Abstract
Addressed herein is a facile method for the preparation of cobalt-silver (Cox–Agy) nanoparticles decorated mesoporous natural phosphate (m-NP). Various Cox–Agy@NP nanocatalysts were prepared in different forms of Ag and Co3O4 including mono- and bimetallic active sites. The bimetallic nanocatalysts were prepared by two different preparation methods, namely in-situ (Ag–Co3O4) and ex-situ (Ag/Co3O4 core–shell). In the first step, colloidal Cox–Agy nanoparticles were synthesized in solution and their formation was monitored by using UV–Visible spectroscopy. Furthermore, the obtained nanoparticles were characterized by XRD and IR spectroscopy. All nanoparticles were deposited on m-NP using a simple wetness impregnation method followed by a calcination at 500 °C. The prepared nanocatalysts were fully characterized by advanced analytical techniques including IR, XRD, XPS, SEM–EDX, FESEM, and TEM. The catalytic reduction of 4-nitrophenol was studied as a model reaction to investigate the effect of synergy created between the metals, oxidation state, catalyst structure, and preparation method on their catalytic activity. Accordingly, reaction kinetics and comparative study of various colloidal Cox–Agy and m-NP supported nanocatalysts in the reduction of 4-nitrophenol was carried out. The optimized conditions were used to study the substrate scope of the catalytic reduction over various nitroarenes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Monometallic nanoparticles are present in diverse sectors considering their electrical, optical, chemical, or biological applications [1,2,3,4]. However, in recent years, bimetallic nanoparticles are highly preferred for various applications due to their unique properties including bi-functionality and synergistic effects between two distinct metal atoms [5,6,7,8]. From the catalytic point of view, bimetallic nanoparticles composed of two distinct metals form outstanding catalytically active sites and chemical stability due to the synergistic effects created between the metal atoms [9,10,11,12].
Recently, special attention is paid to supported metal nanocatalysts which possess high catalytic performance in terms of the activity and stability. They reveal various benefits compared to their homogeneous counterparts in terms of their practical separation, thermal stability and reusability [12,13,14,15,16,17,18]. Consequently, the heterogeneous metal nanocatalysts improve the sustainability of catalytic processes by converting them into the less time- and energy-consuming ones. Furthermore, anchoring of metal nanoparticles on suitable support materials disclose heterogeneous catalysts possessing high activity and selectivity [15, 16]. Additionally, the deactivation of active sites during chemical processes could be declined or eliminated by using of supported metal nanocatalysts, which results in the longer catalytic lifetime. Moreover, supported metal nanocatalysts boost the catalytic efficiency by increasing the surface area and the support material can improve the catalytic efficiency of the anchored nanoparticles via providing strong support-metal interaction and improved distribution and dispersion, making them more sustainable [19].
Many support materials have been reported so far including metal oxides (Al2O3 [20], SiO2 [14, 21], ZnO [13], CeO2 [19]), carbon based materials (commercially available carbon blacks [22], carbon nanotubes [23], graphene [24]), natural clays [25], and polymers [26]. However, natural phosphate (NP), which is an attractive and cost-effective catalyst or catalytic carrier for several chemical transformations, has received little attention as a support material for the metal nanoparticles [15, 16], although hydroxyapatite and fluorapatite have been extensively employed in various catalytic applications [27, 28]. Taking into consideration that mesoporous supports allow high distribution and dispersion of active sites, we have recently reported the use of mesoporous NP (m-NP) as a support material for the palladium nanoparticles [15, 16]. According to the characteristic nanosized particles and pores of m-NP, the developed Pd@NP nanocatalysts showed strong catalytic activity, high stability and recyclability in hydrogen transfer reaction [15] and dehydroaromatization of natural olefines [16]. Silver nanoparticles have been found to be more efficient and easily available materials with diverse contributions in various applications [29,30,31]. The diversity of applications is owing to the tuning of their particle size, shape, surrounding dielectric characteristics, and inter-particle interactions [32]. The potential use of Ag nanoparticles is widely known in catalysis, cancer therapy, electrochemical sensor, drug therapy, and etc. [33]. On the other hand, cobalt and cobalt oxide nanoparticles have also various biomedical applications owing to their distinctive properties [34]. In addition to biomedical applications, they have been widely used in batteries, pigments dyes, electronic thin films, capacitors, and gas sensors [35,36,37]. In the water electrolysis, Co3O4 nanoparticles play a significant role as a catalyst [38]. Moreover, cobalt can exhibit variable oxidation states (Co2+, Co3+, and Co4+) which makes it an attractive transition metal for various catalytic applications. Recently, several eco-friendly, safe, easy, and inexpensive methods has been reported for the synthesis of cobalt and cobalt oxide nanoparticles using a variety of chemical and physical processes [34]. In the last decade, Co-based bimetallic systems has gained considerable importance due to the combined properties of metals coming from their electronic and geometric effects [38, 39]. However, bimetallic cobalt-silver nanoparticles could be deposited on adequate stabilizing supports for a better dispersion and distribution of active sites [40, 41]. Recently, reports are focused on grown cobalt oxide (Co3O4) and silver (Ag) nanoparticles on reduced graphene oxide (rGO) as a supercapacitor electrode material [40]. In addition, another Co3O4–Ag ternary nanocomposite was carried out through the decoration of fibrous polyaniline as a supercapattery battery-type electrode [41]. According to the literature, numerous studies have been reported on the catalytic performance of Ag/Co3O4 bimetallic system [42,43,44], in contrast there is a lack of studies on Ag–Co3O4 supported catalysts. Based on the catalytic performance of supported Co–Ag bimetallic catalysts in selective allylic oxidation of olefins [12,13,14], we have chosen for the first time to study the mono- and bimetallic Co–Ag nanoparticles supported on m-NP as nanocatalysts.
The reduction of 4-nitrophenol in aqueous solution represents an established “model catalytic reaction” for evaluation of the catalytic activity of metal nanoparticles [45, 46]. In wastewater, nitrophenols and other nitro-derivatives are one of the most common organic contaminants due to their use as herbicides, insecticides, and synthetic dyes [47]. Moreover, they are present in many unexploded landmines worldwide as dangerous high-energy explosives [48]. Additionally, nitro compounds are carcinogenic with genotoxic risks to humans and wildlife [49]. In contrast, the amines that are generally synthesized from the catalytic reduction of nitro compounds play a crucial role as important industrial intermediates. According to the significance of the reduction of nitro compounds, the development of an effective and recyclable catalysts has attracted enormous attention in the chemical industry [50, 51]. Recently, several bimetallic nanoparticles have been studied such as Au–Pt [52], Hg–Pd [53], Pt–Pd [54], Au–Ag [55], Ag–Cu [56], Pd–Co [57, 58], NiPd [59], and FePd [60].
Herein, we report the preparation, characterization, and catalytic application of Cox–Agy nanoparticles supported on m-NP. Several mono- and bimetallic colloidal Cox–Agy nanoparticles in solution and supported on m-NP were prepared and tested as nanocatalysts in the 4-nitrophenol reduction. The deposition was carried out using wetness impregnation method to prepare four types of Cox–Agy@NP supported nanocatalysts. The prepared nanocatalysts were fully characterized and their catalytic performance was evaluated in nitroarene reduction as a model reaction.
2 Experimental
2.1 Materials Synthesis
2.1.1 Preparation of Mesoporous Natural Phosphate
The natural phosphate (NP) was obtained from the region of Khouribga, Morocco [61]. The NP was treated by several techniques involving attrition, sifting, calcinations (900 °C), washing and re‐calcination. After its treatment, the NP has the following chemical composition: CaO (54.12%), P2O5 (34.24%), F (3.37%), SiO2 (2.42%), SO3 (2.21%), CO2 (1.13%), Na2O (0.92%), MgO (0.68%), Al2O3 (0.46%), Fe2O3 (0.36%), K2O (0.04%) and numerous trace metals in ppm.
Natural phosphate was subjected to several grinding and sieving as described in our previous studies to obtain a mesoporous support with a particle size of < 45 μm [15, 16]. Due to its nanosized particle size and pores, the m-NP represents a good choice as a catalytic carrier for nanoparticles deposition [15, 16].
2.1.2 Synthesis of Cox–Agy Colloidal Solutions
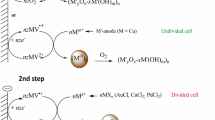
According to our previously described procedure [15, 16], we have prepared four different types of colloidal Cox–Agy nanoparticles in solution. Typically, monometallic Ag nanoparticles (x = 0, y = 1), Co nanoparticles (x = 1, y = 0), in situ Co–Ag nanoparticles (x = 0.5, y = 0.5) and ex-situ Co/Ag (core/shell) nanoparticles (x = 0.5, y = 0.5). UV–Visible spectroscopy was used to monitor the formation of all the prepared colloidal Cox–Agy nanoparticle solutions.
2.1.2.1 Synthesis of Monometallic Silver Nanoparticles
Colloidal silver nanoparticles in solution were synthesized by (i) mixing in a 500 ml flask a solution of AgNO3 (0.6 mmol) in distilled water (50 ml) with a previously stirred solution of sodium carboxymethylcellulose (CMC-Na, 0.222 g) as a surfactant dissolved in a mixture of water/ethanol (40 ml/10 ml). Then, monodispersed Ag (0) nanoparticles were obtained by (ii) adding an additional amount of water/ethanol (110 ml/ 40 ml) to complete 250 ml of solution mixture under stirring that will be (iii) refluxed for 17 h under air.
2.1.2.2 Synthesis of Monometallic Cobalt Nanoparticles
Monodispersed cobalt nanoparticles were synthesized using the same procedure in silver nanoparticles preparation, using Co(NO3)2,6H2O (0,6 mmol) as a precursor of cobalt.
2.1.2.3 Synthesis of Bimetallic Co–Ag Nanoparticles
The in-situ monodispersed bimetallic Co–Ag colloidal solution was synthesized by (i) mixing and stirring two aqueous solutions (0,3 mmol in 25 ml) of metal precursors AgNO3 and Co(NO3)2·6H2O. (ii) In a 500 ml flask, the metal precursors solution was mixed with a previously stirred solution of sodium carboxymethylcellulose (CMC-Na, 0.222 g) in a mixture of water/ethanol (40 ml/10 ml). (iii) The volume was completed to 250 ml by adding water/ethanol (110 ml/40 ml) and (iiii) the solution mixture was refluxed for 17 h under air.
2.1.2.4 Synthesis of Bimetallic Co/Ag Core/Shell Nanoparticles
First, (i) monodispersed silver nanoparticles were synthesized by the same described procedure. Thereafter, (ii) an aqueous solution of Co(NO3)2·6H2O (0.3 mmol in 25 ml) was added to the monodispersed Ag (0) and the mixture was refluxed for 17 h to obtain Co/Ag core/shell nanoparticles.
2.1.3 Deposition of Colloidal Nanoparticles on Mesoporous Natural Phosphate
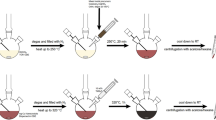
The solution of as-prepared colloidal nanoparticles (Ag, Co, Co–Ag, and Co/Ag) were used as precursors of nanoparticles deposition on m-NP. The deposition was carried out using simple wetness impregnation procedure by stirring m-NP (0.95 g) in the appropriate colloidal solution followed by 3 h of calcination at 500 °C (Scheme S1). The prepared Cox–Agy@NP nanocatalysts are designed Ag@NP, Co@NP, Co–Ag@NP, and Co/Ag@NP, respectively.
2.2 Materials Characterization
Spectrophotometric analysis of the colloidal solutions was investigated using a double-beam scanning spectrophotometer (Shimadzu spectrophotometer, model biochrom). The stretching vibration frequencies of the obtained nanoparticles and nanocatalysts were recorded by FT-IR spectroscopy in the range of 400–4000 cm–1 using a Bruker vertex70 DTGS. Diffraction data were collected at room temperature on a D2 PHASER diffractometer (BRUKER-AXS), with the Bragg–Brentano geometry, using CuKα radiation (λ = 1.5406 Å) with 30 kV and 10 mA. X-ray photoelectron spectra (XPS) were recorded on Thermo Scientific K-Alpha spectrometer using an Aluminum anode (Al Ka¼ 1468.3 eV). The binding energy scale was calibrated by assigning the C 1s signal at 284.4 eV. SEM–EDX measurements were obtained with TESCAN VEGA3-EDAX scanning electron microscope equipped with an energy dispersive X-ray detector (EDX). SEM images were acquired by using a Zeiss Ultra Plus Field Emission Scanning Electron Microscope (FESEM). Transmission electron microscopy (TEM) observations were carried out at 120 kV (TEM, Hitachi HT7800 with EXALENS) working at high-resolution (HR) mode with the magnification range of 10–600 k.
2.3 General Procedure for Catalytic Reduction of Nitroarenes
The prepared Cox–Agy@NP nanocatalysts were investigated toward the catalytic reduction of 4-nitrophenol as a catalytic model reaction and their substrate scope was investigated on other nitroarenes. A typical reaction was carried out in a round bottom flask at room temperature, by adding an aqueous solution of NaBH4 (2.2*10–1 M) as hydrogen source and the aqueous solution of 4-nitrophenol (3.5*10–3 M) in presence of Cox–Agy@NP (catalyst/substrate = 35% wt.). The catalytic reduction was monitored by UV–Visible spectroscopy showing the decrease of absorbance at 400 nm related to 4-nitrophenolate (obtained after the addition of NaBH4) and the revelation of a new band at 300 nm indicating the formation of 4-aminophenol. The optimized conditions were used to study the reaction kinetics in presence of various colloidal Co–Ag nanoparticles and m-NP supported nanocatalysts. The recyclability of the optimum prepared nanocatalysts was carried out. Furthermore, the same conditions were used in the reduction of various nitroarenes. The resulting products were extracted from the aqueous medium and the obtained organic phase was analyzed by gas chromatography.
3 Results and Discussion
3.1 Materials Characterization
3.1.1 Colloidal Cox–Agy Nanoparticles
The UV–Visible spectra of as-prepared Cox–Agy colloidal nanoparticles (Fig. 1) show a wide absorption band around λmax = 420 nm attributed to surface plasmon resonance (SPR) band of Ag nanoparticles (Ag, Co–Ag, and Co/Ag). In contrast to monometallic Ag nanoparticles, the bimetallic Cox–Agy nanoparticles show an additional band around 280 nm that could be assigned to the formation of silver oxide species [23, 62]. Moreover, the silver oxide absorbance was significant compared to SPR band in the case of Co/Ag core–shell nanoparticles. While the in-situ Co–Ag nanoparticles, represents an important SPR absorbance as well as a moderate intensity of silver oxide band. However, the absorbance band around 310 nm in the UV–Visible spectra of colloidal Co and Co–Ag nanoparticles can be attributed to the presence of Co(II) species [63]. As a result of the UV–Visible spectra, we can conclude that ex-situ colloidal solution (Co/Ag) promotes the formation of silver oxide compared to in-situ one (Co–Ag) with absence of cobalt band due to the core/shell structure.
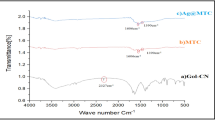
The FT-IR spectra of the as-prepared Cox–Agy nanoparticles and sodium carboxymethylcellulose (CMC-Na) present some slight differences compared to the reference CMC-Na (Fig. 2a), due to the formation of CMC-M (where M = Cox–Agy) in colloidal solutions by the possible interactions between CMC and metal nanoparticles. However, the FT-IR spectra (Fig. 2b) of the obtained nanopowders after purification with washing with distilled water and acetone, confirm the synthesis of pure CMC-M nanoparticles structure (M = Ag; Co; Co–Ag; and Co/Ag). The vibration stretching of the significant band around 3445 cm−1 is attributed to the O–H group of molecular water and O–H groups. Hence, the noticeable increasing of this band intensity (3445 cm−1) indicates the formation of cobalt hydroxide species (Fig. 2b1) [64,65,66]. Furthermore, two main bands at 506 cm−1 and 805 cm−1 are related to the metal–oxygen and the metal-OH bending vibrations (δ(Co–O–H) and γ(Co–O)) with additional bands related to Co–OH at 1059 cm−1, 1097 cm−1, 1263 cm−1, 1384 cm−1, and 1636 cm−1, coming from Co(OH)2 formation [67,68,69,70]. On the other hand, the slight absorption band around 580 cm−1 that appears in Co–Ag and Co/Ag samples can be attributed to Ag–O stretching mode, which corresponds to a slight presence of silver oxide in bimetallic Cox–Agy nanoparticles [71]. The absence of this band in the monometallic Ag nanoparticles confirms the complete reduction of silver precursors to Ag (0), in consistence with UV–Visible spectra. All FT-IR bands of monometallic Ag nanoparticles sample are attributed to CMC-Ag structure owing to the interaction with the silver metallic species.
XRD analyses of the as-prepared nanoparticles indicate the presence of intense peak at 2θ = 29.4° indexed to (104) plane of NaNO3 phase in all samples (Fig. S1). The presence of NaNO3 can be explained by its formation during the interaction between CMC-Na and metal precursors M(NO3)x, which confirms the FT-IR results of CMC-M formation. However, the XRD spectra of the obtained nanopowders after purification, designed CMC-M (M = Ag; Co; Co–Ag; and Co/Ag), reveal the presence of Ag, Ag2O, Co(OH)2, and Co patterns (Fig. 3). We have noticed the presence of peaks located at 2θ = 18.07°, 32.15°, 38.04°, and 39.27°, which can be indexed to (001), (010), (011), and (002) planes of hexagonal Co(OH)2 (JCPDS 96-101-0268), with crystallite size of 39.92, 34.76, 20.23, and 24.47 nm, respectively for CMC-Co (Fig. 3). Furthermore, the presence of Ag (0) nanoparticles was also confirmed by the wide peak around 2θ = 38°, which is readily assigned to (111) plane of face centered cubic metallic silver (JCPDS 96-150-9147), with crystallite size of 2.15, 3.76, and 5.34 nm for CMC-Ag, CMC-(Co–Ag), and CMC-(Co/Ag) respectively. In addition to Ag (0), the XRD spectra show the presence of cubic phase of Ag2O (JCPDS 41-1104) around 2θ = 27.8°, 32.2° and 46.2° indexed to (110), (111), and (211), with crystallite size of 34.41, 40.97, and 42.8 nm for CMC-(Co–Ag) and 34.64, 13.12, and 36.56 nm for CMC-(Co/Ag) respectively. In addition, the bimetallic Co–Ag and Co/Ag nanoparticles point out a remarkable absence of the intense pattern at 2θ = 18.07° related to Co(OH)2, with presence of Ag2O phase which present intense patterns in the case of core–shell nanoparticles. Although, the same patterns could be attributed to AgxCo3−xO4 phase [72]. Accordingly, the observed patterns attributed to Ag, Ag2O, and Co(OH)2 phases are in good agreement with the UV–Visible and FT-IR results.
3.1.2 Cox–Agy@NP Nanocatalysts
The deposition of Cox–Agy nanoparticles on m-NP was carried out using a simple wetness impregnation method followed by calcination at 500 °C for 3 h. The FTIR spectra of m-NP and Cox–Agy@NP nanocatalysts indicate the stability of the crystalline apatite after deposition and reveal the characteristic bands of phosphate groups at 560–600, 960, and 1030–1120 cm−1 (Fig. S2).
The crystallite structure of the as-deposited Cox–Agy nanoparticles on the surface of m-NP was studied by XRD analysis. All the patterns of the prepared Cox–Agy@NP nanocatalysts were not the same compared to the support m-NP (Fig. 4). The peaks appearing at 2θ = 38.46° and 44.39° are indexed to (102) and (110) planes of face centered cubic metallic silver, respectively, confirming the deposition of Ag (0) nanoparticles on m-NP for the prepared nanocatalysts Ag@NP, Co–Ag@NP and Co/Ag@NP. By using the wide peak at 2θ = 38.46°, the crystallite size of silver was calculated to be 53.40 nm, 22.88 nm, and 29.12 nm, respectively. However, the XRD patterns of Co@NP, Co–Ag@NP and Co/Ag@NP confirm the presence of Co3O4 indicated by the peak at 2θ = 46.92°, which can be assigned to (400) plane of cubic phase of the spinel Co3O4 [73]. According to the Scherrer equation [74], the crystallite size of Co3O4 were respectively 27.50, 37.20 and 27.49 nm. On the other hand, XRD region of natural phosphate shows a significant shifting of apatite peaks both in the deposition of monometallic and bimetallic cobalt nanoparticles (Fig. 4b). While the slight shifting of Co@NP patterns may be due to Co3O4 deposition on m-NP surface, the significant shifting for Co–Ag nanocatalysts could be attributed to the presence of silver oxide on the surface. In addition, the shifted peaks of Co–Ag@NP and Co/Ag@NP nanocatalysts (Fig. 4b) might be related to the intercalation with silver oxide peaks Ag2O (111) and AgO (111) at 2θ = 32.3° and 34.1°, respectively (JCPDS 12-0793 and JCPDS 84-1547) [75]. The presence of silver oxide species was detected in bimetallic nanoparticles analysis and could be also explained by the metallic redox interaction during the calcination process [76, 77].
The observed shifting of the characteristic XRD peaks of the natural apatite leads us to study the lattice parameters and the crystallite sizes of m-NP before and after deposition of Cox–Agy nanoparticles (Table S1). The lattice parameters indicates that the deposition of Cox–Agy nanoparticles does not have a significant effect on the crystalline system of the apatite support with a slight difference on lattice parameters for the bimetallic nanocatalysts. However, the used colloidal solutions for the deposition of these nanoparticles shows an improvement resulting in a decrease of m-NP crystallite size. Moreover, the deposited bimetallic nanoparticles show interesting results especially for the deposited Co/Ag core–shell nanoparticles.
To understand the exact local electronic and chemical configuration of the deposited Cox–Agy nanoparticles on m-NP, XPS analyses were carried out. The chemical environment and the oxidation state of silver and cobalt were studied through the Ag 3d and Co 2p regions (Figs. 5 and 6). In addition to the representative elements of m-NP (phosphate, calcium, oxygen, fluor, and carbon), all XPS survey spectra confirm the deposition of Cox–Agy nanoparticles in the prepared Cox-Agy@NP nanocatalysts (Figs. 5 and 6a–d).
The XPS spectrum of monometallic Ag@NP nanocatalysts indicates that the deposited silver nanoparticles are in metallic form (Fig. 5a and a1). While the major Ag 3d5/2 component is centered at binding energy (BE) of 367.39 eV and the second spin–orbit pair of minor intensity Ag 3d3/2 is detected at BE of 373.16 eV. The Ag 3d5/2 and Ag 3d5/2 are presented as a doublet with spin orbit split of ≈ 6 eV, which is in a good agreement with the characteristic data of metallic silver [78]. On the other hand, the monometallic nanocatalyst Co@NP shows two separated peaks, Co 2p3/2 at BE of 779.68 eV and Co 2p1/2 at BE of 795.05 eV with a characteristic spin–orbit split of 15.37 eV (Fig. 6b and b1). The Co 2p XPS spectrum indicates that the cobalt is deposited as Co3O4 [79].
The XPS spectra of both Co–Ag@NP and Co/Ag@NP nanocatalysts were studied too (Figs. 5 and 6c, c1, c2, d, d1 and d2). The Ag 3d spectra of the deposited bimetallic nanoparticles were rather different from monometallic silver one. In addition to the main existence of metallic Ag, the bimetallic Co–Ag@NP shows a minor presence of Ag/Ag2O mixed phase noticed by Ag 3d5/2 (365.1 eV) and Ag 3d3/2 (370.64 eV) peaks [75]. In contrast, the metallic silver represents a minor phase for the deposited bimetallic core–shell nanoparticles, due to the major presence of AgO species confirmed by Ag 3d5/2 (370.65 eV) and Ag 3d3/2 (376.64 eV) [80]. The Co 2p spectra of Co–Ag@NP and Co/Ag@NP nanocatalysts, present the same results observed for cobalt monometallic deposition indicated by the presence of 2p3/2 and 2p1/2 peaks and two satellite peaks (Fig. 6c2 and d2), which is in good agreement with Co3O4 XPS spectra [79].
In order to study the effect of nanoparticles deposition on the structural and electronic chemical configuration of the support material, XPS analyses of all prepared nanocatalysts were also studied for major elements in m-NP structure (P 2p, O 1s, Ca 2p, F 1s, and C 1s) (Fig. S3). The P 2p spectrum shows the typical P 2p3/2 peak of natural phosphate at BE of 133.2 eV with a slight difference in the electronic configuration for bimetallic nanocatalysts and especially a significant shifting observed for bimetallic Co/Ag core/shell nanoparticles. These results indicated the interactions of the deposited bimetallic nanoparticles with the phosphate group in the natural apatite. While O 1s presents mainly two peaks attributed to PO43− + OH− and O2−, with the same remarked peak position modification for bimetallic deposition may be due to the deposition of silver oxide nanoparticles in the surface. The binding energy of Ca 2p3/2 and Ca 2p1/2 matches those reported in the literature for natural phosphate [81]. The differentiation in Ca 2p spectrum for deposited Co/Ag core/shell nanoparticles could be attributed to the interaction of calcium groups with the deposited silver oxide in the surface of m-NP. The spectra of F 1s (684.9 eV for NP) confirm the fluorapatite aspect of the m-NP used as a support for the deposition of nanoparticles. The C1s spectra confirmed the presence of carbonate in m-NP with the same noticed spectrum modification for the core–shell deposition due to the interaction of metallic nanoparticles with the support.
The analysis of XPS spectra of main m-NP elements showed no-significant modification in terms of electronic and chemical configurations for monometallic deposition on m-NP surface. In contrast, the bimetallic deposition reveals some modifications due to the presence of silver oxide species (AgO and Ag2O) and to the important interaction of bimetallic Cox-Agy nanoparticles with the m-NP surface, especially for deposited Co/Ag core/shell nanoparticles.
To investigate the morphology, the dispersion, and the distribution of the deposited Cox-Agy nanoparticles on m-NP, FESEM images were recorded for all prepared Cox-Agy@NP nanocatalysts (Fig. 7). The FESEM images indicated that nanoparticles deposition has no effect on the m-NP morphology (Fig. 7a1, b1, c1 and d1). The monometallic deposition of silver nanoparticles formed well dispersed and distributed nanospheres on the surface Fig. 7a2 and a3). The deposited nanospheres were identified as formed Ag@NP nanocomposites on m-NP surface as showed by EDX and elemental mapping analysis (nanosphere composed by Ag and major m-NP (fluorapatite) elements) (Fig. 8). On the other hand, the deposition of cobalt leads to distributed Co3O4 nanograins on the mesoporous NP surface (Fig. 7b2 and b3). In contrast, the bimetallic nanocatalysts show different results compared to monometallic deposition (Fig. 7c and d). Both in-situ and ex-situ Cox-Agy colloidal solutions lead to the deposition of distributed silver nanospheres decorated Co3O4 nanograins (Fig. 8c3 and d3). In case of Co/Ag core/shell deposition, a slight differentiation in terms of dispersion and distribution is noticed for the obtained silver decorated Co3O4 (Fig. S4).
TEM analyses were carried out to explore the shape and size of the deposited nanoparticles on m-NP (Fig. 9). The TEM images of the monometallic Ag nanoparticles deposited on m-NP showed a well-dispersed nanoparticles with an average particle size of 10 nm, and confirm the observed Ag@NP nanospheres by FESEM-EDX analyses (Fig. 9a). In the case of the monometallic deposition of cobalt, the images revealed the presence of chain-like (deposited nanograins observed by FESEM) and cubic Co3O4 nanoparticles deposited on m-NP surface (Fig. 9b). The deposition of in-situ colloidal solution of Cox–Agy exhibits the decoration of m-NP support by both Ag and Co3O4 nanoparticles possessing good distribution and well-dispersion (Fig. 9c). In contrast, the deposition of ex-situ Cox–Agy colloidal solution exhibits the presence of Co/Ag core/shell nanoparticles formed mainly by Co3O4 nanograins (core) decorated by Ag nanospheres and silver oxide film (shell) (Fig. 9d).
3.2 Catalytic Application
3.2.1 Catalytic Reduction of 4-Nitrophenol
The catalytic activity of Cox–Agy@NP nanocatalysts was evaluated for the reduction of 4-nitrophenol (4-NP) as a well-accepted model reaction. The reduction reactions were carried out using NaBH4 as reducing agent in aqueous medium under ambient conditions (Fig. S5). The direct reduction of 4-NP by NaBH4 is thermodynamically feasible but kinetically restricted due to the lack of an efficient catalyst [82]. The catalytic transformation is simple and very fast with the advantage of monitoring the reduction by UV–visible spectroscopy. The use of mesoporous natural phosphate alone cannot catalyze the catalytic reduction of 4-NP.
In presence of NaBH4, the 4-nitrophenol is converted to 4-nitrophenolate ion which is noticed by the change of color from light yellow to bright yellow, and the absorption peak shifting from 317 to 400 nm (Fig. 10a). After addition of the catalyst, the characteristic absorption peak of 4-nitrophenolate at 400 nm started to decrease while a new absorption band at 300 nm appeared due to 4-aminophenol formation (Fig. 10b). In addition, the change in the color of reaction mixture from bright yellow to colorless solution was also an indication of the catalytic reduction of 4-NP (Fig. S5). The comparison of Cox–Agy@NP catalytic performances indicate that deposited silver nanoparticles (Ag@NP) are more efficient compared to Co3O4 (Co@NP). However, the bimetallic Cox–Agy nanocatalysts reveal a great improvement in catalytic activity in comparison to monometallic Ag@NP nanocatalyst. These results reveal the great effect of bimetallic synergy on the catalytic performance. On the other hand, the deposited Co–Ag nanoparticles gave better catalytic activity than the Co/Ag ones. The less catalytic efficiency in terms of reaction kinetics of Co/Ag@NP nanocatalyst could be due to the presence of silver oxide film on the deposited core–shell nanoparticles. In contrast, Co–Ag@NP presents a uniform distribution of Co3O4 and Ag NPs (See Fig. 9). Furthermore, XPS spectra show silver oxide as predominant oxidation state compared to Co–Ag@NP where metallic silver is mainly the dominant specie. The fact that metallic silver is the highly active catalytic specie can explain the catalytic performance of Co–Ag@NP compared to Co/Ag@NP. Consequently, Co–Ag@NP was chosen as a model nanocatalyst to optimize the 4-NP catalytic reduction.
The catalytic activity of 4-NP was investigated with various amount of NaBH4 (0.036 to 0.392 M) to determine the optimal concentration of the reducing agent, in presence of Co–Ag@NP (Catalyst/Substrate = 50% wt.) as the model nanocatalyst. The reaction time was dependent on NaBH4 concentration with no effect on 4-NP conversion, which indicate a high catalytic efficiency of the model nanocatalyst. Due to the presence of NaBH4 excess, the catalytic transformation rate was assumed to be dependent only on the substrate, which can be fitted using a first-order rate law to 4-NP concentration. During the optimization, the kinetic of each catalytic reaction has been studied following the reaction rate constant (k) calculated using ln (At/A0) = − kt, where At and A0 represent the absorbance at 400 nm at time t and 0 (Fig. 11). The regular use of NaBH4 excess in such catalytic transformation could be explained by the increase of produced BH4− ions which facilitate the catalytic hydrogen transfer process to convert 4-NP to 4-AP.
The effect of catalyst amount on the catalytic reduction of 4-NP was investigated using various catalyst/substrate ratio (10 to 50% wt.) of Co–Ag@NP chosen as the model nanocatalyst. The best result was obtained with 0.221 M NaBH4 concentration (Fig. 12). The total catalytic reduction of 4-NP takes place from a moderate to a fast time. With a small catalytic ratio of 10%, the total conversion of 4-NP with a reaction time of 65 min indicates the efficiency of the developed Co–Ag@NP nanocatalyst. Starting from catalyst/substrate ratio of 30%, high constant rate was obtained with a shorter reaction time. The ratio of 35% was the best optimum amount for the catalytic application with 98% of conversion in 11 min and a rate constant of 0.4542 min−1.
3.2.2 Substrate Scope and Limitations of Catalytic Reduction of Nitroarenes in Presence of Co–Ag@NP Nanocatalysts
The scope and limitations of the developed nanocatalytic system were investigated in the reduction of several other nitroarenes under the optimized conditions. As shown in Table 1, all studied nitroarenes bearing electron withdrawing or donating substituents were converted to the corresponding amino products in a shorter reaction time compared to previously published nanocatalyst Pd@NP [15].
3.2.3 Nanocatalyst Heterogeneity and Recyclability
The heterogeneity of the nanocatalytic system was investigated according to the metal leaching during the catalytic transformation. First, the reaction was carried out in presence of Co–Ag@NP nanocatalyst and kept going for 2 min. Thereafter, the nanocatalyst was removed from the reaction mixture and then the absorbance was measured. After nanocatalyst filtration, the absorbance of 4-nitrophenolate remain constant, indicating that no reduction of 4-NP is occurred even after 120 min of reaction (Fig. S6). This result represents a response on the heterogeneity of the developed nanocatalyst as no silver or cobalt nanoparticles are leached into reaction mixture.
Furthermore, the catalyst stability and recyclability are the key parameters for the heterogeneous catalysts for catalytic applications. The reusability of Co–Ag@NP has been carried out for four consecutive cycles (Fig. 13). After each cycle, the nanocatalyst was recycled by decanting the aminophenol product and adding the same initial amount of 4-NP until the fourth cycle. The decantation is performed using an organic solvent. This adapted recycling method allow us to investigate the reuse of the nanocatalyst but also the reduction agent lifetime during the cycles. Hence, the recycling process is more adaptable to atom economy following green chemistry and circular economy principals. In the last cycle, a depletion of NaBH4 was observed and an adequate amount of reducing agent was introduced to complete the catalytic reduction. The obtained total conversion of 4-NP during the last cycle, confirms the great efficiency in terms of catalytic stability and recyclability of Co–Ag@NP for further cycles.
To confirm the stability in terms of active sites and catalytic structure, the characterization of recycled nanocatalyst after the first and the last cycles were carried out. XRD examination of the nanocatalysts revealed that the crystallinity of the recovered Co–Ag@NP is similar to the fresh one with no-modification of active sites Ag and Co3O4 nanoparticles (Fig. 13b). However, SEM analyses indicate a good stability of the recycled nanocatalysts in terms of surface morphology, dispersion, and distribution of Ag and Co3O4 nanoparticles (Fig. 13c). Moreover, EDX spectra reveal a good stability of nanocatalysts elemental composition and confirms the stable presence of Co–Ag nanoparticles during the recyclability process (Fig. 13c).
3.2.4 Comparison of Catalytic Performance with Other Co- and Ag-Based Catalysts
A comparison of the catalytic performance of the developed Cox-Agy@NP nanocatalysts with recent literature reports on 4-NP reduction catalyzed by various cobalt and silver based catalytic systems is given in Table 2.
4 Conclusion
In a summary, we have reported a successful synthesis of mono- and bimetallic Cox–Agy colloidal nanoparticles and their deposition on mesoporous natural phosphate (m-NP) using wetness impregnation method. The characterizations of Cox–Agy nanoparticles using UV–Visible, IR, and XRD clearly indicated the formation of Ag (0) and Co(OH)2 with presence of silver oxide in the case of bimetallic Cox–Agy. After the deposition of nanoparticles, all prepared Cox-Agy@NP nanocatalysts were fully characterized by IR, XRD, XPS, SEM–EDX, FESEM, and TEM analyses. The structural, chemical, and physical characterizations confirmed the presence of Ag (0) and Co3O4 for monometallic deposition. In contrast, the Cox–Agy bimetallic deposition presented a moderate amount of silver oxides due to metal synergic and redox interactions. On the other hand, the microscopic analyses revealed a stable morphology with uniform distribution and dispersion of crystalline Ag and Co3O4 nanoparticles on the mesoporous NP. Moreover, the Cox–Agy bimetallic deposition forms silver nanospheres decorated Co3O4 nanograins on the surface. The prepared ex-situ nanoparticles present a core–shell form of deposited Co/Ag on m-NP surface. The catalytic performance of the prepared nanocatalysts was carried out in nitroarene reduction using NaBH4, leading to pseudo-first order reaction kinetics. The excellent catalytic activity of bimetallic Co–Ag@NP nanocatalysts with great stability and recyclability, demonstrates the importance of metal synergy and the choice of catalyst preparation method.
References
M. Akca, D. Varisli, Mol. Catal. 485, 110823 (2020)
M. Domaschke, M. Schmidt, W. Peukert, J. Aerosol Sci. 126, 133–142 (2018)
C. Cheephat, P. Daorattanachai, S. Devahastin, N. Laosiripojana, Appl. Catal. A Gen. 563, 1–8 (2018)
F.J. Morales-Leal, J. RiveraDelaRosa, C.J. Lucio-Ortiz, D.A. DeHaroDelRío, M.A. Garza-Navarro, W. Tian, J.E. Herrera, Chem. Eng. J. 426, 131623 (2021)
D. Lahiri, B. Bunker, B. Mishra, Z. Zhang, D. Meisel, C.M. Doudna, M.F. Bertino, F.D. Blum, A.T. Tokuhiro, S. Chattopadhyay, T. Shibata, J. Terry, J. Appl. Phys. 97, 094304 (2005)
D. Wang, Y. Li, Adv. Mater. 23, 1044–1060 (2011)
C.J. Serpell, J. Cookson, D. Ozkaya, P.D. Beer, Nat. Chem. 2011(36), 478–483 (2011)
M. Moskovits, I. Srnová-Šloufová, B. Vlčková, J. Chem. Phys. 116, 10435–10446 (2002)
P. Osorio-Vargas, C.H. Campos, C.C. Torres, C. Herrera, K. Shanmugaraj, T.M. Bustamante, J.N. Diaz de Leon, F. Medina, L.E. Arteaga-Pérez, Catal. Today (2021).
H. Jahromi, S. Adhikari, P. Roy, E. Hassani, C. Pope, T. Oh, Y. Karki, Fuel Process. Technol. 215, 106737 (2021)
S. Mao, C. Wang, Y. Wang, J. Catal. 375, 456–465 (2019)
A. Aberkouks, A.A. Mekkaoui, B. Boualy, S. El Houssame, M. Ait Ali, L. El Firdoussi, Adv. Mater. Sci. Eng. 2018, 1–7 (2018)
A. Aberkouks, A.A. Mekkaoui, B. Boualy, S. El Houssame, M. AitAli, L. El Firdoussi, Mater. Today Proc. 13, 453–457 (2019)
A. Aberkouks, A.A. Mekkaoui, M. Ait Ali, L. El Firdoussi, S. El Houssame, J. Chem. 2020, 1–11 (2020)
A.A. Mekkaoui, S. Jennane, A. Aberkouks, B. Boualy, A. Mehdi, M. Ait Ali, L. El Firdoussi, S. El Houssame, Appl. Organomet. Chem. 33, e5117 (2019)
A.A. Mekkaoui, A. Aberkouks, L. Fkhar, M. Ait Ali, L. El Firdoussi, S. El Houssame, Appl. Organomet. Chem. 34, e5917 (2020)
D.J. Cole-Hamilton, R.P. Tooze, in: Catal. by Met. Complexes, Springer, Dordrecht, 2006, pp. 1–8.
D. Astruc, F. Lu, J.R. Aranzaes, Angew. Chemie Int. Ed. 44, 7852–7872 (2005)
M. Kohantorabi, M.R. Gholami, Ind. Eng. Chem. Res. 56, 1159–1167 (2017)
M. Aazza, H. Ahlafi, H. Moussout, C. Mounir, A. Fadel, A. Addad, J. Environ. Chem. Eng. 8, 103707 (2020)
X. Xu, Y. Liu, Y. Gao, H. Li, Colloids Surf. A Physicochem. Eng. Asp. 529, 613–620 (2017)
J. Speder, A. Zana, I. Spanos, J.J.K. Kirkensgaard, K. Mortensen, M. Arenz, Electrochem. Commun. 34, 153–156 (2013)
M.M. Rahman, H.B. Balkhoyor, A.M. Asiri, H.M. Marwani, Microchim. Acta 183, 1677–1685 (2016)
S. Kapoor, A. Sheoran, M. Riyaz, J. Agarwal, N. Goel, S. Singhal, J. Catal. 381, 329–346 (2020)
V.K. Landge, S.H. Sonawane, M. Sivakumar, S.S. Sonawane, G. UdayBhaskarBabu, G. Boczkaj, Sustain. Energy Technol. Assessments 45, 101194 (2021)
N. Jadbabaei, R.J. Slobodjian, D. Shuai, H. Zhang, Appl. Catal. A Gen. 543, 209–217 (2017)
K. Kaneda, T. Mizugaki, Energy Environ. Sci. 2, 655 (2009)
K. Kaneda, T. Mizugaki, ACS Catal. 7, 920–935 (2017)
S. Basu, H.S. Samanta, J. Ganguly, Soft Mater. 16, 7–19 (2018)
K. Jemal, B.V. Sandeep, S. Pola, J. Nanomater. 2017, 1–11 (2017)
E. Saion, E. Gharibshahi, K. Naghavi, Int. J. Mol. Sci. 14, 7880–7896 (2013)
M. Brust, C.J. Kiely, Colloids Surf. A Physicochem. Eng. Asp. 202, 175–186 (2002)
M.A. Islam, M.V. Jacob, E. Antunes, J. Environ. Manage. 281, 111918 (2021)
A. Waris, M. Din, A. Ali, S. Afridi, A. Baset, A.U. Khan, M. Ali, Open. Life Sci. 16, 14–30 (2021)
B. Raveau, M.M. Seikh, Zeitschrift Fur Anorg. Und Allg Chemie 641, 1385–1394 (2015)
S.M. Ansari, R.D. Bhor, K.R. Pai, D. Sen, S. Mazumder, K. Ghosh, Y.D. Kolekar, C.V. Ramana, Appl. Surf. Sci. 414, 171–187 (2017)
G. Chai, W. Zhang, L.F. Liotta, M. Li, Y. Guo, A. Giroir-Fendler, Appl. Catal. B Environ. 298, 120606 (2021)
Y. Feng, Z. Li, C.-Q. Cheng, W.-J. Kang, J. Mao, G.-R. Shen, J. Yang, C.-K. Dong, H. Liu, X.-W. Du, Appl. Catal. B Environ. 299, 120658 (2021)
B. Guo, J. Ma, Y. Shi, K. Zheng, M. Wu, G. Ren, S. Komarneni, Ceram. Int. 47, 27617–27623 (2021)
J. Iqbal, A. Numan, R. Jafer, S. Bashir, A. Jilani, J. Alloys Compd. 821 (2020) 153452.
J. Iqbal, A. Numan, M. OmaishAnsari, R. Jafer, P.R. Jagadish, S. Bashir, P.M.Z. Hasan, A.L. Bilgrami, S. Mohamad, K. Ramesh, S. Ramesh, Polymers (Basel). 12, 2816 (2020)
H.M.A. Amin, C.J. Bondue, S. Eswara, U. Kaiser, H. Baltruschat, Electrocatalysis 8, 540–553 (2017)
A. Ashok, A. Kumar, Int. J. Hydrogen Energy 46, 4788–4797 (2021)
A. Ashok, A. Kumar, M.A. Matin, F. Tarlochan, ACS Omega 3, 7745–7756 (2018)
P. Hervés, M. Pérez-Lorenzo, L.M. Liz-Marzán, J. Dzubiella, Y. Lu, M. Ballauff, Chem. Soc. Rev. 41, 5577 (2012)
T. Aditya, A. Pal, T. Pal, Chem. Commun. 51, 9410–9431 (2015)
G. Booth, in: Ullmann’s Encycl. Ind. Chem., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000.
J.C. Oxley, in: Explos. Eff. Appl., Springer New York, New York, NY, 1998, pp. 137–172.
G. Eichenbaum, M. Johnson, D. Kirkland, P. O’Neill, S. Stellar, J. Bielawne, R. DeWire, D. Areia, S. Bryant, S. Weiner, D. Desai-Krieger, P. Guzzie-Peck, D.C. Evans, A. Tonelli, Regul. Toxicol. Pharmacol. 55, 33–42 (2009)
Q. Wang, W. Jia, B. Liu, A. Dong, X. Gong, C. Li, P. Jing, Y. Li, G. Xu, J. Zhang, J. Mater. Chem. A 1, 12732 (2013)
Ö. Metin, H. Can, K. Şendil, M.S. Gültekin, J. Colloid Interface Sci. 498, 378–386 (2017)
J. Zhang, G. Chen, D. Guay, M. Chaker, D. Ma, Nanoscale 6, 2125–2130 (2014)
V.K. Harika, H.K. Sadhanala, I. Perelshtein, A. Gedanken, Ultrason. Sonochem. 60, 104804 (2020)
Y. Wang, Q. Li, P. Zhang, D. O’Connor, R.S. Varma, M. Yu, D. Hou, J. Colloid Interface Sci. 539, 161–167 (2019)
H. Chen, X. Fan, J. Ma, G. Zhang, F. Zhang, Y. Li, Ind. Eng. Chem. Res. 53, 17976–17980 (2014)
A.D. Verma, S. Pal, P. Verma, V. Srivastava, R.K. Mandal, I. Sinha, J. Environ. Chem. Eng. 5, 6148–6155 (2017)
T.A. Revathy, T. Sivaranjani, A.A. Boopathi, S. Sampath, V. Narayanan, A. Stephen, Res. Chem. Intermed. 45, 815–832 (2019)
H. Göksu, H. Can, K. Şendil, M.S. Gültekin, Ö. Metin, Appl. Catal. A Gen. 488, 176–182 (2014)
H. Göksu, S.F. Ho, Ö. Metin, K. Korkmaz, A. MendozaGarcia, M.S. Gültekin, S. Sun, ACS Catal. 4, 1777–1782 (2014)
Ö. Metin, A. Mendoza-Garcia, D. Dalmızrak, M.S. Gültekin, S. Sun, Catal. Sci. Technol. 6, 6137–6143 (2016)
Natural Phosphate (NP) Comes from Khouribga Region (Morocco); It Is Readily Available (Raw or Treated) from the Centre for Studies and Research of Mineral Phosphates (CERPHOS) 37, Bd My Ismail, Casablanca, Morocco
T. Chaieb, L. Delannoy, C. Louis, C. Thomas, Catal. Lett. 146, 2622–2629 (2016)
M. Medvidović-Kosanović, A. Stanković, M. Jozanović, M. Drulak, M. Ilić, Croat. Chem. Acta 91, 421–426 (2018)
J.C. Villegas, O.H. Giraldo, K. Laubernds, S.L. Suib, Inorg. Chem. 42, 5621–5631 (2003)
L.-H. Su, X.-G. Zhang, C.-H. Mi, B. Gao, Y. Liu, Phys. Chem. Chem. Phys. 11, 2195 (2009)
R. Xu, H.C. Zeng, J. Phys. Chem. B 107, 12643–12649 (2003)
M. Aghazadeh, H.M. Shiri, A.-A.M. Barmi, Appl. Surf. Sci. 273, 237–242 (2013)
J. Wang, Y. Bao, C. Cui, Z. Zhang, S. Li, J. Pan, Y. Zhang, G. Tu, J. Wang, Z. Li, J. Mater. Sci. 54, 7692–7701 (2019)
M. Aghazadeh, Int. J. Electrochem. Sci. 12, 5792–5803 (2017)
R. Li, Z. Hu, X. Shao, P. Cheng, S. Li, W. Yu, W. Lin, D. Yuan, Sci. Rep. 6, 18737 (2016)
A.I. Oje, A.A. Ogwu, M. Mirzaeian, N. Tsendzughul, J. Electroanal. Chem. 829, 59–68 (2018)
A.V. Salker, M.S. FalDesai, Catal. Sci. Technol. 6, 430–433 (2016)
D.D.M. Prabaharan, K. Sadaiyandi, M. Mahendran, S. Sagadevan, Appl. Phys. A 123, 264 (2017)
P. Scherrer, Nachrichten Math. Phys. 2, 98–100 (1918)
A. Al-Sarraj, K.M. Saoud, A. Elmel, S. Mansour, Y. Haik, S.N. Appl, Sci. 3, 15 (2021)
V. Rico-Pérez, A. Bueno-López, Appl. Sci. 4, 468–481 (2014)
H. Romar, E. Rivoire, P. Tynjälä, U. Lassi, Top. Catal. 60, 1408–1414 (2017)
P. Prieto, V. Nistor, K. Nouneh, M. Oyama, M. Abd-Lefdil, R. Díaz, Appl. Surf. Sci. 258, 8807–8813 (2012)
A. Sarnecki, P. Adamski, A. Albrecht, A. Komorowska, M. Nadziejko, D. Moszyński, Vacuum 155, 434–438 (2018)
A.M. Ferraria, A.P. Carapeto, A.M. Botelho do Rego, Vacuum 86, 1988–1991 (2012)
J. Hidalgo-Carrillo, J. Sebti, A. Marinas, J.M. Marinas, S. Sebti, F.J. Urbano, J. Colloid Interface Sci. 382, 67–73 (2012)
B. Baruah, G.J. Gabriel, M.J. Akbashev, M.E. Booher, Langmuir 29, 4225–4234 (2013)
W. Zhang, F. Tan, W. Wang, X. Qiu, X. Qiao, J. Chen, J. Hazard. Mater. 217–218, 36–42 (2012)
Y. Ma, X. Wu, G. Zhang, Appl. Catal. B 205, 262–270 (2017)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Orfi, H., Mekkaoui, A.A., Sündü, B. et al. Ag, Co3O4, Ag–Co3O4, and Ag/Co3O4 Nanoparticles Decorated Mesoporous Natural Phosphate: Effect of Metal Synergy and Preparation Method on the Catalytic Reduction Reaction. J Inorg Organomet Polym 32, 2192–2208 (2022). https://doi.org/10.1007/s10904-022-02262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02262-8