Abstract
We are developing metallic magnetic calorimeters for beta spectrometry with the aim of determining the shape factors of beta spectra. The beta emitter is enclosed inside the detector absorber and the absorber size is chosen such that the detection efficiency is close to 100% for the entire spectrum.
The nature of the deposit of the beta emitter is critical for precise measurement. A dependence of the shape of the spectrum on the quality of the source has been observed, supposedly due to interactions between the electrons and the deposit itself. We chose for this comparative study a nuclide with an allowed transition whose theoretical spectrum is relatively well-known, 63Ni, a pure beta emitter with an endpoint energy of 66.98 keV.
Results of measurements of 63Ni deposited with different techniques are presented and compared to each other and to the theoretical spectrum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the field of ionizing radiation metrology, the determination of nuclear and atomic data is essential. Therefore, we are developing metallic magnetic calorimeters (MMCs) [1, 2] for beta spectrometry, with the aim of determining the shape of beta spectra. The experimental determination of spectrum shapes has a great importance for beta emitters decaying via forbidden transitions because the theoretical calculation of the corresponding spectrum is complicated [3] and experimental data are rare and not precise enough. Beta spectra are quite difficult to measure precisely with conventional detectors. Indeed, with lithium drifted silicon (Si:Li) detectors, a part of the energy could be lost in the dead layer, involving possible deformation of the spectrum. With liquid scintillation technique, there is a dependence of the detection efficiency on the energy.
Metallic magnetic calorimeters with the beta source embedded in the absorber seem promising for improving the determination of shape factors. In addition to the 4π solid angle, the high stopping power of gold will allow for a detection efficiency better than 99% over the entire spectrum and a threshold at around 1% of the endpoint energy. Besides, it has been demonstrated that the linearity of MMCs can be better than 0.5% over two orders of magnitude in energy [4].
The first step of the development is to verify the potential of our detection system for the measurement of beta spectra. We chose for this validation 63Ni which has an endpoint energy of 66.98 keV and decays via an allowed transition. The theoretical spectrum is considered well-known and precise experimental spectra have already been published [5]. Our first experiments show significant differences between the spectra for different beta sources, even for the same technique of source preparation.
2 Experimental Details
All the beta sources presented here have been made from a single solution of nickel chloride, with a massic activity of 49 kBq/g, 10 μg/g of non-active nickel chloride and 0.1 mol/L of hydrochloric acid. The count rate, 5 Bq, is the same for all the absorbers.
For each of the first two sources, MD6 and MD7, 0.2 μL of the solution has been deposited with a micropipette on a gold foil and then freeze dried. We obtained two deposits with different aspects: MD6 was invisible whereas MD7 exhibited a clearly visible crystallized substance. In order to see the impact of a thicker deposit, we increased by a factor 5 the amount of non-active Ni for the same source activity of 63Ni. The crystallization of this deposit, MD8, was also visible. Although the thickness of such an inhomogeneous deposit is difficult to quantify, the mean thickness must be greater by a factor 5, compared to MD6 and MD7.
These three sources have been deposited on Au foils of 10 μm thickness. To delimit each Ni deposit and to assure the same activity, the edges of the gold foils had previously been covered with a silicon glue, a barrier to stop the flow of the liquid source. The silicon was removed after drying of the solution. Then each deposit was sandwiched with a second gold foil and the two foils were diffusion welded at 670 K.
The last deposit was obtained by electroplating. Since first tests of electroplating on gold were not conclusive, we proceeded to electroplate on copper. In this attempt, the cathode and the anode were both Cu foils and a voltage of 3.1 V (I∼100 μA) was applied during one hour. A shiny deposit was obtained on one side of the Cu foil, referred to as MD9. This Cu foil was folded in two, so as to have the deposit inside, and was embedded in a previously folded Au foil.
However, the deposit on the copper foil was clearly visible, which is not consistent with the concentration of the solution; a thickness below 1 nm was to be expected. A liquid scintillation measurement permitted to verify that radioactive nickel had indeed been deposited. The low count rate, confirmed later by MMC measurement, makes us suspect that the visible deposit is rather copper oxide than metallic nickel.
All the absorbers have an approximate size of 1 mm2×(2×10) μm.
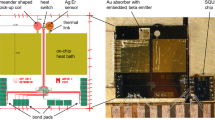
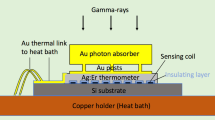
The thermometer made of Au:Er is fixed to the absorber by ultrasonic wedge bonding, thus assuring a metallic contact and a very efficient heat transfer. The detector (absorber + thermometer) is then placed on the SQUID chip, with the thermometer placed inside the SQUID sensing loop (Fig. 1).
The thermal link, which restores the thermal equilibrium, consists in a gold wire (5 μm diameter, ∼800 μm length) wedge bonded to the absorber and to the copper detector holder.
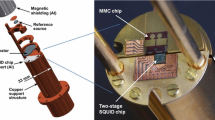
The magnetic field required to magnetize the Au:Er sensor is initially being created with a superconducting coil and then maintained by a superconducting lead ring without injection of a current from outside the cryostat.
The detector together with the readout SQUID is operated in a dilution refrigerator at a temperature below 12 mK.
For energy calibration, an external 241Am source is used. A thin gold foil (60 μm) between the source and the detector stops the α-rays and the numerous Np X-rays which would cover a large part of the beta spectrum. Only the 59.5 keV γ-rays interact in the absorber.
3 Results
Data are recorded continuously, at a sampling rate of 100 kHz, and the analysis including triggering is done offline via a Matlab code.
A band-pass filter is used to reduce noise and rapidly bring the baseline back to zero, i.e. minimize pile-up. The energy of each particle interaction is then simply obtained by the determination of the maximum of each pulse. This method implies the pulse shape to be homogeneous, which is confirmed by a systematic pulse shape analysis on additional data recorded at a sampling rate of 2.5 MHz. A dead time of 6 ms and a (software) trigger threshold at 120 eV are implemented. A rejection of pulses with a duration above threshold shorter than 0.16 ms is added to avoid noise trigger. In order to validate the spectrum obtained with this analysis, it was compared with two other types of analysis. One of them is based on a matched filter. The other one employs a delay line, which consists in subtracting, with a fixed delay, the opposite of the signal from the original signal to quickly restore the baseline. Then, a Gaussian fit is used to obtain the maximum of the pulse. The spectra obtained with the three analyses are all consistent.
The experimental spectra are compared to a theoretical spectrum, calculated by Betashape [3], which analytically computes the energy spectrum of electrons from beta disintegrations. Three corrections are taken into account: the influence of the size of the nucleus, the screening effects and the radiative effects. Another effect may have an influence on the spectrum shape: the exchange effect [6]. It corresponds to the creation of a beta electron into a bound orbital of the daughter atom, with the simultaneous ejection of a corresponding shell electron. This effect leads to a deviation of the spectrum at low energy. The exchange effect for 63Ni had been quantified by the group of Oxford [7] from 5 to 67 keV. Our threshold being lower than 5 keV, these values were fitted and then extrapolated down to 0 keV. Then, this fit was applied to correct the theoretical spectrum calculated from Betashape. The theoretical spectrum compared to the different experimental spectra in Fig. 2 takes into account the exchange effect on the entire spectrum. The theoretical spectrum was normalized with respect to the experimental data by integration between 20 an 55 keV.
(Color online) Measured spectra from different 63Ni sources compared to a theoretical spectrum. Top: freeze-dried deposits with visible crystallization (MD7 and MD8). Bottom: freeze-dried thin deposit (MD6) and electroplated deposit (MD9). The energy calibration at 59.5 keV is obtained by using an external source of 241Am
The measured spectra indicate that the thickness of the deposit has a great influence on the spectrum shape (Fig. 2). MD7 and MD8, whose spectra are considered to be consistent with each other, showed a visible crystallization which may partially explain the deviation at low energies by the creation of athermal states in the crystallized source and hence a shift towards lower energies. The spectra of MD9 and MD6, which are not consistent with those of MD7 and MD8, are in turn rather compatible with each other. In a purely metallic electroplated source, the entire beta energy should be converted to heat, but the supposed copper oxide of MD9 may play the same role as a thin salt deposit as it should be present in MD6. Unlike what one could expect, a thick deposit seems to yield a spectrum more consistent with the theory than a thin one. We have already observed this phenomenon in a previous measurement of 241Pu [8]. A 241Pu deposit thicker than the 63Ni sources gave a spectrum in good agreement with the theoretical spectrum within two third of the energy range but showed a discrepancy at low energy.
Shape factors [3] are used as a tool to quantify the discrepancy between our spectra. They have been calculated by the ratio, bin per bin, of experimental and theoretical spectrum. To compare the shape factors between each other, we normalized them as 1 at the bin corresponding to 27 keV. Then, we fitted this ratio to obtain the shape factor as a third order polynomial function. These different functions are plotted in Fig. 3 for the lower energies, where the discrepancy occurs.
The differences between our experimental spectra make it impossible to obtain a single shape factor. This makes it obvious that a type of source without any influence on the spectrum shape is yet to be found.
4 Conclusions and Perspectives
The measurements of beta spectra of different 63Ni sources have clearly demonstrated an influence of the nature of the source deposit on the spectrum shape and hence on the determination of the shape factor. To perform a precise measurement of beta spectra with metallic magnetic calorimeters, one has to assure that the measured temperature rise is strictly proportional to energy. In this regard, the thickness and the nature of the source are essential. There may be a part of the energy spent in the source for chemical or physical transformations such as changing the configuration of molecules or creating defects of the crystal lattice or metastable states. This energy would be lost for the measurement.
This observation leads us to plan two extreme measurements: with a deposit thick enough to stop beta particles of the entire energy range, and a deposit as thin as possible to be able to consider that none of the electrons interact in the source itself. Concerning a thick deposit, a mixture of the solution with a resin or epoxy is planned. To obtain a thinner deposit, two methods are envisaged: on the one hand, electroplating on gold, the technique used by the Oxford group who obtained results in good agreement with the theoretical spectrum in the energy range from 5 keV seems promising. An appropriate increase of Ni concentration and current might avoid the difficulties of the first attempts. On the other hand, we intend to implant the beta emitter into the absorber matrix. Implantation is probably the most promising source preparation technique, but more time consuming to realize due to the necessity of having access to a mass separator.
References
C. Enss et al., J. Low Temp. Phys. 121, 137–176 (2000)
A. Fleischmann, C. Enss, G. Seidel, Metallic magnetic calorimeters, Cryogenic Particle Detectors, ed. by C. Enss, Topics Appl. Phys., vol. 99 (Springer, Heidelberg, 2005), pp. 151–216
X. Mougeot et al., Calculation of beta spectra for allowed and unique forbidden transitions, in Proc. of Advances in Liquid Scintillation Counting (LSC 2010), Paris, France, 6–10 Sept. 2010. Radiocarbon (2011, to be published)
M. Rodrigues et al., J. Low Temp. Phys. 151, 1080 (2008)
L.C. Angrave et al., in Proc. of the 7th International Workshop on Low Temperature Detectors (LTD-7), ed. by S. Cooper, Munich, Germany, 27 July–2 Aug., (1997) p. 178
M.R. Harston, N.C. Pyper, Phys. Rev. A 45, 9 (1992)
L. Angrave, The physics of non-equilibrium phonons and non-equilibrium superconductivity applied to a precision measurement of the beta spectrum of 63Ni, Trinity Term 2000, Ph.D. thesis, Madgalen College, Oxford, 2000
M. Loidl et al., in Proc. of the 13th International Workshop on Low Temperature Detectors, LTD-13, 20–24 July 2009, Stanford, USA. AIP Conf. Proc., vol. 1185, p. 587 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le-Bret, C., Loidl, M., Rodrigues, M. et al. Study of the Influence of the Source Quality on the Determination of the Shape Factor of Beta Spectra. J Low Temp Phys 167, 985–990 (2012). https://doi.org/10.1007/s10909-012-0607-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10909-012-0607-6