Abstract
In this study, a low-cost thermosetting unsaturated polyester resin (UPR) reinforced with ceramic wastes was produced and employed as a substrate, which was then coated with a hydrophobic solution comprising nano/micro scale ZnO particles. Ultra-water-repellent composite substrate surfaces were produced by a two-step process. Firstly, the composite surfaces were abraded with 600 SiC paper in order to create rough surface. In order to lower their surface energy and create unique surface topography, the textured composite substrate surfaces were next covered with a single or double layer of hydrophobic solution containing nano/micro ZnO particles. Contact angle measurements, surface free energy calculations, field emission scanning electron microscopy (FESEM), energy-dispersive X-ray spectrum (EDS), and optical profilometer were all used in the surface characterisation process. The highest contact angle was obtained as 157.8° when a double layer of micro ZnO-containing hydrophobic coating was applied. Surfaces coated with nano ZnO particles did not have a self-cleaning effect, whereas surfaces coated with micro ZnO additions had both superhydrophobic and self-cleaning properties. The hydrophobic coating, which contains ZnO particles of various sizes, also plays a vital role for the contact angle and specific surface energy, highlighting how crucial it is to acquire and create the right texture and surface chemistry. The synergistic effect of the processes on superhydrophobic and self-cleaning properties has been verified and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the most widely used matrix materials in polymer composite materials is unsaturated polyester resin (UPR). Cured unmodified UPR typically has poor strength and elongation at break and is prone to stress cracking and brittle fracture [1]. To get the needed qualities, ceramic reinforcement is added, or a second polymeric phase is included. Adding a ceramic phase to an unsaturated polyester resin is a particularly appealing technique to achieve desired features. In theory, including these materials into the thermoset UPR matrix can enhance physico-mechanical qualities. Therefore, polyester matrix composites are widely employed in high-performance components in the construction, sanitaryware, biomedical, marine, aviation, and automotive industries [2,3,4].
Ceramic reinforced polymer matrix composites have recently risen to prominence in sanitaryware due to advantages over ceramics such as higher impact resistance, non-sharp-edged fracture morphology, easier to achieve functional properties (anti-bacterial, phosphorescent, easy cleaning, etc.), and the ability to be produced at much lower temperatures (exothermic reaction), yielding thin-section, light, and aesthetic products [4]. However, employing reinforcement has the drawback of making ceramic reinforced polymer matrix composite materials hydrophilic which in turn causes adverse effects such as dirt retaining, easy staining, etc.
Imparting hydrophobicity to the surfaces looks to be a solution. Many research methods have been used to prepare superhydrophobic surfaces [5,6,7,8,9,10,11,12,13,14,15,16]. High water contact angles (> 150°) and low sliding angles (< 10°) are characteristics of superhydrophobic and self-cleaning surfaces [11]. Due to the low adhesion to the surface caused by the micro- and nanoscopic surface structures (hierarchical structures), this wetting behaviour is also known as the "Lotus effect," which results in the elimination of pollution particles by the rolling water droplets. The relationship between increased surface roughness caused by hierarchical structures and increased contact angle has been revealed. However, due to the hydrophobic nature of UPR, coating of this surfaces with hydrophobic solution is still a challenge. There are a few studies in the literature to obtain superhydrophobicity on polyester matrix composites [17]. Surface modification of the surface to be coated is critical when applying a hydrophobic coating on a hydrophobic polyester surface. UV irradiation, plasma pre-treatment, or chemical hydrolysis treatments are the methods that have been used to improve coating performance by increasing roughness, specific surface area, and wettability [18, 19].
In order to create superhydrophobic surfaces, research on the design of hierarchical surface structures has mainly focused on the employment of a wide variety of material combinations and coating strategies. The hydrophobic property can be produced by covering the surface with a low surface energy polymer solution, but the self-cleaning feature may not be accomplished by such methods [20]. Some studies indicate that mathematical models based on roughness structures induced on mildly hydrophobic surfaces have been constructed and optimized, leading to superhydrophobicity [21]. The simulations showed that the optimal surface geometries for self-cleaning qualities are double (or multiple) roughness structures or slender pillars. After a theoretical analysis, some researchers concluded that in order to produce roughness-induced superhydrophobicity, the asperities needed to have a large surface area, be free of sharp edges, be densely packed, and be smaller than a water droplet [22, 23]. Li and co-workers [17] used plasma based processes to improve hydrophobicity of polyester resin based composites with the roughness of the resulting surfaces that was within the range of 20–400 nm. They tested both CF4-plasma enhanced chemical vapour deposition and oxygen plasma techniques. The CF4-PECVD studies demonstrated that it can significantly increase the hydrophobicity of unsaturated polyester resins that are not filled. However, when the amount of inorganic filler added to composites increased, the rise in hydrophobicity became less noticeable, and the effect of the CF4-PECVD treatment was essentially negligible when the filler amount was 70%. This was attributable to the fluorinated film's failure to develop in the region of the inorganic filler. The contact angle for 70% marble filled composite was 155°, when oxygen plasma and OTS self-assembled monolayer synthesis were combined.
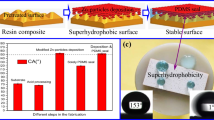
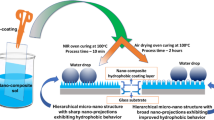
Another way to develop superhydrophobic surfaces is to fabricate composite surfaces by biomimicry of lotus leaf structure (adjustment of surface chemistry with wax crystals and a combination of micro/nano roughness). Composite surfaces are of particular interest since the addition of nano/micro particles provides roughness and the hydrophobic solution reduces surface free energy and hence wettability. Therefore, in this work composite structure consist of a polymer hydrophobic solution containing nanoscale and microscale zinc oxide (ZnO) particles was applied to the textured composite surface. ZnO particles are of particular interest due to their proven hydrophobic, antimicrobial and antifouling properties in different applications due to their hierarchical roughness success [16, 24,25,26]. The hydrophobicity of a rough surface can be increased by increasing the ratio of the air/water interface. In this case, micro/nano additives and air composite rough surface structure can also greatly increase the hydrophobicity of the films. Accordingly, both low surface free energy and high surface roughness contribute to superhydrophobicity and self-clean property. For this reason, a commercial polymeric composition (ECC-4000) consisting of 10% fluoropolymer, 60% alkoxysilane, and 30% ethanol was used as a matrix solution. This is modified with micro and nano scale zinc oxide particles to provide roughness and chemistry. Through this treatment, composite substrate surface has become superhydrophobic. The UPR surface was roughened before coating since the hydrophobic nature of the polyester surface means that the coating solution will not cling to it well. The water repellency behaviour of the treated composite substrates is discussed with reference to the surface texture depending on the size (micro and nano) of the ZnO powders and the coating thickness.
2 Experimental Procedures
2.1 Materials
In the study, unsaturated isophthalic polyester casting resin (Polipol 383T) from the Poliya firm was used as a matrix component. Methyl Ethyl Ketone Peroxide (MEKP) was used as hardener and Co-Octoate (Poliya Company) was used as accelerator. As a filler phase, scrap ceramic sanitaryware was utilised (Fig. 1). The Fritsch Vibrating Cup Mill Pulverisette 9 was used to grind the ceramic waste materials for two minutes at 900 rpm. The Fritsch Vibratory Sieve Shaker Analysette 3 Spartan was used to dry sieve the ground waste for 5 min, separating it into particles smaller than 90 µm.
Coating solution consist of as the matrix (polymer solution) to be modified with zinc oxide particles, a commercial polymeric composition (ECC-4000) consisting of 10% fluoropolymer, 60% alkoxysilane, and 30% ethanol was utilized. Micro scale ZnO powder (99.99%, d50: 2.25; Akcoat, Türkiye) and nano size ZnO powder (99.99%, d50: 20 nm; EgeNanotek, Türkiye) was used in this study. SEM-SE images of the ZnO powders were seen in Fig. 2. As can be seen from the SEM images, micro ZnO powder are in elongated and nano powder is in nearly spherical and needle like shape.
2.2 Production of UPR Based Composites and Specifications
Firstly, the accelerator (Cobalt octoate) was added into the UPR resin and mixed 5 min. After that ceramic powders were added into polyester resin. In the production of composite, polyester resin and ceramic powders were mixed gradually for 15 min at 500–1000–1500 rpm using a mixer and vacuum process was applied for 5 min after mixing. The composite was then combined with hardener (MEKP) before being cast into moulds and then again mixed. The casting was done in moulds using a mould release agent. After the samples coming out of the mould were kept at room temperature for 1 day, they were subjected to heat treatment in an oven at 70 °C for 2 h for curing. The specifications of the UPR and UPR based composites are given in Table 1.
2.3 Surface Preparation
The desired surface roughness was produced by grinding, this will also improve the adhesion of the coatings to the composite surfaces and contribute to the wetting behaviour as it creates roughness. For this purpose, SiC polishing discs with a roughness degree of 600 were used.
2.4 Preparation of the Coating Solution and Coating
A commercial polymeric solution (ECC-4000) consisting of 10% fluoropolymer, 60% alkoxysilane, and 30% ethanol was utilized as a matrix. Nanoscale and microscale zinc oxide (ZnO) particles were added (8 wt%) to the hydrophobic solution for an expected effect of lowering the surface energy. To make a homogenous mixture, it was mixed for 30 min in a magnetic stirrer at 300 rpm. After that, an ultrasonic homogenizer was used for 5 min. The composite surfaces were coated with ZnO modified hydrophobic solution. The substrates were heated at 120 °C for 30 min before spray coating. The modified hydrophobic solution (with zinc oxide particles) was applied onto 3 cm × 3 cm neat polyester and composite samples. To acquire reliable findings, five samples were coated. Weight difference and SEM-BSE images were used to determine the thickness of the coatings [16]. After coating, the samples were cured for 24 h at room temperature.
2.5 Surface Characterization Techniques
Using the optical microscope and scanning electron microscope (SEM, ZEISS Supra 40VP), the surface morphology of the ground and coated surfaces was investigated. The thicknesses of the coatings were determined by using SEM-BSE imaging mode. SEM–EDX analysis was carried out to confirm the homogeneous distribution of the ZnO particles in the hydrophobic solution and the sample surfaces. An optical profilometer (Fimmetrics, Profilm 3D model) was used to measure surface properties after the substrate surfaces had been ground and coating. Topographic scans were processed in the same way for each experiment using the Profilm3D program. Using 3 µl drops of water and diiodomethane as liquids, the drop shape analyzer (Kruss, DSA-25) was utilized to obtain contact angle and surface free energy (SFE) values. Young's equation and a two-component model proposed by Fowkes [27] and Owens, Wendt, Rabel, and Kaelble (OWRK approach) [28, 29] were used to calculate SFE. In our prior paper, we described the calculations in great detail [16].
3 Results and Discussion
The target surface roughness was produced by grinding and for the modification of the surface for increasing hydrophobicity (Fig. 3). For this, SiC polishing disc with roughness grade of 600 was employed. This treatment can modify material surface physically while the bulk properties of materials keep unchanged.
Table 2 shows the contact angles and specific surface energies of the smooth and rough uncoated UPR and composite. The results showed that smooth UPR surface with a has 80.70° ± 2.57 contact angle and 60.38 mJ/m2 specific surface energy. The contact angle of the UPR surface decreased to 60.80° ± 3.01 with the addition of the hydrophilic ceramic phase. As the surface roughness of the UPR surface grew after abrasion, the contact angle increased from 80.70° ± 2.57 to 84.90 ± 2.81 and the specific surface energy dropped from 60.38 to 47.11 mJ/m2. Neat UPR surface exhibits behaviour close to hydrophobicity. Coating these surfaces with a hydrophobic solution is difficult due to UPR's hydrophobic nature. This has been also experienced in our studies.
The contact angle of the flat UPR composite surface was measured as 60.80° ± 3.01, the contact angle increased to 66.21 ± 2.98 with the increase in the surface roughness level, and the specific surface energy was decreased from 79.85 to 72.54 mJ/m2. In other words, the contact angle of the composite samples is lower than the UPR surfaces. Addition, on abraded surfaces, higher contact angles were obtained with the increase of surface roughness [16]. In order to evaluate other parameters, the study was continued with composite samples.
The area roughness function in Profilm3D was used to determine the surface average roughness. The arithmetic mean surface roughness’s (Sa) were found as 0.367 for smooth composite surface and 0.7064 for abraded composite surface and shown in Fig. 4. In comparison to the untreated samples, the surface roughness of the composite samples rises after grinding treatment. It is widely acknowledged that raising the surface roughness to a certain level increase the surface's hydrophobicity [30]. In our situation, the contact angle increased from 60.80° to 66.21° when the composites' surface roughness value increased from 0.3670 to 0.7064.
Figure 5 displays the SEM images (500×) utilized to analyse the surface topography of the composite samples. The untreated composite sample surfaces have a smoother surface than the abraded composite surfaces.
The lower surface energies of the hydrophilic surfaces modified with ZnO powders are 5.4 mJ/m2 caused higher contact angle of 145°–150° [16]. As a result, as demonstrated experimentally, it was necessary to minimize the surface free energy in order to obtain a superhydrophobic surface based on theoretical considerations. As far as we have access, no study has been found in the literature on coating the surface of polyester matrix ceramic reinforced composites with ZnO doped solution and examining the obtained properties.
To lower the surface energy, a hydrophobic solution containing microscale and nanoscale zinc oxide (ZnO) particles was applied as a single layer. The textured composite surfaces formed was used to assess the effect of the chemistry on contact angle and hence wettability. The SEM images of the coated composite surfaces was given in Fig. 6. The coating material is consisting of 8 wt% microscale/nanoscale ZnO particles and 10% fluoropolymer, 60% alkoxysilane, and 30% ethanol solution and the substrate is ceramic reinforced polyester matrix, the two materials have different ability to reflection of electron in SEM imaging, hence the brighter areas show zinc oxide particles and the darker part is polymer matrix composite substrate. As can be seen from the SEM images of the surface obtained by using the spraying method, a nearly homogeneous coating surface was obtained. However, after applying both nano and micro ZnO added solutions to the surface in a single layer, voids were noticed in some locations. In particular, it was determined that the coating containing nano powder was cracked.
The variation of contact angle and specific surface energies of the rough surfaces of the composite substrates after single layer nano/micro ZnO particles incorporated coating is shown in Table 3. The water contact angle rose dramatically with the coating comprising both nano and micro scale ZnO particles. Thanks to this process, the contact angle of the abraded composite surface increased from 66.21° ± 2.98 to 143.2° ± 3.09 with nano scale ZnO containing coating application and to 147.4° ± 5.12 with micro scale ZnO containing coating application. ZnO nanoparticles are dispersed over the entire surface causing abundant air pockets under the droplets. In this case, the wetting behaviour is closer to the Cassie-Baxter regime for hydrophobic surfaces. Because the nano ZnO incorporated coating is not evenly distributed on the surface and there are agglomerations and cracks, the standard deviation of the contact angle value is larger. According to the SEM pictures in Fig. 6, the high contact angles achieved after coating might be attributable to the microscale surface topography formed by the synergistic effect of abrading the composite surface and integrating micro/nano scale ZnO powder. The contact angles were well associated with the surface free energy of the nano/micro ZnO modified and abraded composite surfaces. Quantitative data gathered for the specific surfaces under consideration revealed that a lower surface energy resulted in a greater contact angle. In order to achieve a superhydrophobic surface that adheres to theoretical principles, it was therefore crucial to reduce the surface free energy, as empirical evidence has demonstrated. According to Young's model, a smooth surface is hydrophilic when γsl < γsg and hydrophobic when γsl > γsg. The coated composites had rough hydrophobic surfaces with water contact angles between 143° and 147°. This is feasible according to the Cassie-Baxter model when air pockets are trapped between the liquid drop and the rough surface, and a chemically hydrophilic surface can become hydrophobic or superhydrophobic due to surface topography when γlg > γsg.
While the hydrophobic solution modified the surface chemistry on its own, the ZnO particles in the solution contributed to the superhydrophobic effect by increasing surface roughness. The similar results are obtained in literature [16]. There are several examples of superhydrophobic plant or animal shell surfaces in nature that are caused by surface topography rather than surface chemistry, such as lotus flower leaves and snail shells [31, 32]. As a result, it was established that the induced hydrophobicity was caused by the specific surface topography formed by the crystalline zinc oxide particles rather than the surface's chemical structure. On the other hand, the microscopic characteristics of the nano scale ZnO incorporated surfaces did not fulfil the necessary conditions for self-cleaning behaviour since the unsteady surfaces with agglomeration and cracks. These surfaces show high hydrophobicity but the drop did not slide from the surface and drops pinned to the surface hence the hysteria is higher than 10°.
Since voids were observed on the surface after single layer coating process, double layer coating process was applied. The SEM image of the double layer coated composite surfaces are given in Fig. 7. The composite substrate was thoroughly covered after two applications of the coating solution were applied. Gaps were found in certain places, though.
The cross-sectional SEM-BSE images of the sample containing the nanoparticle ZnO with single and double coating is shown in Fig. 8. Although the initial coating that was put to the rough surface adhered nicely to the surface, difficulties were seen when the second layer was applied. The single layer coating thickness for the nanoscale ZnO doped coating was observed to be around 3.84 ± 0.38 µm, however the double layer coating's coating was not uniform and had a maximum thickness measurement of 7.3 ± 1.28 µm. Moreover, weight difference was used to calculate the thickness of the coatings. For single coatings, the amount of coating material applied to the surface ranges from 0.0038 ± 0.0004 to 0.0073 ± 0.0009 g/cm2 for double coatings. With reference to the composite mixing rule (dried polymer coating density is 0.99 g/cm3 and zinc oxide density is 5.61 g/cm3) the thicknesses of the coatings were determined to be 4.29 ± 0.45 µm for the single coatings and 8.23 ± 1.01 µm for the double coatings [16]. Coating thickness calculations made by weight difference and SEM images support each other. As seen in Fig. 8a, the formation of the gap between the hydrophobic coating and the hydrophobic moulding polyester resin is a generally observed phenomenon due to their inherent repulsion.
SEM–EDS area analysis image taken from the coating surface containing double-coated micro particulate ZnO is given in Fig. 9a. According to quantitative analysis the zinc, oxygen and carbon elements were detected. The zinc and oxygen element comes from micro ZnO particles and carbon element from hydrophobic solution.
The surface average roughness values of the samples after single and double layer with nano and micro scale ZnO containing solution were given in Fig. 10. The arithmetic mean surface roughness for the single-layer coating containing nano ZnO particles was measured as 0.854 (Fig. 10a). The surface roughness increased to 3.148 after applying the same solution to the surface as a second layer (Fig. 10b). Such an increase in surface roughness is due to the agglomeration of nano powders. This is also confirmed by SEM images (Fig. 7a, b). The single-layer coating comprising micro ZnO particles had an arithmetic mean surface roughness of 1.51 (Fig. 10c). Because the powder size is at the micron level, the surface roughness is increased compared to a single nano ZnO coated solution. After applying the same solution to the surface as a second layer, the surface roughness increased to 1.73 (Fig. 10d). While there are voids on the surface after a single layer coating (Fig. 6c, d), the increase in surface roughness is minimal since the surface is entirely covered. The homogeneity increases when a second layer was coated (Fig. 7c, d).
Table 4 displays the changes in contact angle and specific surface energy of the composite substrates' rough surfaces following the application of a coating containing double-layer nano/micro ZnO particles. The contact angle increased slightly from 143° ± 5.12 to 155° ± 8.23 after the second layer coating process using nano ZnO particles and became a superhydrophobic surface. The contact angle had a significant standard deviation (± 5.12 and ± 8.23) due to the insufficient surface homogeneity (see Fig. 10a, b). When a second layer of the micro ZnO-containing solution was applied to the composite surface, the contact angle increased from 147° ± 1.28 to 157 ± 1.61. Contrary to the situation when a solution containing micro ZnO is applied, the standard deviation values of the contact angles are low (± 1.28 and ± 1.61). The micro ZnO doped coating is sufficiently homogenous on the surface, therefore the contact angle value's standard deviation is fairly small. Li and co-workers [17] used plasma based processes to improve hydrophobicity of polyester resin based composites with the roughness of the resulting surfaces was within the range of 20–400 nm. The contact angle for 70% marble filled composite was 155°, when oxygen plasma and OTS self-assembled monolayer synthesis were combined. Although identical contact angles are obtained, the method we used in our investigation is significantly more cost effective.
The specific surface energy of double layer nano ZnO incorporated surface is relatively higher (9.57 mJ/m2) than double layer micro ZnO incorporated surface (8.42 mJ/m2). The contact angles were well associated with the surface free energy of the nano/micro ZnO modified and abraded composite surfaces. Quantitative data collected for the individual surfaces in question demonstrated that a lower surface energy resulted in a greater contact angle. The surface roughness did not influence the surface energy but it influences the surface area. Roughness does not accurately reflect surface chemistry. The surface chemistry in this work was regulated by the use of a composite coating, which is a mixture of polymer and inorganic micro/nano ZnO particles.
On the other hand, a surface contact angle greater than 150° is not sufficient for a self-cleaning surface. In the study, micro scale ZnO incorporated surfaces shows a self-clean effect, since the contact angle of over 150° is obtained, the drop moves on the coated surface and the difference between the angles of progress and separation directions of the drop on the solid surface are below 5°. However, self-clean effect was not obtained with nano ZnO particles since the double layer coating does not adhere well on nanoscale ZnO doped surfaces, the water drop cannot roll on the surface due to the inhomogeneities on the surface even if the contact angle is high. In this investigation, the hydrophobic coating solution comprising micro ZnO particles had a synergistic effect that caused the contact angle to approach 155° and the hysteria (ΔƟ) to be about 5°.
4 Conclusions
The wettability and easy-to-clean properties of ceramic reinforced polyester matrix composite surfaces were investigated by spraying a hydrophobic solution incorporating nano/micro scale ZnO particles creating a unique roughness pattern on the substrates. The following findings were collected at the conclusion of the investigation.
-
Clean UPR surfaces display behaviour that is nearly hydrophobic. With the addition of the hydrophilic ceramic phase, the contact angle of the UPR surface fell from 80.70° to 60.80°.
-
After the surfaces were abraded, the contact angles increased; the composite surface's contact angle was 66.21° and the UPR surface's contact angle was 84.90°.
-
Coating the surface with a single layer of hydrophobic solution comprising nano and micro ZnO particles enhanced the contact angle and brought it close to superhydrophobicity (143°–147°).
-
Coating with a hydrophobic solution containing micro ZnO particles performed better than coating with nano ZnO particles. Defects on the surface were discovered because the nano ZnO particles were not adequately distributed.
-
While the surfaces covered with solution containing nano ZnO particles did not have a self-clean effect, the surfaces with micro ZnO additions had both superhydrophobic and self-clean effects.
-
Surface roughness rises throughout the grinding process as a large number of nano/microcraters form on the composite surface. Due to the impacts of roughening and changes in surface chemistry, the applied coating stuck better to the composite surface as air pockets developed via micro/nano craters on the composite surfaces, the contact angle increased, and the specific surface energy reduced.
-
In the study, the best results were obtained by applying a double layer of micro ZnO containing coating (CA: 157.8° and ∆Ɵ is around 5°).
References
H. Kramer, in Ullmann’s Polymers and Plastics: Products and Processes. ed. by B. Elvers (Wiley, Weinheim, 2016), p.781
B. Dholakiya, in Polyester. ed. by H.E.D.M. Saleh (Rijeka, Intech, 2012), p.167
P.W.R. Beaumont, C.H. Zweben, Comprehensive Composite Materials II, 2nd edn. (Elsevier, Amsterdam, 2018), pp.1–624
G. Acikbas, H. Gocmez, Mater. Test. 59, 11–12 (2017)
G. Acikbas, N. Calis Acikbas, Appl. Phys. A 127, 1–11 (2021)
A. Hozumi, O. Takai, Thin Solid Films 303, 1–2 (1997)
A. Nakajima, K. Abe, K. Hashimoto, T. Watanabe, Thin Solid Films 376, 1–2 (2000)
J.T. Han, Y. Jang, D.Y. Lee, J.H. Park, S.H. Song, D.Y. Ban, K. Cho, J. Mater. Chem. 15, 30 (2005)
H.Y. Erbil, A.L. Demirel, Y. Avcı, O. Mert, Science 299, 1377–1380 (2003)
J.T. Han, D.H. Lee, C.Y. Ryu, K. Cho, J. Am. Chem. Soc. 126, 15 (2004)
G. Acikbas, N Calis Acikbas. J. Asian Ceram. Soc. 9, 2 (2021)
S.S. Latthe, C. Terashima, K. Nakata, A. Fujishima, Molecules 19, 4 (2014)
L. Zhai, F.C. Cebeci, R.E. Cohen, M.F. Rubner, Nano Lett. 4, 7 (2004)
X. Wu, L. Zheng, D. Wu, Langmuir 21, 7 (2005)
G. Acikbas, N. Calis Acikbas, J. Am. Ceram. Soc. 105, 2 (2022)
S. Ozcan, G. Acikbas, N. Calis Acikbas, Appl. Surf. Sci. 438, 136 (2018)
G. Li, X. Wei, W. Wang, T. He, X. Li, Appl. Surf. Sci. 257, 1 (2010)
R.M.S. Attar, M. Alshareef, R.M. Snari, O. Alaysuy, A.M. Aldawsari, S. Abu-Melha, N.M. El-Metwaly, J. Mater. Res. Technol. 21, 11–12 (2022)
O.A.S. Adeakin, A.V. Popoola, K.K. Ajekwene, Fibers Polym. 23, 11 (2022)
H. Sun, Y. Wang, J. Chen, H. Hillborg, Silicone rubber with improved hydrophobicity. (Curran Associates Proceedings IEEE, 2015), https://ieeexplore.ieee.org/document/7352048. Accessed 23 July 2023
N.A. Patankar, Langmuir 29, 19 (2004)
M. Nosonovsky, B. Bhushan, Langmuir 24, 4 (2008)
M. Nosonovsky, B. Bhushan, Langmuir 11, 535–549 (2005)
L. Al-Naamani, S. Dobretsov, J. Dutta, J.G. Burgess, Chemosphere 168, 408–417 (2017)
M.S. Selim, H. Yang, F.Q. Wang, N.A. Fatthallah, Y. Huang, S. Kuga, Appl. Surf. Sci. 466, 40–50 (2019)
S. Ouir, H. Lachenani, F. Boudeffar, A. Bouaoua, H. Cheraga, F. Zermane, Z. Benmaamar, Appl. Phys. A 129, 1 (2019)
F.M. Fowkes, Ind. Eng. Chem. 56, 12 (1964)
D.K. Owens, R.C. Wendt, J. Appl. Polym. Sci. 13, 8 (1969)
D.H. Kaelble, J. Adhes. 2, 2 (1970)
Z. Zhan, Q. Zhang, F. Lu, Y. Liu, W. Liu, Z. Li, Q. Xie, AIP Adv. 9, 4 (2019)
C. Neinhuis, W. Barthlott, Ann. Bot. 79, 6 (1997)
E.H. Ishida, Channelling the forces of nature–human and earth conscious materials may create new waves. (Qualicer Ceramic tile quality, 2004), https://www.tib.eu/en/search/id/TIBKAT%3A392004119/QUALICER-2004-VIII-World-Congress-on-Ceramic-Tile. Accessed 21 July 2023
Funding
This work was financially supported by Mersin University, Scientific Research Fund [Project Number: 2022-1-TP2-4574].
Author information
Authors and Affiliations
Contributions
Gokhan Acikbas: Conceptualization, Validation, Visualization, Resources, Investigation, Methodology, Writing—original draft and review; Halil Hindi: Methodology.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Acikbas, G., Hindi, H. The Effect of a Composite Hydrophobic Coating with Zinc Oxide on the Characteristics of Polyester Matrix Composite Surfaces. J Inorg Organomet Polym 34, 419–429 (2024). https://doi.org/10.1007/s10904-023-02842-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02842-2