Abstract
Insects of the family Hydrophilidae produce egg cocoons (a silk-like substance that surrounds the eggs). In several genera, the female attaches these egg cocoons to their abdomen and carries them until the larvae hatch. In this study, we examined whether the egg cocoon-carrying behavior of the water scavenger beetle, Helochares nipponicus (Coleoptera: Hydrophilidae), functions to protect the egg cocoon from physical, biological, and environmental stressors. The number of hatchlings from egg cocoons that were removed from female scavenger beetles and floated on the water surface did not differ from those of the that remained attached. However, few eggs hatched from egg cocoons submerged in water and from those placed in a dry environment. When first instar larvae of the conspecific species were put into small Petri dishes with egg cocoons still attached to the female, the number of hatchlings did not differ from those without larval cohabitation. In contrast, when the egg cocoons were removed from the females, the presence of conspecific larvae significantly reduced the number of hatchlings. First instar larvae fed on the eggs by inserting their heads into the egg cocoons. These results strongly suggest that the egg cocoon-carrying behavior of H. nipponicus functions to regulate air and water to the eggs and avoid cannibalism by conspecific larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The care of offspring by insect parents can take many forms, including egg care. Egg care can be broadly classified into “egg attendance” and “egg brooding” (Smiseth et al. 2012). As opposed to egg attendance, wherein insect parents stay with their eggs in a specific location, such as a nest, egg brooding, also referred to as egg carrying, is defined as the retention and carrying of eggs by the insect parents outside or inside their body until the eggs hatch (Smiseth et al. 2012). The primary form of external egg carrying in egg brooding has been observed in three orders, Blattodea, Coleoptera, and Hemiptera, and 175 genera of insects (Machado and Trumbo 2018). Egg brooding has also been reported in other arthropods, such as spiders (Bristowe 1976). Egg brooding is a form of care with a relatively high degree of freedom, which includes the ability to move when the environment becomes unsuitable and the parents can engage in other activities, such as foraging. It suggests that the fitness of the parents and offspring in egg brooding is higher than that in egg attendance. However, the occurrence of the egg brooding trait in insects is limited compared with egg attendance, which is widely reported in 585 taxa in 12 orders (Machado and Trumbo 2018). Understanding what selection pressures and evolutionary constraints keep egg brooding in this current state is critical to understand the evolution of parental care behavior.
Insects of the family Hydrophilidae currently comprise more than 3100 species in 181 genera worldwide (Short and Fikáček 2011; Mađarić et al. 2013). Hydrophilids produce an egg cocoon, a pouch-like object containing eggs. These egg cocoons are classified into several types based on their characteristics, such as the oviposition site and cocoon shape (Wichard et al. 2002). Larger species, such as Hydrophilus, float their egg cocoons on the surface of the water with a “mast” to supply air. Many genera, including Berosus, attach their egg cocoons to the surface of stones and water plants. In contrast, the females of several genera, including Helochares, Helobata, and Peltochares, carry their egg cocoons by attaching them to their abdomen until they hatch (Hansen 2000). Why did these genera evolve egg brooding behavior, although most species of hydrophilids have a form in which the egg cocoons remain in the oviposition site? In general, egg brooding functions to increase offspring fitness by defending eggs against predators, other egg-eating conspecifics, parasites and pathogens, desiccation, flooding, and hypoxia (Smiseth et al. 2012). However, this has not been verified in Coleoptera.
A water scavenger beetle, Helochares nipponicus (Coleoptera: Hydrophilidae), is small and approximately 4 mm in length. Females of this species carry egg cocoons by attaching them to the underside of their abdomen and retaining them until they hatch (Nakajima et al. 2020). This species inhabits wetlands and breeds from spring to summer. In this study, we observed the egg cocoon-carrying behavior of H. nipponicus in an aquarium and measured the hatchability of their egg cocoons in different physical and biological environments.
Materials and Methods
Insect
Adults of H. nipponicus were collected from April to June 2022 from a reservoir in Tsubata-machi, Kahoku-gun, Ishikawa and a puddle in Asahi-cho, Shimoniikawa-gun, Toyama. The individuals were confined without separating the sexes in plastic insect-rearing containers (100 mm in diameter and 40 mm in height) filled with water and dead leaves of the common reed, Phragmites australis (Poales: Poaceae). Females who oviposited their egg cocoon during rearing or already brooded at the time of collection were reared individually in a 30 mm-diameter Petri dish with water and common reed leaves at 25 °C in a light:dark (L:D) cycle of 16:8 in a breeding room. Afterwards, the females were placed in other insect rearing containers and reared outdoors. Some of the females used in the experiment oviposited more than one egg cocoon, and these individuals were also used in the next experiment. The eggs turned black a few days before hatching, and larvae hatched from the egg cocoon in approximately seven days at 25 °C. When the oviposition date was confirmed, the egg cocoons up to five days old were used in the experiment. If the eggs were not black, females with egg cocoons during field collection were used in the experiments. Larvae that hatched from egg cocoons used in the immersion experiment described below, were also used in the larval coexistence experiment. Larvae were reared by brood at 25 °C in a L:D cycle of 16:8 in a 30 mm-diameter Petri dish containing water and dead leaves of common reed. The larvae were fed freeze-dried bloodworms from 0 days post-hatching, and two- to five-day-old individuals were used in the experiments.
Immersion Experiment
We examined whether egg cocoon-carrying behavior is a function of protection from abiotic environmental factors. The experiment was conducted from May to July 2022. On the first day of the experiment, egg cocoons were gently removed from the females using tweezers. Eggs were counted through the cocoon under a stereomicroscope. Next, the egg cocoons were placed in a 30 mm-diameter Petri dish and then separated into three groups (floated, immersed, and dried groups) (Fig. 1). A group in which egg cocoons were not removed from the females was designated as the control group. A 20 mm-piece of dead leaf of common reed was added to the Petri dish and covered with a lid in every group. In the floated group, the egg cocoon was floated on the surface of the water in such a way that the back of the egg cocoon was in contact with the air. In the immersed group, the egg cocoon was completely immersed in water by inverting the dorso-ventral axis of the egg cocoon in the water and holding for several tens of seconds. In the dried group, water was not added to the Petri dish. The egg cocoons were observed daily for two weeks from the start of the experiment. The number of hatched larvae was counted, and the hatched larvae were removed from the Petri dishes daily.
Schematic diagram of the immersion experiment. A piece of common reed leaf and an egg cocoon were placed in the small Petri dish filled with water, and the number of hatched larvae was counted after the two-week experiment. a Control group, in which egg cocoons were attached to the females; b floated group, in which egg cocoons were floated on the surface of water; c immersed group, in which egg cocoons were submerged in water; d dried group, in which no water was added
Larval Coexistence Experiment
We determined whether the H. nipponicus female’s carrying behavior functions to avoid cannibalism from conspecific larvae. The experiment was conducted from May to July 2022. Ten first instar larvae were placed in 30 mm-diameter Petri dishes and separated into two groups (care and no-care groups) on the first day of the experiment. The experimental conditions of Petri dishes were the same as those of the immersion experiment. In the care group, the female carrying the egg cocoon was placed in the Petri dish containing larvae, and in the no-care group, only a floated egg cocoon was placed in the Petri dish. After 24 h, all larvae were removed, and the number of eggs damaged by larval feeding was counted. Subsequently, the egg cocoons continued to be reared and observed daily for two weeks. After the egg cocoons hatched, the number of hatched larvae were counted until the end of the experiment. The data obtained from the control group in the immersion experiment were used in the larval coexistence experiment.
Statistics
All analyses were performed in R (version 4.2.2; R Core Team 2020). Results were considered to be significant at P < 0.05. The number of hatched larvae in the immersion experiment and larval coexistence experiments were compared using Tukey’s honestly significant difference test (Tukey’s HSD test). The number of larvae 24 h after the start of the experiment in the larval coexistence experiment was compared by an unpaired t-test (two-tailed, equal variance).
Results
Egg Cocoons and Carrying Behavior
The H. nipponicus female attaches the egg cocoons on the ventral surface of the abdomen immediately after oviposition and carries it until the larvae hatch. Egg cocoons were attached to the back of the hind femora in several places by silky strings (Fig. 2a). Females spent most of the day and night in the water in a tank filled with dead leaves of common reed. As a result, egg cocoons were completely submerged most of the time. A layer of air was observed between the ventral surface of the female abdomen and the back of the egg cocoon being carried, the same air layer was observed in males and females that did not carry egg cocoons (Fig. 2b); we observed this in two cases of egg cocoon hatching. In these cocoons, hatching occurred underwater, during which the female remained motionless but held onto a leaf with her head above the water. Hatching occurred almost simultaneously, with almost all larvae exiting the egg cocoon 1 h after the first emergence.
Female of Helochares nipponicus and egg cocoon. Egg cocoon was attached only to the hind legs with strings (arrowheads); hence a the ventral side of the female’s abdomen and the egg cocoon are not attached. In water, b a layer of air (an arrow) was observed on the ventral surface from the head to the abdomen with and without the egg cocoon. c From the dorsal view of egg cocoon, the embryo heads were observed to be arranged in a row, and from d the ventral view, several eggs were observed to be arranged in a lying position. When the egg cocoon was split along the dorso-ventral axis, e the head of the ventral eggs was facing slightly inward. The scale bar represents 1 mm
The egg cocoon was flat and half-moon shaped. Most eggs were placed in an egg cocoon in a standing position, with the embryo head close to the dorsal side of the egg cocoon, i.e., close to the air layer of the ventral side of the female abdomen (Fig. 2c-e). From one egg cocoon, 48.1 ± 10.3 eggs (mean ± s.d., N = 53) were observed. There was no difference in the number of eggs per cocoon in the four groups of immersion experiments and in the three groups of the larval coexistence experiment (Tukey’s HSD test, P > 0.05), where the number of eggs could be counted before the start of the experiment. Egg cocoons were white in color immediately after egg-laying but turned brown during embryogenesis. Eggs in the cocoon immediately after egg-laying were milky white and turned gray as development progressed, eventually turning dark gray, and the eggs usually hatched the next day. Eggs hatched in Petri dishes at 25 °C after 6.6 ± 1.0 days (mean ± s.d., N = 19).
Immersion Experiment
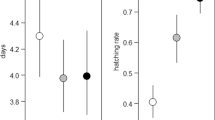
The number of hatchlings varied greatly depending on the conditions in which the egg cocoons were placed (Fig. 3a). In the control group, the egg cocoons were either half submerged or completely submerged in water but in contact with an air layer on the ventral surface of the female’s abdomen (Fig. 3b). The egg cocoons in the floated group remained suspended on the water surface during the two weeks of the experiment (Fig. 3c). Egg cocoons in the immersed group were completely submerged in water until the end of the observation, and more water mold and Vorticella were observed in the immersed group than that in the other groups (Fig. 3d). The egg cocoons in the dried group lost a considerable amount of moisture and wilted one day after the start of the experiment (Fig. 3e). In the control group, all 14 egg cocoons hatched and 47.9 ± 2.9 larvae (mean ± s.e.) emerged from each cocoon. In the floated group, 12 of 13 egg cocoons hatched and 43.4 ± 4.4 larvae emerged from each cocoon. Only 1 of 13 egg cocoons hatched in the immersed group, and 4.3 ± 4.1 larvae emerged from that cocoon. No egg cocoons hatched in the dried group. Compared with the number of hatchlings in the control group, no significant difference was observed in the floated group, but significant differences were observed between the immersed and dried groups (Tukey’s HSD test: floated group, N = 13, P = 0.79; immersed group, N = 13, P < 0.0001; dried group, N = 13, P < 0.0001).
a Differences in the number of hatchling larvae and b-e the egg cocoons on the expected hatching date in the immersion experiment. In the floated group, where egg cocoon was suspended on the water surface, most of the eggs hatched, as in the control group. In contrast, the number of hatchlings significantly decreased in the immersed and dried groups. Error bars indicate standard errors; letters (a and b) indicate significant differences based on Tukey’s HSD test. The scale bar represents 1 mm
Larval Coexistence Experiment
When first instar larvae were present with eggs, the number of hatchlings was fewer in the egg cocoons when the female did not carry the cocoon (Fig. 4a). First instar larvae placed in Petri dishes were mostly localized on common reed leaves or the walls near the water surface. In the care group, in which larvae coexisted with egg cocoons carried by the female, the females did not have a noticeable response to larval contact with egg cocoons. However, when the larvae came in contact with their bodies, they occasionally exhibited aversive behaviors such as leaning toward the larvae or walking. In addition, females carrying egg cocoons showed defensive behavior by tilting their dorsal sides in response to stimulation from tweezers. Microscopic observation of egg cocoons at 24 h after the start of the experiment revealed that 0.5 ± 0.4 eggs (mean ± s.e., N = 13) were consumed in the care group and 10.6 ± 1.4 eggs (N = 14) were consumed in the no-care group. In the no-care group, larvae clung to the egg cocoons and were observed feeding on the eggs by inserting their heads into the egg cocoons (Fig. 4b, Online Resource 1). After larvae were removed, the damaged egg turned black, and sometimes the eggs fell out. All egg cocoons of each groups hatched. At the end of the experiment, 49.5 ± 2.1 larvae (mean ± s.e., N = 13) emerged in the care group and 35.7 ± 3.0 larvae (N = 14) emerged in the no-care group. Compared with the control group, there was no significant difference in the number of hatched larvae in the care group, but a significant decrease in the no-care group (Tukey’s HSD test: care group, N = 13, P = 0.93; no-care group, N = 14, P = 0.01). Twenty-four hours after the beginning of the experiment, we counted the number of artificially introduced larvae in the care and no-care group and found that some larvae were probably predated by other individuals or starved to death. The number of larvae counted in the care group was 6.3 ± 0.7 (mean ± s.e.) and that in the no-care group was 8.0 ± 0.5. There was no significant difference between the two groups, but the survival rate after 24 h was lower in the care group (t test: t = − 1.91, df = 25, P = 0.068).
a Differences of the number of hatchling larvae and b larvae feeding on an egg cocoon in the larval coexistence experiment. The number of hatchlings in the no-care group, where the egg cocoons were removed from the females and larvae were allowed to coexist, significantly decreased compared to that of the control and care groups. The first instar larvae of the conspecific species ate the eggs inside the floating egg cocoon by breaking through the egg cocoon. Error bars indicate standard errors; letters (a and b) indicate significant differences based on Tukey’s HSD test
Discussion
In this study, we determined that the egg cocoon-carrying behavior of a water scavenger beetle H. nipponicus has at least three functions: providing air and water to the eggs and avoiding cannibalism from conspecific larvae. To the best of our knowledge, this study is the first to experimentally clarify the function of egg brooding in Coleoptera.
All Hydrophiloidea species and several species of Hydraenidae produce egg cocoons with the eggs covered by a silky substance (Hansen 2000; Wichard et al. 2002) classified seven oviposition types based on oviposition site and egg cocoon shapes. According to Hansen (2000), the evolutionary scenario is that the most ancestral type of egg cocoon is the type with a “mast”, which is a respiratory tube that can provide access to air, as egg cocoons were buried in the soil, followed by a type that adhered to the surface of plants, and then a type that floated on the water surface. The basic evolutionary pathway is assumed to have led to the derivation of egg cocoon-carrying behavior by females in three taxonomic group of the superfamily Hydrophiloidea (Helochares, Epimetopid, and Spercheid types). In Hydrophilidae, egg cocoon-carrying species have been reported in the genera Aulonochares, Helobata, Peltochares, and Radicitus, in addition to the genus Helochares, which includes H. nipponicus (Short and García 2014; Girón and Short 2021). Helochares sp. is characterized by an egg cocoon anchored to the femora by two string-like fibers (Hansen 2000). All the genera of the family Epimetopidae, namely, the genera Epimetopus (Hansen 2000), Eumetopus (Ji and Jäch 1998), Eupotemus (Fikáček et al. 2021), and Spercherus of the Spercheidae (Darilmaz and Kiyak 2011) have also been reported to hold and carry their egg cocoons under their abdomen, similar to that in Hydrophilidae. In other words, the carrying behavior is a behavioral trait that has evolved in at least nine genera of Hydrophiloidea.
Supply of Air and Water
A representative group of aquatic insect egg carriers is belostomatines of the Hemiptera subfamily Belostomatinae. In belostomatine insects, the female lays her eggs on the male’s back, and the male cares for them until they hatch. One of the characteristic care behaviors is called “surface-brooding,“ in which the eggs are positioned at the interface between air and water (Smith 1997). This behavior provides water to the egg and oxygen to the embryo for respiration. Smith (1997) discussed the following evolutionary pathway as the reason for the evolution of the surface-brooding in belostomatines: egg size increased because of interspecific competition with other predatory aquatic insects, and as a result, the larger egg could no longer obtain sufficient oxygen in the water, but constant exposure to the air would inevitably lead to desiccation. Water levels fluctuate greatly depending on the weather conditions in the wetlands inhabited by the belostomatines. To eliminate these risks and protect the eggs from drowning and desiccation, emergent-brooding evolved first, in which the parent laid eggs on emerged plants near the water’s edge and guarded it. Subsequently, back-brooding evolved, in which eggs are laid on the back of insects for surface-brooding. The requirement for adequate respiration and water in an environment with a large fluctuation in water level is also true for Hydrophilids that have evolved egg cocoon-carrying behavior. One basis for this is the “mast” found in the egg cocoons of many species of Hydrophiloidea that do not carry egg cocoons (Hansen 2000). The mast of the egg cocoon enables the supply of oxygen necessary for the development of the eggs in the egg cocoon (Angus 1973), but this mast is not found in the egg cocoon-carrying species. In our observations, the dorsal surface of the egg cocoon of H. nipponicus was in contact with a layer of air held on the ventral surface of the female’s abdomen. Anderson (1976) reported that in Helochares tristis, the dorsal surface of the egg cocoon is always surrounded by an air bubble when the female is submerged. We believe the egg cocoons of carrying species allow oxygen to be supplied directly to the egg cocoon without the mast. In addition, we observed that females spent most of their time in shallow water during egg carrying, and the ventral surface of the egg cocoon was in contact with the water. The egg carrying species of Hydrophilidae must provide a suitable abiotic environment for the embryo.
Defense Against Cannibalism
Cannibalism has been observed in many animal species with a variety of diets, including herbivores and predators (Polis 1981; Elgar and Crespi 1992; Fox 1975) found that most cannibalistic predators are fishes and insects in freshwater. Moreover, cannibalism between larvae has been reported in several species of Hydrophilidae, although it has not been investigated in egg cocoon-carrying species. For example, the number of larvae killed by cannibalism has been experimentally confirmed to increase with prolonged starvation and differences in body size in the water scavenger beetle, Sphaeridium spp. (Sowig 1997). Larvae of Dactylosternum abdominale (Koppenhöfer and Schmutterer 1993) and Enochrus quadripunctatus (Hosseinie 1995), which also belong to water scavenger beetles, are severely cannibalistic under rearing conditions. Such cannibalism between larvae has also been observed in our rearing of H. nipponicus. Schulte (1985) observed that larvae of the genus Cercyon of the hydrophilids migrate immediately after hatching before beginning to feed, which he presumed is an adaptation to avoid cannibalism between siblings. In the present study, we observed that the egg cocoons of H. nipponicus are preyed upon by the conspecific larvae and that the egg cocoons are carried by the female to avoid cannibalism by the conspecific larvae. To the best of our knowledge, larval predation on eggs of the conspecific or other species has never been reported in Hydrophilidae, but cannibalism in many insects is reported to be directed at siblings in the unprotected egg stage (Branquart et al. 1997; Via 1999; Sigsgaard et al. 2002). It should be noted that the larvae of hydrophilids that are egg predators lay their egg cocoons on substrates such as plants. Attention should be paid to Hydrophilid larvae that are egg predators, even in species with other oviposition types (species that do not carry egg cocoons).
In addition to defense against intraspecies predation, carrying behavior of H. nipponicus may possibly function as a defense against parasites and predators of different species. For example, in the golden egg bug Phyllomorpha laciniata, females have been reported to lay eggs on the bodies of conspecific females and are able to avoid egg parasitism by their motility (Carrasco and Kaitala 2009). Trichogrammatid parasitoid bees have been reported to parasitize the eggs of Hydrophilidae species that lay their egg cocoons on the surface of floating water plants (Fursov 2004; Albertoni et al. 2016). A few cases in which egg predators have been identified in Hydrophilidae have been reported, such as the water scavenger beetle, Coelostoma stultum, whose eggs are preyed upon by the grey garden slug, Deroceras laeve, by puncturing the egg cocoon (Matsushima and Haga 2021); thus, egg cocoon carrying is thought to be a defensive adaptation against parasitic and predatory attacks.
Egg brooding, or egg carrying, is a phenomenon reported in 175 insect genera (Machado and Trumbo 2018), but its function has not been fully verified. In addition to the three functions of avoiding drowning and desiccation, and defending against conspecific predators, which were experimentally verified in this study, egg brooding may have other functions, such as thermoregulation (Tourneur 2022). In our experiments, egg cocoons removed from females and immersed in water were significantly infested with water mold. Therefore, the carrying behavior of Hydrophiloidea may possibly function as a defense against microorganisms. In future studies, other functions will have to be examined to understand why the egg brooding trait of egg or egg cocoon carrying evolved only in certain taxa.
Data Availability
All data analyzed during this study are included in this published article and its supplementary information file.
References
Albertoni FF, Steiner J, Zillikens A (2016) The associated beetle fauna of Hohenbergia augusta and Vriesea friburgensis (Bromeliaceae) in southern Brazil. J Nat Hist 50:2917–2939
Anderson JME (1976) Aquatic Hydrophilidae (Coleoptera). The biology of some Australian species with descriptions of immature stages reared in the laboratory. Aust J Entomol 15:219–228
Angus RB (1973) The habitats, life histories and immature stages of Helophorus F. (Coleoptera: Hydrophilidae). Trans R Entomol Soc Lond 125:1–26
Branquart E, Hemptinne JL, Bauffe C, Benfekih L (1997) Cannibalism in Episyrphus balteatus (Dipt.: Syrphidae). Entomophaga 42:145–152
Bristowe WS (1976) The world of spiders (revised edition). Collins, London, p 304
Carrasco D, Kaitala A (2009) Egg-laying tactic in Phyllomorpha laciniata in the presence of parasitoids. Entomol Exp Appl 131:300–307
Darilmaz MC, Kiyak S (2011) A study of the family Spercheidae (Coleoptera) from Turkey. Turk J Zool 35:441–444
Elgar MA, Crespi BJ (1992) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, Oxford, p 361
Fikáček M, Matsumoto K, Perkins P, Prokin A, Sazhnev A, Litovkin S, Jäch MA (2021) The family Epimetopidae (Coleoptera: Hydrophiloidea): review of current knowledge, genus-level phylogeny, and taxonomic revision of Eupotemus Acta Entomol Mus Natl Pragae 61:1–34
Fox LR (1975) Cannibalism in natural populations. Annu Rev Ecol Syst 6:87–106
Fursov VN (2004) New data on the biology and distribution of the Lathromeroidea silvarum Nowicki, 1937 (Chalcidoidea: Trichogrammatidae)-an egg parasitoid of water beetles (Hydrophilidae and Dytiscidae). Russ Entomol J 13:165–169
Girón JC, Short AEZ (2021) The Acidocerinae (Coleoptera, Hydrophilidae): taxonomy, classification, and catalog of species. ZooKeys 1045:1–236
Hansen M (2000) Observations on the immature stages of Georissidae (Coleoptera: Hydrophiloidea), with remarks on the evolution of the hydrophiloid egg cocoon. Invertebr Syst 14:907–916
Hosseinie SO (1995) Life history, behavior and morphology of the immature stages of Enochrus quadripunctatus Herbst in the laboratory (Coleoptera: Hydrophilidae) I. Life history and behavior. J Sci Islam Repub Iran 6:195–206
Ji L, Jäch MA (1998) Epimetopidae: Synopsis of the genus Eumetopus Balfour-Browne (Coleoptera). In: Jäch MA, Ji L (eds) Water Beetles of China, vol 2. Zoologisch-Botanische Gesellschaft in Österreich and Wiener Coleopterologenverein, Wien, pp 195–205
Koppenhöfer AM, Schmutterer H (1993) Dactylosternum abdominale (F.)(Coleoptera: Hydrophilidae): a predator of the banana weevil. Biocontrol Sci Technol 3:141–147
Machado G, Trumbo ST (2018) Parental care. In: Córdoba-Aguilar A, González-Tokman D, González-Santoyo I (eds) Insect behavior: from mechanism to ecological and evolutionary consequences. Oxford University Press, Oxford, pp 203–218
Mađarić BB, Stanković VM, Čorak L, Ugarković Đ, Komarek A (2013) Contributions to molecular systematics of water scavenger beetles (Hydrophilidae, Coleoptera). J Zool Syst Evol Res 51:165–171
Matsushima R, Haga T (2021) Predation on the egg of Coelostoma stultum (Coleoptera: Hydrophilidae) by the alien species Deroceras laeve (Mollusca: Gastropoda: Agriolimacidae). Molluscan Res 41:254–261
Nakajima J, Hayashi M, Ishida K, Kitano T, Yoshitomi H (2020) Aquatic Coleoptera and Hemiptera of Japan. Bun-ichi Sogo Shuppan, Tokyo, p 351
Polis GA (1981) The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst 12:225–251
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org. Accessed 18 Jan 2023
Schulte F (1985) Eidonomie, Ethökologie und Larvalsystematik dungbewohnender Cercyon Species (Coleoptera: Hydrophilidae). Entomol Gen 11:47–55
Short AEZ, Fikáček M (2011) World catalogue of the Hydrophiloidea (Coleoptera): additions and corrections II (2006–2010). Acta Entomol Mus Natl Pragae 51:83–122
Short AEZ, García M (2014) A new genus of egg case-carrying water scavenger beetle from the Guiana Shield (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 3835:251–262
Sigsgaard L, Greenstone MH, Duffield SJ (2002) Egg cannibalism in Helicoverpa armigera on sorghum and pigeonpea. BioControl 47:151–165
Smiseth PT, Kölliker M, Royle NJ (2012) What is parental care? In: Royle NJ, Smiseth PT, Kölliker M (eds) The evolution of parental care. Oxford University Press, Oxford, pp 1–17
Smith RL (1997) Evolution of paternal care in the giant water bugs (Heteroptera: Belostomatidae). In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, pp 116–149
Sowig P (1997) Predation among Sphaeridium larvae: the role of starvation and size differences (Coleoptera Hydrophilidae). Ethol Ecol Evol 9:241–251
Tourneur JC, Cole C, Vickruck J, Dupont S, Meunier J (2022) Pre-and post-oviposition behavioural strategies to protect eggs against extreme winter cold in an insect with maternal care. Peer Commun J 2:e21. https://doi.org/10.24072/pcjournal.104
Via S (1999) Cannibalism facilitates the use of a novel environment in the flour beetle, Tribolium castaneum Heredity 82:267–275
Wichard W, Arens W, Eisenbeis G (2002) Order: Coleoptera-Beetles. In: Wichard W, Arens W, Eisenbeis G (eds) Biological atlas of aquatic insects. Apollo Books, Stenstrup, pp 136–181
Acknowledgements
We would like to thank Mr. M. Kasai (Ishikawa Prefectural University) for his assistance in the laboratory experiments.
Funding
This research was supported by JSPS KAKENHI Grant Number 20K06097.
Author information
Authors and Affiliations
Contributions
MH conceived the idea and designed the study. YN performed the experiment and analyzed the data. Both authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishijima, Y., Hironaka, M. Egg Cocoon-Carrying Behavior in Female Water Scavenger Beetle, Helochares nipponicus (Coleoptera: Hydrophilidae), Functions to Increase Offspring Fitness and Survivability. J Insect Behav 36, 133–141 (2023). https://doi.org/10.1007/s10905-023-09828-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-023-09828-5