering [12,13,14]. As such, effective methods should be developed to improve \(T_{C}\) of \(\hbox {MgB}_{2}\).
Chemical doping, which can increase the pinning force, can also be applied to improve the critical current density of \(\hbox {MgB}_{2}\). Significant progress has been made in this regard [15, 16]. However, practical applications of \(\hbox {MgB}_{2}\) have yet to be implemented. First, chemical doping generally involves the direct mixing of a raw material (B and Mg) with a dopant. This dopant may react with B or Mg during subsequent experimental processes. Second, a B-rich phase may form during the in situ solid sintering because the melting point of Mg is relatively low and Mg vapor forms in this stage. Thus, excessive Mg is added during \(\hbox {MgB}_{2}\) preparation, especially in large-scale production, which leads to the formation of residual Mg in the final product. Dopant and residual Mg greatly decrease the superconducting transition temperature and increase the transition width [17,18,19,20,21,22]. In order to make a material in the superconducting state during practical applications, we should set a temperature lower than the offset temperature of the material. Therefore, many difficulties impede the practical applications of \(\hbox {MgB}_{2}\) even though its critical current density is large.
Meta-materials are a type of composite materials with artificial structures that can provide special functions that are absent in nature [23,24,25]. Researchers have started to try to improve \(T_{C}\) via the meta-material method. In 2007, our group proposed that doping electroluminescent materials into a superconducting material to form a smart meta-superconductor may be an effective method to improve \(T_{C}\) [26, 27]. Zhang et al. [28] prepared \(\hbox {MgB}_{2}\) doped with an efficient electroluminescent material \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\) by an in situ solid sintering process. Tao et al. [29] prepared \(\hbox {MgB}_{2}\) doped with \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\) via a modified ex situ process and confirmed that this method is favorable for \(T_{C}\) improvement. According to homogeneous system theory [30], Smolyaninov et al. proposed that a superconducting meta-material with an effective dielectric response function that is less and approximately equal to zero may exhibit high \(T_{C}\) [31], and they verified this theory in their subsequent experiments [32, 33]. Meta-superconductor has attracted lots of concerns.
Based on our previous work, combining a superconductor and an inhomogeneous phase to form a smart meta-superconductor may be an effective method for \(T_{C}\) improvement. The results show that the concentration, dimensions, and distribution of the inhomogeneous phase all affect the critical temperature of the smart meta-superconductor. In order to systematically investigate the influence of inhomogeneous phase on the superconductivity, six kinds of dopants with different sizes were prepared through a hydrothermal method in this study [34]. \(\hbox {MgB}_{2}\) doped with these dopants were prepared via a modified ex situ solid sintering. Results indicate that these dopants display a limited effect on the onset temperature but elicit a remarkable influence on the offset temperature. Among these samples, the sample doped with 2.0 wt% dopant A yields the highest \(T_{C}^{{ off}}\), which is 1.6 K higher than that of pure \(\hbox {MgB}_{2}\).
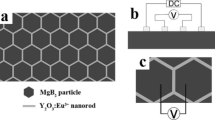
Microstructure model of the smart meta-superconducting \(\hbox {MgB}_{2}\) [29]
2 Model
Figure 1 shows the microstructure model of smart meta-superconducting \(\hbox {MgB}_{2}\), which consists of \(\hbox {MgB}_{2}\) matrix and the inhomogeneous phase such as the EL material \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) or \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\). The hexagons in this figure represent \(\hbox {MgB}_{2}\) particles consisting of \(\hbox {MgB}_{2}\) multi-grains. The inhomogeneous phase is dispersed around the \(\hbox {MgB}_{2}\) particles, just like the gray area in this figure. The thickness of the inhomogeneous phase in the model must satisfy the coherence length conditions. In the present study, \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) disperses around the \(\hbox {MgB}_{2}\) particles to form a meta-structure. Adjusting the applied electric field to control the EL of \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) or \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\) may alter \(T_{C}\) of this smart meta-superconductor.
3 Experiment
-
1.
Preparation of the dopants
0.153 g of \(\hbox {Y}_{2}\hbox {O}_{3}\) and 0.012 g of \(\hbox {Eu}_{2}\hbox {O}_{3}\) were weighed and transferred to a beaker. Approximately 4 mL of concentrated nitric acid was then added to the beaker. A kind of white precursor was formed after heating the solution at \(70\,^{\circ }\hbox {C}\). Afterward, a certain quantity of the precursor was dissolved in 4 mL deionized water to form a transparent solution, which was designated as L1. Another solution, which was designated as L2, was prepared by dissolving 0.577 g of ammonium oxalate in 14 mL deionized water. Solution L1 was added dropwise to solution L2. A white precipitate then formed. After adding \(0.221\hbox { g Na}_{3}\hbox {VO}_{4}\), the solution pH was adjusted to 9 by adding NaOH. Subsequently, the solution was transferred to a reaction kettle. A hydrothermal reaction then occurred at \(160\,^{\circ }\hbox {C}\) and was allowed to proceed for a certain period of time to form a liquid with precipitates. After washing several times with deionized water and absolute ethanol, we can get a kind of yellow precipitate, which was sintered at \(800\,^{\circ }\hbox {C}\) for 2 h to form the final \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) flake. The size and the composition of the dopants would been altered by changing the temperature for doping L1 into L2 and adjusting the time for the hydrothermal reaction. We have synthesized six kinds of dopants designated as A–F. Table 1 shows the corresponding experimental conditions and dimensions for these dopants. The composition of dopant A and B is \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\), and the composition of dopant C–F is \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\) [34].
-
2.
Preparation of \(\hbox {MgB}_{2}\)-based superconductor
\(\hbox {MgB}_{2}\) powder was purchased from Luoyang Tongrun Information Technology Co., Ltd, and its purity is 95%. The weights of \(\hbox {MgB}_{2}\) and the dopant were calculated based on the content of the dopant. Then, \(\hbox {MgB}_{2}\) and the dopant were, respectively, weighed and added into alcohol to form two suspensions, which were sonicated for 10 min. Afterward, the dopant was added dropwise to \(\hbox {MgB}_{2}\). The resulting suspension was further sonicated for 20 min. The final suspension was transferred into a culture dish, which was then placed in a vacuum oven for 1 h at \(60\,^{\circ }\hbox {C}\) to yield a black powder. Finally, the powder was pressed into a tablet and placed in a small tantalum container, which was annealed at \(800\,^{\circ }\hbox {C}\) for 2 h in the high-purity argon atmosphere with no external pressure. Ten doping samples were prepared with varying dopant and doping concentrations. The samples were designed as S1, S2, S3, S4, S5, S6, S7, S8, S9, and S10. A pure \(\hbox {MgB}_{2}\) sample, which was marked as S, was likewise produced via the ex situ process. To ensure the reliability of the experimental results, we synthesized more than three samples of different kinds of \(\hbox {MgB}_{2}\). \(T_{C}^{{ off}}\) of each sample was measured more than twice. The dopant and doping concentration of the different samples are listed in Table 2.
4 Results and Discussion
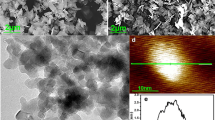
Figure 2a–f shows the scanning electron microscopy (SEM) images of dopants A–F, respectively. The composition of dopant A and B is \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\), and the composition of dopant C–F is \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\). It can be seen that all the dopants have square flake micromorphologies. Meanwhile, it can confirm that adjusting the doping temperature and the hydrothermal reaction time can change the dopant size. One dopant, however, does not have a strictly uniform flake structure. The size of dopant A is approximately 1–2 \(\upmu \hbox {m}\) and that of dopant B is approximately 2–3 \(\upmu \hbox {m}\). The sizes of dopants C, D, E, and F are all under \(1\,\upmu \hbox {m}\), which decrease in turn. The difference in the sizes of these four dopants is not large. The thickness of these flakes is about one tenth of the length. Figure 2g shows the EL spectra of all the samples under the same excitation voltage. The EL intensities in order from the weak to the strong are: C, B, D, A, E, and F.
Figure 3a shows the SEM image of pure \(\hbox {MgB}_{2}\). It can be seen that the size of pure \(\hbox {MgB}_{2}\) particles is 0.2–2 \(\upmu \hbox {m}\) with irregular micromorphology. There are obvious boundaries around the particles, which greatly enlarge the superconducting transition width (\(\Delta T = 4.6\hbox { K}\)), as shown in Fig. 3b. Figure 3b presents the corresponding R–T curve in a low-temperature range. The superconducting critical transition temperature was determined by measuring the resistivity of the samples. The R–T curve was measured using the four-probe method in a liquid helium cryogenic system made by the Advanced Research Systems Company. Figure 3b shows that the onset (\(T_{C}^{{ on}}\)) and offset (\(T_{C}^{{ off}}\)) critical temperatures of pure \(\hbox {MgB}_{2}\) are 38.2 and 33.6 K, respectively. The \(T_{C}\) is lower than the theoretical value and \(\Delta T\) is larger than the theoretical value. The inset of Fig. 3b is the X-ray diffraction (XRD) spectrum of the pure \(\hbox {MgB}_{2}\). The spectrum indicates that the main phases in the raw material are \(\hbox {MgB}_{2}\) and Mg. Meanwhile, a small amount of MgO can be found in this raw material. The main source for Mg is the residual Mg used to prepare \(\hbox {MgB}_{2}\). Mg vapor is generated during the solid sintering of \(\hbox {MgB}_{2}\) as the melting point of Mg \((650\,^{\circ }\hbox {C})\) is relatively low. To fully react with B to form \(\hbox {MgB}_{2}\), excess Mg is added during preparation. Residual Mg, which acts as an impurity phase in \(\hbox {MgB}_{2}\), is a main factor for the decrease in \(T_{C}\) and the increase in \(\Delta T\) [17,18,19]. Furthermore, the red curve is the R–T curve of \(\hbox {MgB}_{2}\) doped with 2.0 wt% \(\hbox {Y}_{2}\hbox {O}_{3}\) prepared by the method in [29]. Results shows that doping non-EL material \(\hbox {Y}_{2}\hbox {O}_{3}\) decreases the superconducting transition temperature.
Figure 4 shows the R–T curves of \(\hbox {MgB}_{2}\) doped with dopant A at concentrations of 0.5, 1.0, 1.5, 2.0, and 3.0 wt%. The corresponding samples are marked as S1, S2, S3, S4, and S5. All the five curves clearly show the superconducting transition. When the doping concentration is lower than 2 wt%, the resistivity values for the normal state of the four samples are not obviously different. However, the increase in resistivity value is large at a doping concentration of 3 wt%. All of the samples were pressed into small pieces with a diameter of 12 mm and a thickness of 1 mm. The distance among the four probes is 1 mm, and the resistance measurement of all of the samples is under the same condition. Thus, the difference in normal resistance should not be related to the variation of dimensions in the samples.
The onset temperature of these five samples is approximately 0.2 K lower than that of pure \(\hbox {MgB}_{2}\). Furthermore, the offset temperature of these samples is greatly affected by the doping concentration, as shown in the inset of Fig. 4. The inset has listed three measurements for the \(T_{C}^{{ off}}\) of each sample. The abscissa of the inset image represents the five samples. The ordinate represents the value of \(T_{C}^{{ off}}\). It can be seen that some measurement results are overlapping and the measurement for \(T_{C}^{{ off}}\) of all of the samples exhibited good reproducibility. The ordinate value of the dotted line in the inset image is 33.6 K, which is the \(T_{C}^{{ off}}\) of pure \(\hbox {MgB}_{2}\). The result shows that the value of \(T_{C}^{{ off}}\) rises first and then decreases with the increase in doping concentration and most of the measurements are higher than that of pure \(\hbox {MgB}_{2}\). When the doping concentration is 2.0 wt%, \(T_{C}^{{ off}}\) is as high as 35.2 K, which is 1.6 K higher than that of pure \(\hbox {MgB}_{2}\). The \(T_{C}^{{ off}}\) of the other samples has also been improved by different degrees, except for S2, whose \(T_{C}^{{ off}}\) is basically equal to that of pure \(\hbox {MgB}_{2}\). The EL material \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) is the only difference between the doped samples and pure \(\hbox {MgB}_{2}\). Thus, the differences in \(T_{C}^{{ off}}\) should be induced by the EL exciting effect of \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) [29]. These results indicate that doping \(\hbox {YVO}_{4}{:}\hbox {Eu}^{3+}\) to \(\hbox {MgB}_{2}\) is favorable to the improvement in \(T_{C}^{{ off}}\). Moreover, the optimum doping concentration is 2.0 wt%, which is consistent with the results of our previous works [28, 29].
Figure 5 presents the R–T curves of the samples S6, S7, S8, S9, and S10. The multiple measurements for the offset temperature of each sample are presented in the inset image. The \(T_{C}^{{ off}}\) of pure \(\hbox {MgB}_{2}\) is also marked by dotted line. The doping concentrations of these five samples are all 2.0 wt% with different dopants, as shown in Table 2. The difference in resistivity values for the normal state of these samples is not obvious. The onset temperatures of all samples are all 38.0 K, which is 0.2 K lower than that of pure \(\hbox {MgB}_{2}\). Except for S6, the offset temperatures of these samples are improved with different degree. The main reason for the difference in \(T_{C}^{{ off}}\) is that these dopants have different thicknesses and EL intensities, which will result in a different EL exciting effect. The improvement in \(T_{C}^{{ off}}\) is remarkable compared with \(\hbox {MgB}_{2}\) doped with non-EL materials, such as \(\hbox {Y}_{2}\hbox {O}_{3}\), because doping always decreases \(T_{C}\). Similar results were described in our previous study [29], which revealed that \(T_{C}\) of \(\hbox {MgB}_{2}\) doped with the EL material \(\hbox {Y}_{2}\hbox {O}_{3}{:}\hbox {Eu}^{3+}\) is 2.8 K higher than that of \(\hbox {MgB}_{2}\) doped with \(\hbox {Y}_{2}\hbox {O}_{3}\). Similar to the experimental results in Fig. 4, Fig. 5 indicates that doping EL materials into \(\hbox {MgB}_{2}\) improves \(T_{C}^{{ off}}\).
The six kinds of dopants are all materials with good EL. During the R–T curve measurement, electrons would excite the dopants to generate EL, causing the EL exciting effect, which is favorable for the improvement in superconducting transition temperature [28, 29]. However, as an impurity phase, the dopants would also introduce lattice defects and cause lattice distortion, which decreases the superconducting transition temperature. Thus, in order to improve the critical temperature as much as possible, the dopants should be uniformly dispersed in the sample to decrease the impurity effect and increase the EL exciting effect.
Figure 6a presents the SEM image for S4. Figure 6b shows the energy dispersive spectroscopy results. Mg, B, O, V, Y, and Eu, are detected in this sample. However, it is difficult to distinguish the inhomogeneous phase in the \(\hbox {MgB}_{2}\) matrix from the SEM image; thus, EDS elemental mapping was chosen to display the dispersion of inhomogeneous phase. The distribution images of the metallic elements are presented in Fig. 6c–f. The corresponding element is listed in the lower right corner of each image. Lots of Mg conform to the XRD and energy dispersive spectroscopy results. Mg is uniformly dispersed throughout the image, which is not the same with the dispersion of V, Y, and Eu as shown in Fig. 6d–f. It can be seen that Fig. 6d–f is divided into many small black areas by V, Y, and Eu, respectively. These small areas consist of \(\hbox {MgB}_{2}\) particles. Given that no chemical reaction occurred during sample preparation, the dispersion of the elements V, Y, and Eu can represent the dispersion of the dopants. Thus, Fig. 6d–f shows that the dopants are distributed around the \(\hbox {MgB}_{2}\) particles to form a kind of superstructure the same with Fig. 1. Such a distribution is able to decrease the impurity effect and increase the EL exciting effect. The increase in the offset temperature is attributed to the EL exciting effect induced by the dopants during resistivity measurement.
When the doping concentration is 2.0 wt%, different dopants exert different effects on the increase in \(T_{C}^{{ off}}\). If the dopant is A, the \(T_{C}^{{ off}}\) is 35.2 K, which is 1.6 K higher than that of pure \(\hbox {MgB}_{2}\). \(T_{C}^{{ off}}\) will change to 33.6 K when dopant A is replaced with dopant B. Sample S4 has the highest offset temperature because the dopant A owns a strong EL intensity. Although dopant B also own a strong EL intensity, S6 does not have a high offset temperature because of the large thickness of dopant B, which is not conducive to inducing a strong EL exciting effect. In addition, the size and thickness of dopants C–F decrease in turn and their EL intensities increase in turn. The \(T_{C}^{{ off}}\) of \(\hbox {MgB}_{2}\) doped with dopants C, D, E, and F are 34.0, 34.0, 34.8, and 34.2 K, respectively. The results showed that the offset temperature of the samples doped with dopants C–F with same concentration does not strictly increase in turn. The main reason is that as the size and thickness decrease, the dopant flakes cannot be uniformly dispersed easily but are more likely to aggregate.
The effect of doping EL materials into \(\hbox {MgB}_{2}\) on the superconductive transition temperature has been investigated in our previous study. At first, an in situ process has been applied to synthesize the samples [28]. The results showed that doping EL materials improves \(T_{C}\). However, the dopant can react with boron to form \(\hbox {YB}_{4}\) during the in situ process. Thus, an ex situ process was then applied to synthesize the samples to avoid such a reaction [29]. The results showed that the ex situ method can prevent the generation of \(\hbox {YB}_{4}\). Meanwhile, the results confirmed that doping EL materials improves \(T_{C}\). However, the improvement in \(T_{C}\) in our previous works is not obvious. In this study, we have systematically investigated the influence of the inhomogeneous phase on \(T_{C}\) and prepared the samples with higher superconducting transition temperature. Although, the superconducting transition width of 4.6 K is too large as for the pristine material, this experiment focuses on the change in \(T_{C}\) of the doped samples compared with that of pure \(\hbox {MgB}_{2}\). Moreover, the raw materials of all samples are the same; thus the difference between these samples is the inhomogeneous phase, which is the inducing factor for different \(T_{C}\). The result indicates that doping EL material favors the improvement in \(T_{C}^{{ off}}\). The largest improvement in \(T_{C}^{{ off}}\) is 1.6 K. Actually, we have got the similar result even if the \(\Delta T_{C}\) of pure \(\hbox {MgB}_{2}\) is 1.8 K [29]. The large superconducting transition width should not change the experimental results. This study is also valuable in using commercial \(\hbox {MgB}_{2}\).
5 Conclusion
The inhomogeneous phase of a smart meta-superconductor has a great effect on its superconductivity. In order to systematically investigate the influence of the inhomogeneous phase on \(T_{C}\), six kinds of dopants with different sizes are prepared via the hydrothermal method. \(\hbox {MgB}_{2}\)-based meta-superconductors are prepared via a modified experimental method. EDS elemental mappings show that the inhomogeneous phases are comparatively uniformly dispersed around the \(\hbox {MgB}_{2}\) particles; thus V, Y, and Eu were accumulated at a small area. The superconducting transition temperatures for the samples are determined from the R–T curves, which are measured by the four-probe method. Results indicate that these six kinds of dopants have a different effect on the improvement in \(T_{C}^{{ off}}\). The optimum doping concentration is 2.0 wt%. Among all the samples, S4 doped with 2.0 wt% dopant A owns the highest \(T_{C}^{{ off}}\), which is 1.6 K higher than that of pure \(\hbox {MgB}_{2}\). Although the mechanism that increases \(T_{C}^{{ off}}\) remains to be clarified, the improvement in \(T_{C}^{{ off}}\) is likely related to the size, thickness, and EL intensity of the inhomogeneous phase of the \(\hbox {MgB}_{2}\) smart meta-superconductor. This experiment provides a novel approach for improving \(T_{C}\) and expands the practical application of superconducting materials.
References
J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani, J. Akimitsu, Nature 410, 63–64 (2001)
C. Buzea, T. Yamashita, Supercond. Sci. Technol. 14, R115–R146 (2001)
J.S. Slusky, N. Rogado, K.A. Regan, M.A. Hayward, P. Khalifah, T. He, K. Inumaru, S.M. Loureiro, M.K. Haas, H.W. Zandbergen, R.J. Cava, Nature 410(6826), 343–345 (2001)
H. Luo, C.M. Li, H.M. Luo, S.Y. Ding, J. Appl. Phys. 91(10), 7122–7124 (2002)
R.J. Cava, H.W. Zandbergen, K. Inumaru, Physica C 385, 8–15 (2003)
S.M. Kazakov, R. Puzniak, K. Rogacki, A.V. Mironov, N.D. Zhigadlo, J. Jun, C. Soltmann, B. Batlogg, J. Karpinski, Phys. Rev. B 71(2), 024533 (2005)
A. Bianconi, Y. Busby, M. Fratini, V. Palmisano, L. Simonelli, M. Filippi, S. Sanna, F. Congiu, A. Saccone, M. Giovannini, S. De Negri, J. Supercond. Nov. Magn. 20(7), 495–501 (2007)
O. de la Peña, A. Aguayo, R. de Coss, Phys. Rev. B 66(1), 012511 (2002)
J. Kortus, O.V. Dolgov, R.K. Kremer, A.A. Golubov, Phys. Rev. Lett. 94(2), 027002 (2005)
Y.G. Zhao, X.P. Zhang, P.T. Qiao, H.T. Zhang, S.L. Jia, B.S. Cao, M.H. Zhu, Z.H. Han, X.L. Wang, B.L. Gu, Physica C 361, 91–94 (2001)
M. Monni, M. Affronte, C. Bernini, D. Di Castro, C. Ferdeghini, M. Lavagnini, P. Manfrinetti, A. Orecchini, A. Palenzona, C. Petrillo, P. Postorino, A. Sacchetti, F. Sacchetti, M. Putti, Physica C 460–462, 598–599 (2007)
A.V. Sologubenko, N.D. Zhigadlo, S.M. Kazakov, J. Karpinski, H.R. Ott, Phys. Rev. B 71(2), 020501 (2005)
K. Rogacki, B. Batlogg, J. Karpinski, N.D. Zhigadlo, G. Schuck, S.M. Kazakov, P. Wägli, R. Puźniak, A. Wiśniewski, F. Carbone, A. Brinkman, D. van der Marel, Phys. Rev. B 73(17), 174520 (2006)
A.V. Sologubenko, N.D. Zhigadlo, J. Karpinski, H.R. Ott, Phys. Rev. B 74(18), 184523 (2006)
S.X. Dou, S. Soltanian, J. Horvat, X.L. Wang, S.H. Zhou, M. Ionescu, H.K. Liu, P. Munroe, M. Tomsic, Appl. Phys. Lett. 81(18), 3419–3421 (2002)
J.H. Kim, S. Zhou, M.S.A. Hossain, A.V. Pan, S.X. Dou, Appl. Phys. Lett. 89(14), 142505 (2006)
P. Postorino, A. Congeduti, P. Dore, A. Nucara, A. Bianconi, D. Di Castro, S. De Negri, A. Saccone, Phys. Rev. B 65(2), 020507 (2001)
T. Takenobu, T. Ito, D. Hieu Chi, K. Prassides, Y. Iwasa, Phys. Rev. B 64(13), 134513 (2001)
A. Bianconi, S. Agrestini, D. Di Castro, G. Campi, G. Zangari, N.L. Saini, A. Saccone, S. De Negri, M. Giovannini, G. Profeta, A. Continenza, G. Satta, S. Massidda, A. Cassetta, A. Pifferi, M. Colapietro, Phys. Rev. B 65(17), 174515 (2002)
J.Q. Li, L. Li, F.M. Liu, C. Dong, J.Y. Xiang, Z.X. Zhao, Phys. Rev. B 65(13), 134426 (2002)
W. Mickelson, J. Cumings, W.Q. Han, A. Zettl, Phys. Rev. B 65(5), 052505 (2002)
R.H. Wilke, S.L. Bud’ko, P.C. Canfield, D.K. Finnemore, R.J. Suplinskas, S.T. Hannahs, Phys. Rev. Lett. 92(21), 217003 (2004)
H. Liu, X.P. Zhao, Y. Yang, Q.W. Li, J. Lv, Adv. Mater. 20(11), 2050–2054 (2008)
Y.P. Qiao, X.P. Zhao, Y.Y. Su, J. Mater. Chem. 21(2), 394–399 (2011)
X.P. Zhao, J. Mater. Chem. 22(19), 9439–9449 (2012)
W.T. Jiang, Z.L. Xu, Z. Chen, X.P. Zhao, J. Funct. Mater 38, 157–160 (2007) (in Chinese). http://www.cnki.com.cn/Article/CJFDTOTAL-GNCL200701046.htm
S.H. Xu, Y.W. Zhou, X.P. Zhao, Mater. Rev. 21, 162–166 (2007) (in Chinese). http://www.cnki.com.cn/Article/CJFDTotal-CLDB2007S3048.htm
Z.W. Zhang, S. Tao, G.W. Chen, X.P. Zhao, J. Supercond. Nov. Magn. 29(5), 1159–1162 (2016)
S. Tao, Y.B. Li, G.W. Chen, X.P. Zhao, J. Supercond. Nov. Magn. 30(6), 1405–1411 (2017)
D.A. Kirzhnits, E.G. Maksimov, D.I. Khomskii, J. Low Temp. Phys. 10(1–2), 79–93 (1973)
I.I. Smolyaninov, V.N. Smolyaninova, Adv. Condens. Matter Phys. 91(9), 479635 (2014)
V.N. Smolyaninova, B. Yost, K. Zander, M.S. Osofsky, H. Kim, S. Saha, R.L. Greene, I.I. Smolyaninov, Sci. Rep. 4, 7321 (2014)
V.N. Smolyaninova, K. Zander, T. Gresock, C. Jensen, J.C. Prestigiacomo, M.S. Osofsky, I.I. Smolyaninov, Sci. Rep. 5, 15777 (2015)
W.C. Qi, G.W. Chen, C.S. Yang, C.R. Luo, X.P. Zhao, J. Mater. Sci.: Mater. Electron. 28(13), 9237–9244 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China for Distinguished Young Scholar under Grant No. 50025207.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Chen, H., Qi, W. et al. Inhomogeneous Phase Effect of Smart Meta-Superconducting \(\hbox {MgB}_{2}\). J Low Temp Phys 191, 217–227 (2018). https://doi.org/10.1007/s10909-018-1865-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10909-018-1865-8