Abstract
Male and female Spathius agrili Yang were tested for attraction to the synthetic male pheromone. Lures consisting of a 3-component pheromone blend were placed in the center of a white filter paper target used to activate upwind flight in the wind tunnel. When virgin males and females were tested for attraction, both sexes were attracted to the lure prior to mating. However, only males were attracted to the pheromone lures after mating. In another experiment, of females that flew to the lure as virgins, half were subsequently mated and the other half were not, and mated females were no longer attracted. Then both mated and virgin females were provided with host material (emerald ash borer larvae in sticks of ash) to determine if oviposition affected attraction. They were supplied with fresh hosts ad libidum and subsequently tested for attraction for 55 days, and results showed that oviposition did not affect attraction to the pheromone. The key factor in attraction to the pheromone was mating. Because this pheromone is released by one sex and is attractive to both sexes for the purpose of mating, it qualifies as an “aggregation-sex pheromone”.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) is an invasive species of wood boring beetle from Asia, that seriously threatens north American ash trees (Kovacs et al. 2010). Introduced and discovered near Detroit, MI and Windsor, Ontario in 2002, the beetle has spread relatively unchecked over the last decade, and so far has been found in 18 states in the US, causing significant economic and ecological damage (Haack et al. 2002; Poland and McCullough 2006; Kovacs et al. 2010). The EAB larvae feed beneath the bark of healthy ash trees, creating numerous galleries that eventually girdle and kill the trees. North American ash trees have little in the way of adaptations for host plant resistance to protect against EAB attack. Therefore, classical biological control efforts have been made to reduce the enormous impact of EAB on the native ash population. This has involved three parasitoid species from Asia (Bauer et al. 2008). All three species were recovered from release sites more than a year after their release, indicating establishment (Bauer et al. 2011; Gould et al. 2011; Hooie et al. 2015). One of these species, Oobius agrili Zhang & Huang (Hymenoptera: Encyrtidae), is an egg parasitoid (Liu et al. 2007). The remaining two species, Spathius agrili Yang (Hymenoptera: Braconidae) and Tetrastichus planipennisi Yang (Hymenoptera: Eulophidae), both attack EAB during its late larval stages (Liu et al. 2007; Ulyshen et al. 2010). There is evidence that both T. planipennisi and S. agrili could be effective biological control agents, although the range of S. agrili would likely be limited to regions south of the 40th parallel (Ulyshen et al. 2010; Duan et al. 2011; Hooie et al. 2015). More cold-tolerant parasitoids such as Spathius galinae Belokobylskij & Strazanac are being considered for release in more northerly locations (Hooie et al. 2015).
The first record of a parasitoid attacking EAB was that of S. agrili in China (Yang et al. 2005). Adults locate their host habitat through attraction to ash tree volatiles. Subsequently, S. agrili females walk on the bark and locate the EAB larvae feeding beneath by detecting vibrations from their feeding and movement (Wang et al. 2010). Females of S. agrili oviposit through the ash bark onto late instar EAB larvae, which are paralyzed in the process, rendering them less likely to be super-parasitized subsequently (Wang et al. 2010). S. agrili is a gregarious ectoparasitoid, and a single clutch typically contains 1–10 eggs, but as many as 20 (Yang et al. 2010). Fertilized eggs become females, and unfertilized eggs become males. They go through five instars on their host and then spin cocoons inside the gallery where they pupate. Emerging adults locate one another for mating by a male-produced pheromone that is attractive to both sexes (Cossé et al. 2012). This life cycle may be repeated up to five times per year.
A classical biological control program was initiated with S. agrili releases in the U.S. in 2007 (Bauer et al. 2008). Assessing establishment involves the destructive and labor intensive process of felling ash trees and either rearing out insect specimens from logs, or peeling bark to look for parasitized larvae. Another approach is the use of yellow soap pan traps which capture numerous non-target species, including other members of the genus Spathius, which then must be identified. With the discovery of an attractive pheromone, there is the possibility of developing a species-specific pheromone-baited trap to evaluate the presence and establishment of S. agrili in areas where it has been released (Cooperband et al. 2013). However, little is known about the physiological conditions required for attraction to this pheromone. This study examines the physiological state of S. agrili males and females required for attraction to the male-produced pheromone.
Materials and Methods
Insects
Rearing of S. agrili occurred at the mass-rearing facility in the Brighton laboratory (USDA-APHIS-PPQ, Brighton, MI), inside walk-in environmental chambers with 16 L:8D photoperiod, at 26.5 ± 1 °C and 22.5 ± 1 °C, respectively, full spectrum lighting, and relative humidity of 70 ± 5 %. For a complete description of rearing conditions, see Cooperband et al. (2013). Pupae were placed in individual containers for emergence and subsequently separated into containers of only males or females, and virgin adults were shipped to the Otis Laboratory (USDA-APHIS-PPQ-CPHST, Buzzards Bay, MA) where behavioral bioassays were conducted in an insect containment facility. There, males and females were stored in separate environmental chambers at 20–25 °C, 75 % RH with 16 L:8D light regime. Upon arrival at the Otis laboratory, the adult wasps were approximately 5 days old.
Wind Tunnel Bioassays
A push-pull wind tunnel (120 × 91 × 75 cm) was constructed for use inside a walk-in environmental chamber to evaluate attraction (see Cooperband et al. 2012, 2013). Air was cleaned of odors as it passed through carbon filters upon entering and leaving the wind tunnel. White paper discs (5 cm diam.) with 15 holes punched in them were used as landing targets. Red rubber septa (11 mm, Wheaton, Millville, NJ) pheromone lures were made in the USDA ARS laboratory (Peoria, IL), consisting of 1 mg (Z)-10-heptadecen-2-one, 450 μg racemic (E)-11-tetradecen-4-olide, and 100 μg dodecanal (Cossé et al. 2012). A lure was placed in the center of a paper disc, and the holes around it allowed the air to flow around the lure creating a broad turbulent plume that was carried downwind to the release stage at a wind speed of 30 cm/s. After initial data collection was complete, twelve replicates of each of virgin and mated males were added to increase the overall number of male replicates. For those additional males, pheromone was emitted from a piezo-electric sprayer adjusted to a release rate similar to the septa (Cossé et al. 2012; El-Sayed et al. 1999). A solution of the 3-component pheromone blend (same ratios as for rubber septa) in hexane was fed (10 μl/min) by a motor-driven (CMA/Microdialysis, N. Chelmsford, MA) syringe into a glass capillary surrounded by the filter paper landing disk. The frequency of flight by males to the sprayer-delivered pheromone was nearly the same as that using the rubber septa (50.0 and 48.1 %, respectively), so data from the two delivery methods were combined. Insects were placed individually on the release stage and allowed 3 min to respond. This was repeated three times for each flight test. Insects that flew upwind in the plume were considered to have responded, whereas insects that remained on the stage, or flew to the ceiling, wall, or floor of the wind tunnel were considered as non-responders. Males and females were tested for attraction when they were between 5 and 30 day old, but on average 12 days old. This was considered a reasonable age due to the relatively long lifespan of this species (Yang et al. 2010; Cooperband et al. 2013).
Mating
To evaluate whether mating status had an effect on attraction to the pheromone lure, males and females were either virgin or mated. To ensure mating took place, male and female wasps were paired and placed in a 1.5 ml Eppendorf tube or a 5 cm diam. petri dish and watched until mating was observed. Wasps were tested in the wind tunnel first as virgins to ensure they were responsive to the pheromone. After that, half of the wasps were mated, and then all wasps were tested again in the wind tunnel.
Effects of Oviposition on Attraction to Pheromone
We tested the effect of oviposition on attraction to the pheromone, and whether females will regain attraction to the pheromone after oviposition. Ten females were split into two groups, five that would be mated and five that would remain virgins. They were all tested as virgins to verify that they would fly to the pheromone. Then half of them were observed mating as described above. They were all tested again in the wind tunnel to evaluate changes in attraction to the pheromone. Then each female was placed individually in a plastic jar (1.9 L PVC Pinch Grip-It, US Plastic Corp., Lima, OH). Each jar contained a drop of honey and a young shoot of fresh green ash, Fraxinus pennsylvanica (collected in Livingston, Washtenaw, or Oakland County in Michigan, and cut to approx. 18 cm long and 3–4 cm diam.), artificially infested with two host EAB larvae for oviposition. In each ash stick, two larval chambers were formed by peeling back 3 cm of the bark and boring out a channel in the exposed wood. A late instar EAB larva was placed in each chamber and covered with the bark flap which was held in place by a rubber band. This ensured that each female always had appropriate hosts available.
Several days per week, the EAB larvae were inspected, and any S. agrili eggs or larvae were counted. EAB larvae that were dead, paralyzed, or parasitized were replaced with fresh EAB larvae, and ash sticks were replaced as needed. The S. agrili females were tested in the wind tunnel to evaluate flight response to the pheromone. Mated and virgin females with unlimited host material for oviposition were tested several days per week for attraction to the pheromone in the wind tunnel until 55 days had elapsed or until they died.
Statistical Analysis
Statistical analysis was conducted to test the null hypothesis that mated and virgin wasps responded at equal frequencies using the Students T-test at α = 0.05 (Microsoft Office Excel 2007 SP2).
Results
Mating
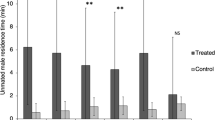
Of 56 virgin females, 43 (76.8 %) were activated to fly upwind in the pheromone plume, and 37 (66.1 %) resulted in a successful landing on the target. Conversely, of 42 females that had mated, only two flew upwind and one landed on the target (4.8 and 2.4 %, respectively). Although virgin females were strongly attracted to the pheromone lure, females were no longer attracted to the lure after mating (Student’s T test, P < 0.001). In contrast, of 118 virgin males that were tested, 57 (48.3 %) flew upwind in the pheromone plume and 24 (20.3 %) landed at the source, and of the 35 mated males tested, 17 (48.6 %) flew upwind and 11 (31.4 %) landed at the source (Table 1). There were no significant differences in flight and landing frequencies between virgin and mated males (Student’s T test, P > 0.05). Therefore, female attraction to the pheromone was lost after mating, whereas male attraction to the pheromone was not significantly affected by mating status.
Oviposition
The vibrations produced by the chewing of EAB larvae in the ash sticks stimulated all S. agrili females to oviposit (Wang et al. 2010). There was no difference between mated and virgin wasps in their survival, number of days they were tested, number of days eggs were present, and total number of eggs. However, virgin wasps flew upwind and landed on the target significantly more than mated wasps, whose attraction to the pheromone ceased after mating (Table 2). In the 50 day of oviposition, mated S. agrili females did not regain attraction to pheromone. Virgin and mated females seemed to have similar life expectancies and oviposited at approximately the same rate. Due to the short supply of EAB larvae, the number of replicates was small. Females also died at different ages, making it difficult to see patterns in behavior over time. However, virgin females oviposited and flew to the pheromone until death.
Discussion
We demonstrate in this study attraction by both sexes to a male-produced pheromone, and a shift in physiological state and behavior which results in a loss of attraction after mating only by females. Because this pheromone is attractive at a distance to both males and females, it is likely used as an “aggregation-sex pheromone” (Cardé 2014), whereby males both produce the pheromone and are attracted to it, and thereby form an aggregation, swarm, or lek, and virgin females are attracted to the pheromone for the purpose of locating a mate. This recently described classification of aggregation-sex pheromones is applicable to a pheromone in another hymenopteran species, Sirex noctilio (Hymenoptera: Siricidae) that functions in a similar manner (Cooperband et al. 2012). Aside from the loss of attraction to the pheromone, we found that change in mating status did not affect other aspects of the female reproductive biology such as fecundity, rate of egg production, and longevity. In preliminary studies using SPME fibers, pheromone was collected in abundance from both mated and virgin males (Cossé and Zilkowski, unpublished data), indicating that in addition to continued attraction after mating, mated males also continued to produce the pheromone, a sign of active mate seeking. This suggests that males of this species likely mate multiple times. Interestingly, Cossé et al. (2012) reported that groups of male S. agrili produced less pheromone than individual males. A possible advantage of male-male attraction is that males may take advantage of pheromone produced by other males and allot fewer resources to pheromone production themselves. In addition to being attracted to the pheromone of other males, males may have a feedback mechanism to reduce their own pheromone production in the presence of other males.
The study of sex pheromones in non-social Hymenoptera has been expanding over the past half century since the first discovery of pheromones in moths in 1959 (Butenandt et al. 1959; reviewed by Quicke 1997; Ayasse et al. 2001). Most commonly, sex pheromones are produced by females to attract males (Vinson 1972; Jewett et al. 1976; Weseloh 1976; Swedenborg and Jones 1992; Bergström et al. 1995; Sullivan 2002; van Beek et al. 2005). In wasps, a few examples exist of males that produce sex pheromones to attract females (Gonzalez et al. 1985; Consoli et al. 2002; Ruther et al. 2007; Cooperband et al. 2012; Cossé et al. 2012). There have been reports of wasp species of which both sexes produce volatiles attractive to one or both sexes (Swedenborg and Jones 1992; Onagbola and Fadamiro 2011). However, only one species of Hymenoptera, the wheat stem sawfly, is currently known where both sexes produce and are attracted to the same pheromone (Cossé et al. 2002). There is little doubt that as this field of research continues to expand, more examples of each strategy will become known.
References
Ayasse M, Paxton RJ, Tengö J (2001) Mating behavior and chemical communication in the order hymenoptera. Annu Rev Entomol 46:31–78
Bauer LS, Liu H, Miller D, Gould J (2008) Developing a classical biological control program for Agrilus planipennis (Coleoptera: Buprestidae), an invasive ash pest in North America. News Mich Entomol Soc 53:38–39
Bauer L, Gould J, Duan J, Hansen J, Cossé A, Miller D, Abell K, Van Driesche R, Lelito J, Poland T (2011) Sampling methods for recovery of exotic emerald ash borer parasitoids after environmental release. In: Proceedings of the 22nd USDA Interagency Research Forum on Invasive Species, pp. 2–4
Bergström G, Wassgren A-B, Adnerbrant O, Fägerhag J, Edlund H, Hedenström E, Högberg H-E, Geri C, Auger MA, Varama M et al (1995) Sex pheromone of the pine sawfly Diprion pini (Hymenoptera: Diprionidae): chemical identification, synthesis and biological activity. Experientia 51:370–380
Butenandt A, Beckmann R, Stamm D, Hecker E (1959) Über den sexual-lockstoff des seidenspinners Bombyx mori. Z Naturforsch 14b:283
Cardé RT (2014) Defining attraction and aggregation pheromones: teleological versus functional perspectives. J Chem Ecol 40:519–520
Consoli FL, Williams HJ, Vinson SB, Matthews RW, Cooperband MF (2002) trans-Bergamotenes - male pheromone of the ectoparasitoid Melittobia digitata. J Chem Ecol 28:1675–1689
Cooperband MF, Böröczky K, Hartness A, Jones TH, Zylstra KE, Tumlinson JH, Mastro VC (2012) Male-produced pheromone in the European woodwasp, Sirex noctilio (Hymenoptera: Siricidae). J Chem Ecol 38:52–62
Cooperband MF, Hartness A, Lelito JP, Cossé AA (2013) Landing surface color preferences of Spathius agrili (Hymenoptera: Braconidae), a parasitoid of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). J Insect Behav 26:721–729
Cossé AA, Bartelt RJ, Weaver DK, Zilkowski BW (2002) Pheromone components of the wheat stem sawfly: identification, electrophysiology, and field bioassay. J Chem Ecol 28:407–423
Cossé AA, Petroski RJ, Zilkowski BW, Vermillion K, Lelito JP, Cooperband MF, Gould JR (2012) Male-produced pheromone of Spathius agrili, a parasitoid introduced for the biological control of the invasive emerald ash borer, Agrilus planipennis. J Chem Ecol 38:389–399
Duan JJ, Oppel CB, Ulyshen MD, Bauer LS, Lelito J (2011) Biology and life history of Tetrastichus planipennisi (Hymenoptera: Eulophidae), a larval endoparasitoid of the emerald ash borer (Coleoptera: Buprestidae). Fla Entomol 94:933–940
El-Sayed AM, Gödde J, Arn H (1999) Sprayer for quantitative application of odor stimuli. Environ Entomol 28:947–953
Gonzalez JM, Matthews RW, Matthews JR (1985) A sex pheromone in males of Melittobia australica and M. femorata (Hymenoptera: Eulophidae). Fla Entomol 68:279–286
Gould J, Bauer L, Duan J, Fraser I, Hansen J, Ulyshen M, Lelito J (2011) Release and recovery of parasitoids of the emerald ash borer (EAB), Agrilus planipennis in MI, OH, and MD. In: Proceedings of the 22nd USDA Interagency Research Forum on Invasive Species, pp. 24–25
Haack RA, Jendeck E, Liu H, Marchant KR, Petrice TR, Poland TM, Ye H (2002) The emerald ash borer: a new exotic pest in North America. News Mich Entomol Soc 47:1–5
Hooie NA, Wiggins GJ, Lambdin PL, Grant JF, Powell SD, Lelito JP (2015) Native parasitoids and recovery of Spathius agrili from areas of release against emerald ash borer in eastern Tennessee, USA. Biocontrol Sci Tech 25:345–351
Jewett D, Matsumura F, Coppel HC (1976) Sex pheromone specificity in the pine sawflies: interchange of acid moieties in an ester. Science 192:51–53
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578
Liu H, Bauer LS, Miller DL, Zhao T, Gao R, Song L, Luan Q, Jin R, Gao C (2007) Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae) in China. Biol Control 42:61–71
Onagbola EO, Fadamiro H (2011) Response of pteromalus cerealellae to conspecific odor: evidence for female- and male-produced pheromones? J Stored Prod Res 47:393–398
Poland TM, McCullough DG (2006) Emerald ash borer: invastion of the urban forest and the threat to North America’s ash resource. J For 104:118–124
Quicke DLJ (1997) Parasitic wasps. Chapman & Hall, London, UK
Ruther J, Stahl LM, Steiner S, Garbe LA, Tolasch T (2007) A male sex pheromone in a parasitic wasp and control of the behavioral response by the female’s mating status. J Exp Biol 210:2163–2169
Sullivan B (2002) Evidence for a sex pheromone in bark beetle parasitoid Roptrocerus xylophagorum. J Chem Ecol 28:1045–1063
Swedenborg PD, Jones RL (1992) Multicomponent sex pheromone in Macrocentrus grandii Goidanich (Hymenoptera: Braconidae). J Chem Ecol 18:1901–1912
Ulyshen MD, Duan JJ, Bauer LS (2010) Interactions between Spathius agrili (Hymenoptera: Braconidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae), larval parasitoids of Agrilus planipennis (Coleoptera: Buprestidae). Biol Control 52:188–193
van Beek TA, Silva IMMS, Posthumus MA, Melo R (2005) Partial elucidation of Trichogramma putative sex pheromone at trace levels by solid-phase microextraction and gas chromatography–mass spectrometry studies. J Chromatogr A 1067:311–321
Vinson SB (1972) Courtship behavior and evidence for a sex pheromone in the parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Environ Entomol 1:409–414
Wang X-Y, Yang Z-Q, Gould JR, Wu H, Ma J-H (2010) Host-seeking behavior and parasitism by Spathius agrili Yang (Hymenoptera: Braconidae), a parasitoid of the emerald ash borer. Biol Control 52:24–29
Weseloh RM (1976) Dufour’s gland: source of sex pheromone in a hymenopterous parasitoid. Science 20:695–697
Yang Z-Q, Strazanac JS, Marsh PM, Van Achterberg C, Choi W-Y (2005) First recorded parasitoid from China of Agrilus planipennis: a new species of Spathius (Hymenoptera: Braconidae: Doryctinae). Ann Entomol Soc Am 98:636–642
Yang Z-Q, Wang X-Y, Gould JR, Reardon RC, Zhang Y-N, Liu G-J, Liu E-S (2010) Biology and behavior of Spathius agrili, a parasitoid of the emerald ash borer, Agrilus planipennis, in China. J Insect Sci 10:1–13
Acknowledgments
We thank Juli Gould and Tracy Ayer for their assistance and with S. agrili. We also extend thanks to David Lance and Russ Bullock for their comments on the manuscript. The parasitoids were produced and supplied from the United States Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS), Plant Protection and Quarantine (PPQ) EAB parasitoid rearing facility in Brighton, MI. Mention of a commercial product does not constitute an endorsement or recommendation for its use by USDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooperband, M.F., Hartness, A., Zilkowski, B. et al. Attraction of Spathius Agrili Yang (Hymenoptera: Eulophidae) to Male-Produced “Aggregation-sex Pheromone”: Differences Between the Sexes and Mating Status. J Insect Behav 28, 167–174 (2015). https://doi.org/10.1007/s10905-015-9492-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-015-9492-6