Abstract
Copper-based metal–organic-frameworks with open metal sites have received increasing research interest as heterogeneous catalysts for various organic transformations. A copper-based metal organic framework (1) built with L-NO2 ligand (L-NO2 = 4,4′-dicarboxy-4″-nitrotriphenylamine) was selected for catalyzing aerobic homocoupling of arylboronic acid toward biaryl products given its structural robustness and 1-D channels lined with rich open metal sites. The experimental results show that MOF (1) exhibits pronounced size selectivity over arylboronic acid molecules, which is only effective for short arylboronic acid molecules (e.g. phenylboronic acid, p-methylphenylboronic acid and p-fluorophenylboronic acid), giving the corresponding biaryl products in good yields. Moreover, MOF (1) also demonstrates a good recyclability which only shows a small decay in the catalytic performance after five repeated runs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The past decades have witnessed an ever-growing research interest in employing metal–organic-frameworks (MOFs) as heterogeneous catalysts for a variety of organic transformations [1, 2]. MOFs are a unique class of porous materials which are composed of metal ions (or clusters) and organic ligands joined by coordination bonds. Porous materials (e.g. 3D pure inorganic solids) have found a diverse range of applications such as shape-selective catalysis, pollutant removal, gas storage, energy device, and even antibacterial agents [3,4,5,6]. However, different from conventional porous materials, the unmatched combination between metal ions and organic ligands endows MOFs the unprecedented diversities in compositions, structures and topologies, which also provide vast opportunities for tailored design of MOF-based catalysts toward specific reactions [7]. Thanks to their three-dimensional ordered structure, MOFs can ensure uniform catalytic sites either on metal ions or organic linkages, helping improve the reaction selectivity with minimal side reactions. The intrinsic porosity and large surface area with MOFs render efficient contact between substrates and catalytic sites, boosting the reaction turn-over-frequency (TOF). Moreover, the catalytic activity and selectivity of MOFs towards various reactions can be readily tuned by pre-synthesis or post-modification of MOFs [8]. Besides, heterogeneous MOFs catalysts with thermal and chemical robustness can be easily separated from the products, and repeatedly used with almost constant catalytic performance. In short, MOFs perfectly merge the advantages of both heterogeneous and homogeneous catalysts.
Symmetrical and unsymmetrical biaryls are important structural motifs widely found in pharmaceuticals, dyes and organic semi-conductor molecules [9,10,11,12], which are typically prepared through palladium-catalyzed aryl C–C (sp2-sp2) coupling reactions [13, 14] such as Suzuki reaction, and Hiyama-Kumada reaction. Despite their high efficiency, these reaction methods suffer from some limitations including the use of expensive palladium catalysts, the need of stoichiometric oxidants to complete the catalytic cycle, and relatively rigorous reaction conditions (e.g. high temperature) [15,16,17,18]. To this point, copper-based MOFs with open metal sites (OMSs) have emerged as a promising class of heterogeneous catalysts for such type organic transformation. For example, Yaghi et al. reported the Cu3(BTC)2 (BTC = 1,3,5-Benzenetricarboxylic acid) catalyzed homocoupling of arylboronic acids with the assistance of external cyclohexylamine base [19]. Pitchumani et al. demonstrated that copper-terephthalate MOF is an efficient, environmentally-benign and reusable heterogeneous catalyst that can achieve the aerobic homocoupling transformation of arylboronic acids to symmetrical biaryls under mild conditions [20]. The reaction mechanism is proposed to involve transmetallation between Cu (II) OMSs and arylboronic acid to generate Ar-Cu(II)-Ar intermediate, which further undergoes aerobic oxidation and reductive elimination to generate the final biaryl product.
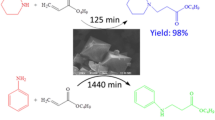
Encouraged by these findings, we herein utilized a copper-based MOF (1) constructed by the L-NO2 ligand to catalyze the aerobic homocoupling arylboronic acids toward biaryls (Scheme 1). As reported in our previous report [21], MOF (1) is constructed by paddle-wheel Cu2(CO2)4 nodes separated by L-NO2 linkages, which shows one-dimensional channels lined with open Cu(II) coordination sites (occupied by removable water molecules). This current work reveals that MOF (1) exhibits a pronounced size-selective effect toward aerobic homocoupling reaction of arylboronic acids, which is only effective for those arylboronic acids with short molecule length (e.g. phenylboronic acid, p-methylphenylboronic acid, and p-fluorophenylboronic acid).
2 Experimental
2.1 Materials and Methods
All reagents and solvents were commercially available and used as received. MOF (1) was synthesized according to our reported method [21]. Powder X-ray diffraction (PXRD) data were recorded on XRD diffractometer (Ultima IV, Rigaku Corporation) with Cu Kα radiation (λ = 1.54056 Å). 1H-NMR spectrum was performed on a Bruker Avance DPX 500 MHz spectrometer using DMSO-d6 solution.
2.2 Synthesis of MOF (1)
0.1 mmol of Cu(NO3)2·3H2O, 0.05 mmol of L-NO2 and three drops of fluoroboric acid were added into 11 mL of mixed solvent comprising H2O-DMF-CH3CN (in 8:2:1 volume ratio), and sealed in a Parr Teflon-lined stainless steel vessel (25 mL). The mixture was heated at 120 °C for 3 days. Upon cooled to room temperature at a rate of 5 °C/h, crystals of MOF (1) were collected by filtration, washed with CH3CN and dried in air. Yield 53% (based on L-NO2 ligand).
2.3 Aerobic Homocoupling of Arylboronic Acids
Under the air condition, 12 mg of MOF (1), and arylboronic acid (2 mmol) were added into 2 mL of DMF, and stirred at room temperature for 18 h. The suspended MOF (1) was collected by filtration, and the filtrate was added with 50 mL of water followed by extraction with DCM. The organic phase was separated, and dried over anhydrous Na2SO4. The homocoupling product was obtained by flash chromatography over silica gel with the petroleum as the eluent, and structurally confirmed by 1H-NMR spectra (Fig. S1–S3).
3 Results and Discussion
Copper-based MOF (1) was obtained as green crystal powders under the solvothermal condition according to our previous report [21], which shows identical PXRD patterns with the simulated one from crystal data (Fig. S4). At the beginning, aerobic homocoupling of phenylboronic acid was selected as the model reaction for initial evaluation of the catalytic activity of MOF (1). The coupling reaction was conducted in DMF solvent under the air circumstance at room temperature, and the experimental results are summarized in Table 1. Satisfyingly, an 85% yield of 1, 1′-biphenyl was achieved upon chromatography separation (Entry 4). The reaction time has a profound effect on the reaction yield (Entry 5). When the reaction time is prolonged from 8 to 18 h, the yield dramatically increases from 30 to 85%. The optimal period for this reaction is 18 h, beyond which little enhancement is gained on the output. Besides, the catalyst amount is another important factor influencing the reaction yield (Entry 6). When the catalyst dosage increases from 6 to 10 mg, the reaction yield jumps from 40 to 80%. Further improving the catalyst loading, only a small enhancement is resulted in the yield. Finally, the optimized catalyst amount is set to 12 mg with respect to 2 mmol of phenylboronic acid, which achieves an 85% yield. Furthermore, three control experiments were carried out (Entries 1–3) to convince the catalytic role of MOF (1) in this reaction. It discloses that the coupling reaction is unable to happen in the presence of copper salt, or L-NO2 ligand, or air alone. These control experiments unambiguously point the catalytic importance of the coordination assembly between Cu (II) ion and L-NO2 ligand. Meanwhile, the contrast experiment also confirms that oxygen is indispensable to this reaction since no reaction takes place under the N2 atmosphere (Entry 7). The effect of solvents on this reaction was also screened (Entries 8–16), revealing that this reaction can only work in the DMF solvent. The solvent-dependence on this reaction agrees well with the literature result [20], which has been in part accounted by the better solubility of these solvents for the arylboronic acids which significantly slow their binding to the metal sites for effective homocoupling. Finally, comparison between MOF (1) and some typical Cu (II)-based MOFs for aerobic homocoupling of phenylboronic acid is summarized in Table S1.

In order to verify the heterogeneous nature of MOF (1) in this reaction, the Sheldon test was subsequently performed (Fig. 1). The reaction was conducted under the optimal condition. After reaction for 8 h, the catalyst was isolated from the reaction through filtration, and the filtrate was further stirred for additional 10 h. It was found that the yield of 1, 1′-biphenyl product is barely changed, proving the heterogeneous nature of MOF (1). The reusable performance of MOF (1) is pertinent to its practical use, and the recycling experiments demonstrate that after the first three runs the catalytic performance of MOF (1) keeps almost unchanged in terms of the reaction yield. After five recyclable runs, the reaction yield only slightly drops from 85 to 74% (Fig. 1). Particularly, the PXRD pattern of MOF (1) remains intact after five runs, suggesting its structure robustness (Fig. 1).
Given that phenylboronic acids with ortho-substituent are not good candidates for this reaction due to the steric hindrance which impedes the formation of Cu-aryl radical intermediate [20], some normal phenylboronic acids with different para-substituent were further selected for MOF (1)-catalyzed homocoupling reaction (Table 2). Under the optimal condition employed for homocoupling phenylboronic acid, the reaction remains effective for p-methylphenylboronic acid and p-fluorophenylboronic acid, giving the coupling product in good yields (Table 2, Entries 1–2). Surprisingly, no reaction is observed for phenylboronic acids with those para-substituents including –CHO, –CN, –OCH3, –COOEt) (Table 2, Entries 3–6). On the basis of the proposed mechanisms in literatures, it involves the formation of Cu (III)-Ar intermediate followed by the formation of homocoupling product through reduction-elimination mechanism [20]. In our case, the open Cu (II) metal sites in MOF (1) reside within the 1-D channels, protruding toward the interior [21]. Therefore, for successfully effecting the homocoupling reaction, the arylboronic acid molecule should first diffuse into the 1-D channel, then interact with the Cu (II)-metal open site, and finally generate the key Cu (III)-Ar intermediate with the assistance of oxygen. Besides, it has proven that the electronic nature of the para-substituent on phenylboronic acid has little effect on the reaction efficiency [20]. Accordingly, in our case we attributed the stark reactivity difference of MOF (1) toward these phenylboronic acids with different para-substituents to the size effect, that is, the dimension of 1-D channel in MOF (1) restricts the entrance of those substrates with a long molecular length, thus limiting the cascade steps toward the final homocoupling product (Scheme 1). To our knowledge, the size effect is not evident in the reported copper-terephthalate-MOF-mediated coupling reaction of arylboronic acids [20], and the current work represents a rare copper-based MOF showing the size effect toward the coupling substrate [22].
In conclusion, a 3-D copper-based MOF (1) with open metal sites lining with the 1-D channels can promote the aerobic homocoupling reaction of phenylboronic acid to generate the symmetrical biphenyl in a good yield. Particularly, it shows pronounced size selectivity which is only effective for short para-substituted phenylboronic acids. The current work represents an interesting example of size-selective aerobic homocoupling reaction of arylboronic acids mediated by a copper-based MOF catalyst.
Data Availability
All data generated or analysed during this study are included in this article [and its supplementary information files].
References
V. Pascanu, G.G. Miera, A.K. Inge, B. Martin-Matute, J. Am. Chem. Soc. 141, 7223 (2019)
A. Bavykina, N. Kolobov, I.S. Khan, J.A. Bau, A. Ramirez, J. Gascon, Chem. Rev. 120, 8468 (2020)
D.W. Bruce, D. Oare, R.I. Walton, Porous Materials (Wiley, Hoboken, 2010)
S. Subhadarshini, E. Pavitra, G.S.R. Raju, N.R. Chodankar, D.K. Goswami, Y.-K. Han, Y.S. Huh, N.C. Das, A.C.S. Appl, Mater. Interfaces 12, 29302 (2020)
S. Subhadarshini, E. Pavitra, G.S.R. Raju, N.R. Chodankar, A. Mandal, S. Roy, S. Mandal, M.V.B. Rao, D.K. Goswami, Y.S. Huh, N.C. Das, Ceram. Int. 47, 15293 (2021)
S. Subhadarshini, R. Singh, D.K. Goswami, A.K. Das, N.C. Das, Langmuir 35, 17166 (2019)
L. Jiao, J.Y.R. Seow, W.S. Skinner, S. William, Z.U. Wang, H.L. Jiang, Mat. Today. 27, 43 (2018)
T. Islamoglu, S. Goswami, Z.Y. Li, A.J. Howarth, O.K. Farha, J.T. Hupp, Account Chem. Res. 50, 805 (2017)
Y. Liao, W. Hung, T. Hou, C. Lin, K. Wong, Chem. Mater. 19, 6350 (2007)
J. Hassan, M. Sevignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 102, 1359 (2002)
B. Yuan, Y. Pan, Y. Li, B. Yin, H. Jiang, Angew. Chem. Int. Ed. 49, 4054 (2010)
D. Lee, J.H. Kim, B.H. Jun, H. Kang, J. Parkand, Y.S. Lee, Org. Lett. 10, 1609 (2008)
A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.M. Basset, V. Polshettiwar, Chem. Soc. Rev. 40, 5181 (2011)
N.S.V. Stanley, J.S. Reis, I.M. de Oliveira, M.N. Balfour, H.A. Stefani, Tetrahedron 75, 1865 (2019)
M. Shimizua, I. Nagao, Y. Tomioka, T. Kadowaki, T. Hiyama, Tetrahedron 67, 8014 (2011)
S.Y. Xu, Y.B. Ruan, X.X. Luo, Y.F. Gao, J.S. Zhao, J.S. Shen, Y.B. Jiang, Chem. Commun. 46, 5864 (2010)
S.R. Cicco, G.M. Farinola, C. Martinelli, F. Naso, M. Tiecco, Eur. J. Org. Chem. 2010, 2275 (2010)
A. Prastaro, P. Ceci, E. Chiancone, A. Boffi, G. Fabrizi, S. Cacchi, Tetrahedron Lett. 51, 2250 (2010)
O. M. Yaghi, A. U. Czaja, B. Wang, Z. Lu, US Patents, 20120130113 (2012)
P. Puthiaraj, P. Suresh, K. Pitchumani, Green Chem. 16, 2865 (2014)
X.F. Yang, X.L. Chen, H.B. Zhu, Y. Shen, Polyhedron 157, 367 (2019)
S. Parshamoni, J. Telangae, S. Sanda, S. Konar, Chem. Asian J. 11, 540 (2016)
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (NSFC) (Grant No. 21171036), and the Fundamental Research Funds for the Central Universities (Grant No. 3207047406).
Funding
All funding information has been listed in the acknowledgement section.
Author information
Authors and Affiliations
Contributions
X-FY and L–LZ performed the experiment. H-BZ is responsible for data analysis and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
We have no any conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, XF., Zhang, LL. & Zhu, HB. Size-Selective Homocoupling of Arylboronic Acids Mediated by a Copper-Based Metal–Organic-Framework. J Inorg Organomet Polym 31, 4623–4627 (2021). https://doi.org/10.1007/s10904-021-02056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02056-4