Abstract
Two luminescent coordination polymers (LCPs), namely [CdL2]n (1) and [ZnL2]n (2) based on a new ligand 2-(2-methyl–benzimidazole-1-yl)-benzoic acid (HL), have been synthesized and characterized by single crystal X-ray diffraction, powder X-ray diffraction, infrared spectroscopy, elemental analysis, and thermogravimetric analysis. 1 and 2 exhibit similar 1D looped chains with different coordination modes of L. Both LCPs show strong fluorescent emission in the visible region. The fluorescence quenching response of 1 and 2 towards Fe3+ over other metal ions in DMF has been demonstrated by quenching titrations. Thus, 1 and 2 can be used as potential and highly selective luminescent sensors for Fe3+ ions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Iron is one of the most important elements in the metabolic process for all living system [1]. The deficiency or excess of the iron element relative to the normal limits could induce serious diseases, such as Alzheimer’s, Huntington’s or Parkinson’s disease [2]. The considerable importance of iron in biological systems has promoted the development of methods to selectively detect and quantify Fe3+. However, most traditional analytical techniques for detecting Fe3+, including spectrophotometry, atomic absorption spectrometry and chromatography, are time-consuming, labor-intensive or indirect-viewed [3, 4]. In recent years, luminescent chemical sensors have emerged as reliable alternatives for detection of metal ions, [5,6,7] which are technologically friendly, easy to integrate to the existing infrastructures, have a low detection limit, and can selectively identify targeted molecules. In particularly, they have potential applications in analytical chemistry and biomedical diagnostic [8,9,10,11].
The ability of luminescent coordination polymers (LCPs) to propagate the host guest interaction to detectable changes as a function of their luminescence makes them promising candidates for sensing metal ions. LCPs have several advantages including simple one-pot and straightforward synthesis, remarkable structural tailorability and functionalizability as well as their enormous practical applications, such as biomedical assays and time-resolved microscopy, fluorescent lighting, and luminescent sensing for chemical species [7, 10, 12, 13]. Therefore, efforts have been made to cultivate LCPs to detect metal ions and explore sensing mechanisms [14,15,16]. Generally, the detection process of metal ions is primarily based on fluorescence quenching via donor–acceptor electron transfer, resonance energy transfer or structural damage mechanism [17,18,19].
It is well-known that many benzimidazole and its deriatives have been used as efficient fluorophores, which have high fluorescence quantum yields, large Stokes shifts, and excellent coordination ability with transition metals [20]. For example, according to Barnard, benzimidazole derivatives can be used as carbene precursors to construct carbene complexes with good fluorescent properties [21]. Moreover, benzimidazole and its deriatives can also be considered as ligands to build LCPs. Zhang’s group has reported Cu(I) compounds, in which the alteration of Cu···Cu and π···π interactions in the structure, may lead to luminescent changes [22]. The luminescence of Mg-based and Zn-based LCPs reported by Zheng occurs a significant red shift compared with ligand, which is probably due to the ligand-to-metal charge-transfer (LMCT) transition [23]. Based on these reasons, the 2-(2-methyl–benzimidazole-1-yl)-benzoic acid (HL) ligand is selected to build LCPs with the following characteristic features: (1) carboxylic group easily participate in coordination, and form various complexes with variable coordination modes; [24] (2) N atoms from benzimidazole moiety also can coordinate with metal ions by providing lone electron pairs; [25] (3) there is a large steric hindrance between carboxylic group and methyl from o-substituted 2-methyl-benzimidazole moiety, so there exist a relatively large twist angle; [26] (4) large π electronic systems will change the fluorescence properties; [27] (5) up to now, this ligand has not been investigated to construct complexes. Herein, based on HL, we report two 1D looped LCPs, namely, [CdL2]n (1) and [ZnL2]n (2). The crystal structures, infrared (IR) spectra, thermogravimetric analysis (TGA), luminescent spectra and fluorescent sensing for Fe3+ were investigated. Both LCPs exhibit high thermostability up to 330 and 350 °C, respectively. The most striking phenomenon is that Fe3+ ion causes a very significant quenching effect on luminescence of 1 and 2. Thus, 1 and 2 can be used as potential and highly selective luminescent sensors for Fe3+ ions. In addition, a possible sensing mechanism for both LCPs is also discussed.
2 Experimental
2.1 Materials and Equipment
All chemicals were available commercially (HL was purchased from Jinan Henghua Science & Technology Co., Ltd.) and utilized without further purification. The C, H and N microanalyses were carried out with a Perkin–Elmer 2400 C elemental analyzer. IR spectrum was recorded from KBr pellets in the 4000–400 cm−1 range on a Nicolet Avatar 360 FTIR spectrometer. TGA were performed on a Netzsch TG209F3 equipment (30–700, 10 °C min−1, N2 gas flow). The powder X-ray diffraction (PXRD) was recorded in the angular range of 2θ = 5–50° with a Bruker D8 ADVANCE X-ray powder diffractometer (Cu-Kα, 1.5418 Å). Luminescence spectra for the solid samples and liquid samples were taken with a Perkin–Elmer LS55 luminescence spectrometer. UV–Vis spectroscopic studies were measured on a Hitachi U-3310 spectrometer.
2.2 Syntheses
2.2.1 Preparation of [CdL2]n (1)
A mixture of the HL (0.0252 g, 0.1 mmol) and Cd(OAc)2·2H2O (0.0131 g, 0.05 mmol) in NMP (N-methyl-2-pyrrolidone)/H2O (2/8, 10 mL) was placed in a Parr Teflon-lined stainless steel vessel and heated to 105 °C for 3 days. After the mixture was cooled to room temperature at the rate of 0.1 °C min−1, the colorless crystals of 1 were collected to give 68% yield based on HL. Anal. Calcd for 1 (Mr = 307.47 g mol−1): C, 58.59; H, 3.57; N, 9.10%. Found: C, 58.52; H, 3.49; N, 9.06%. IR (cm−1, KBr pellet): 3436 (w), 1593 (s), 1556 (s), 1500 (m), 1457 (w), 1390 (s), 1324 (w), 1032 (w), 845 (w), 745 (m).
2.2.2 Preparation of [ZnL2]n (2)
A mixture of the HL (0.0252 g, 0.1 mmol) and Zn(OAc)2·2H2O (0.0110 g, 0.05 mmol) in CH3CN/H2O (2/8, 10 mL) was placed in a Parr Teflon-lined stainless steel vessel and heated to 120 °C for 3 days. After the mixture was cooled to room temperature at the rate of 0.1 °C min−1, the colorless crystals of 2 were collected to give 36% yield based on HL. Anal. Calcd for 2 (Mr = 283.94 g mol−1): C, 63.39; H, 3.87; N, 9.86%. Found: C, 63.32; H, 3.85; N, 9.80%. IR (cm−1, KBr pellet): 3450 (w), 1629 (s), 1500 (m), 1457 (w), 1405 (w), 1354 (s), 1032 (w), 845 (w), 742 (m).
2.3 X-ray Crystallography
Crystallographic data of the LCPs were collected on a Bruker SMART APEX II CCD diffractometer equipped with a graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) at room temperature. The structures were solved by direct methods and refined on F 2 via the full-matrix least-squares technique by applying the program SHELX-97 [28, 29]. All the non-hydrogen atoms have suffered from the anisotropic refinement. And all hydrogen atoms were located in the architectural calculation at desired positions with a riding model and refined isotropically. Crystallographic data and experimental details for structural analyses are shown in Table 1. Selected bond lengths and bond angles are listed in Table 2. The CCDC: 1521923 and 1521924 for 1 and 2.
3 Results and Discussion
3.1 Crystal Structure Description
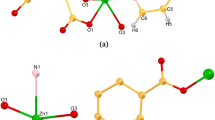
1 crystallizes in the orthorhombic pccn space group, showing a 1D looped chain. There are a half of Cd(II) ions, one L ligand in the asymmetric unit (Fig. 1a). The six-coordinated Cd(II) locates in a distorted octahedral coordination environment [CdN2O4], tightly bonded by four O atoms from two chelate coordinated carboxylic groups of two L ligands and two N atoms from another two L ligands. The Cd–O bond lengths are 2.261(3) and 2.516(3) Å and Cd–N bond length is 2.320(3) Å, which are in accordance with those in other related articles [30]. Each Cd(II) ion is bonded to two adjacent Cd(II) ions through two pairs of bridging L, to constitute an infinite
a The coordination environment around Cd(II) ion in (1) Symmetry mode: A = 1/2−x, 1/2−y, z. B = x, 1/2−y, 1/2 + z. b The coordination environment around Zn(II) ions in (2) Symmetry mode: A = x, 3/2−y, 1/2 + z. B = 1/2−x, y, 1/2 + z. C = 1/2−x, 3/2−y, z c View of the 1D looped chain extending along the b axis. Cd, green; C, yellow; N, blue; O, red. d The 3D supramolecular framework formed by C–H···C hydrogen bond
1D double chain parallel to c axis (Fig. 1c) with the neighboring interchain Cd···Cd separation being 7.144(3) Å. A 3D supramolecular architecture constructed from the 1D chain is stabilized by the hydrogen bonding interactions C9–H9···C9 and C15–H15···C15 (Fig. 1d). The dihedral angle between benzene ring and benzimidazole moiety is ca. 70° (Fig. S1).
The single crystal X-ray analysis reveals that structures of 1 and 2 are similar. However, some differences still exist. In 2, each Zn(II) ion is four-coordinated with two monodentate coordinated carboxylic oxygens and two benzimidazole nitrogen donors from four distinct L ligands to furnish a distorted tetrahedral coordination geometry [ZnN2O2] (Fig. 1b). The reasons for the change of coordination modes of carboxyl from chelate in 1 to monodentate in 2 may include: (1) Zn···O2 separation (2.867(2) Å) exceeds the Zn–O normal distance [31, 32]. (2) The radius of Cd(II) is larger than that of Zn(II), thus Cd(II) can connect more active atoms, resulting in different coordination modes. The Zn–O and Zn–N bond lengths are 1.949(3) and 2.065(3) Å, well comparable to the reported values [33]. The neighboring interchain Zn·Zn separation is 7.255(2) Å. The dihedral angle between benzene ring and benzimidazole moiety is ca. 72° (Fig. S1).
3.2 The PXRD and IR Spectra
PXRD patterns for 1 and 2 were measured on bulk crystalline powders to identify the phase purity of the product. The experimental PXRD patterns of 1 and 2 are in good agreement with simulated ones, respectively, demonstrating the consistency of as-synthesized bulk samples and the testing single crystals (Fig. S2). The discrimination of intensity might be owing to the powder samples’ preferred orientation. The IR spectra of 1 and 2 display the characteristic asymmetric (νas) and symmetric (νs) stretching vibrations of the carboxylate groups (Fig. S3). The difference (Δ = νas−νs) between νas and νs has been widely used as a diagnosis of the coordination modes of the carboxylate group. In 1 and 2, the νas(COO−) vibration appears at 1593 and 1629 cm−1, and νs(COO−) vibration appears at 1393 and 1354 cm−1, respectively. The intermediate Δ value [200 cm−1 (1) and 275 cm−1 (2)] is consistent with the chelating and monodentate coordination modes of the carboxylate group [34]. Absorption bands at 1500 cm−1 are due to C=C of ligand stretching vibrations. In addition, the spectra of the 1 and 2 show bands at 1032 and 1457 cm−1 due to the C–N and C=N stretching modes of the benzimidazole-1-yl ligands [35]. The absence of any strong absorption bands around 1720–1690 cm−1 confirms the complete deprotonation of the carboxyl groups of the HL ligands during the reactions [36].
3.3 Thermogravimetric Analysis
TGA were performed to determine the thermal stability of 1 and 2. The TGA was carried out in the temperature range of 30–700 °C under a flow of nitrogen atmosphere with a heating rate of 10 °C min−1 (Fig. 2). TGA curves of 1 and 2 had no obvious weight loss before 330 and 350 °C, respectively, indicating high thermostability. And then an obvious weight loss of 14.40% for 1 (15.39% for 2) from 330 to 370 °C (from 350 to 490 °C for 2) were observed, corresponding to the release of carboxyl moiety (14.31% for 1, and 15.49% for 2). The further heating led to the structural collapse, while the remaining weight corresponds to CdO for 1 (obsd 41.72%, calcd 41.76%). For 2, the remaining weight observed was 66.04%, which was more than 28.66% (the residue is ZnO) less than 78.93% (the residue is Zn3N2), corresponding to mixture of ZnO and Zn3N2, but the specific ratio of mixture cannot be obtained because of limited ability.
3.4 Luminescent Properties
The excitation and emission spectra of LCPs 1, 2 and ligand in the solid-state at room temperature are shown in Fig. 3. It can be seen that ligand display an intense emission band at 415 nm upon excitation at 355 nm, which may be attributed to the π*–n or π*–π transition as previously reported [37]. Interestingly, 1 and 2 exhibit the maximum emission at 435 nm, excited at 340 nm. Compared to the ligand, 1 and 2 indeed result in a large red shift of 20 nm for maximum emission, which indicates that the emission of 1 and 2 at 435 nm may have originated from ligand to metal charge–transfer transitions [38,39,40]. The red shift can be attributed to coordination of the ligands to metal ions which effectively increases the rigidity of the ligands and reduced the loss of energy via radiation less thermal vibration decay [41,42,43].
The crystalline samples of 1 and 2 are stable in air and insoluble in common solvents such as DMF, DMA, NMP, CH2Cl2, CH3OH, CH3CH2OH, DMSO, CH3CN and H2O. In order to examine the potential application of 1 and 2 in sensing cations, 1/2 (5 mg) was immersed in DMF solutions (3 mL) of metal ions (5 mg, Zn(NO3)2, Cd(NO3)2, Pb(NO3)2, Ca(NO3)2, NaNO3, Co(NO3)2, Mn(NO3)2, Ni(NO3)2 and Fe(NO3)3), treated by ultrasonication for 30 min, and then aged for 1 h to generate stable suspensions before the fluorescence study. Naturally, the luminescent properties of 1 and 2 in different metal ions suspensions are measured. Interestingly, it was found that Cd2+, Zn2+, Pb2+ ions slightly enhanced luminescence intensity of 1 and 2, while Na+, Ca2+ ions had a trivial effect on the luminescence intensity, other metal ions (Co2+, Ni2+, Mn2+) decreased luminescence to a different extent. The most striking phenomenon is that Fe3+ ion can almost completely quench to the system (Fig. 4a). The obvious change of luminescent intensities implies that 1 and 2 can be considered as a promising candidate for selective probing of Fe3+. The PXRD patterns conform that the framework of 1 and 2 immersed in metal ion solutions remains unchanged (Fig. S4). Fe3+ shows strong absorption from 200 to 450 nm, which has an overlap with the emission spectra of 1 and 2, indicating the existence of resonance energy transfer (Fig. S5). As a result, the quenching mechanism caused by Fe3+ mainly depends on resonance energy transfer [44].
a Room-temperature luminescent intensity of 1 and 2 at 435 nm in DMF suspension upon addition of various metal ions. b Fluorescence spectra of 1 (left) and 2 (right) in DMF with increasing amounts of Fe3+. Insets: the linear correlation for the plot of I 0/I vs concentration of Fe3+ ions in low concentration range
To assess the sensitivity of 1 and 2 toward Fe3+ in detail, varying concentrations of Fe3+ were introduced into emulsions of 1 and 2 dispersed in DMF and the emissive responses were monitored. The emission intensities decrease gradually with increasing Fe3+ concentrations (Fig. 4b). The plot of I 0/I vs concentration of Fe3+ ion does not match with the Stern–Volmer equation, indicating the coexistence of the dynamic and static quenching processes, [45, 46] which can be well fitted by I 0/I = 5.22 × exp (c/0.74)–5.38 for 1 and I 0/I = 1.63 × exp (c/0.61)–0.55 for 2 (I 0 and I are the luminescent intensity of 1 and 2 in the absence and presence of Fe3+, respectively; and c is the molar concentration of Fe3+) (Fig. S6). Thereby, the quenching process completely depends on the concentration of Fe3+ ion. Meanwhile, this function in the range of 0–0.5 mmol/L conforms to a good linear correlation. As shown in Fig. 4b, the fluorescence intensity vs [Fe3+] insert plot can be linear–fitted into I 0/I–1 = Ksv[Fe3+] – 0.12 for 1 and I 0/I–1 = Ksv[Fe3+]–0.11 for 2, closed to the Stern–Volmer equation: I 0/I–1 = Ksv[M]. ([M] is the metal ion molar concentration; Ksv is the quenching efficiency to quantitatively evaluate the performance of LCPs as sensor.) Base on the experimental data, the Ksv value is calculated to be 4106.81 L mol for 1 and 3945.98 L mol for 2. Both Ksv values are comparable to those in well-designed solution base organic complexes for sensing of Fe3+ (typical Ksv of about 104 L/mol) [47]. The results indicate that 1 and 2 can be used as promising luminescence sensors for Fe3+ ions.
4 Conclusion
In summary, under solvothermal conditions, we have successfully synthesized two new 1D looped LCPs 1 and 2 with different coordination modes of carboxyl based on 2-(2-methyl–benzimidazole-1-yl)-benzoic acid (HL) ligand. Both LCPs are thermally stable up to 330 and 350 °C, and exhibit strong luminescence emissions in the solid state and in a DMF suspension at room temperature. The experimental results and data show that 1 and 2 display high selectivity and quantitative detection for Fe3+ through fluorescence quenching, suggesting that both LCPs may be used as luminescent probes for Fe3+.
References
M. Zheng, H.Q. Tan, Z.G. Xie, L.G. Zhang, X.B. Jing, Z.C. Sun, Appl. Mater. Interfaces 5, 1078–1083 (2013)
Y.H. Hung, A.I. Bush, R.A. Cherny, J. Biol, Inorg. Chem. 15, 61–76 (2010)
Z.O. Tesfaldet, J.F. van Staden, R.I. Stefan, Talanta 64, 1189–1195 (2004)
A. Ohashi, H. Ito, C. Kanai, H. Imura, K. Ohashi, Talanta 65, 525–530 (2005)
Y. Rachuri, B. Parmar, K.K. Bishta, E. Suresh, Dalton Trans. 45, 7881–7892 (2016)
X. Shen, B. Yan, RSC Adv. 6, 28165–28170 (2016)
L. Wang, Z.Q. Yao, G.J. Ren, S.D. Han, T.L. Hu, X.H. Bu, Inorg. Chem. Commun. 65, 9–12 (2016)
L.L. Wen, X.F. Zheng, K.L. Lv, C.G. Wang, X.Y. Xu, Inorg. Chem. 54, 7133–7135 (2015)
X.D. Wang, O.S. Wolfbeis, R. Meier, Chem. Soc. Rev. 42, 7834–7869 (2013)
Z.C. Hu, B.J. Deibert, J. Li, Chem. Soc. Rev. 43, 5815–5840 (2014)
J. Rocha, L.D. Carlos, F.A.A. Paz, D. Ananias, Chem. Soc. Rev. 40, 926 (2011)
X.D. Wang, O.S. Wolfbeis, R.J. Meier, Chem. Soc. Rev. 42, 7834–7869 (2013)
L. Basabe-Desmonts, D.N. Reinhoudt, M. Crego-Calama, Chem. Soc. Rev. 36, 993–1017 (2007)
Y. Xiao, Y. Cui, Q. Zheng, S. Xiang, G. Qian, B. Chen, Chem. Commun. 46, 5503–5505 (2010)
Z. Hao, X. Song, M. Zhu, X. Meng, S. Zhao, S. Su, W. Yang, S. Song, H. Zhang, J. Mater. Chem. A 1, 11043–11050 (2013)
J.M. Zhou, W. Shi, H.M. Li, H. Li, P.J. Cheng, Phys. Chem. C. 118, 416–426 (2014)
H.B. Xu, S.H. Zhou, L.L. Xiao, H.H. Wang, S. Li, Q.J. Yuan, Mater. Chem. 3, 291–297 (2015)
P.Y. Wu, Y.H. Liu, Y. Liu, J.R. Wang, Y. Li, W. Liu, J. Wang, Inorg. Chem. 54, 11046–11048 (2015)
B.B. Kang, N. Wei, Z.B. Han, RSC Adv. 5, 1605–1611 (2015)
F.A.S. Chipem, S.K. Behera, G. Krishnamoorthy, Sens. Actuators B 191, 727–733 (2014).
G.J. Barbante, P.S. Francis, C.F. Hogan, P.R. Kheradmand, D.J.D. Wilson, P.J. Barnard, Inorg. Chem. 52, 7448–7459 (2013)
T. Wen, D.X. Zhang, J. Liu, R. Lin, J. Zhang, Chem. Commun. 49, 5660–5662 (2013)
J. Li, C. C. Ji, L. F. Huang, Y. Z. Li, H. G. Zheng, Inorg. Chem. Acta 371, 27–35 (2011)
L.M. Fan, W.L. Fan, B. Li, X.Z. Liu, X. Zhao, X.T. Zhang, RSC Adv. 5, 14897–14905 (2015)
J. Yang, G.D. Xie, X.F. Chen, D. Wu, X.M. Lin, G. Zhang, Y.P. Cai, CrystEngComm 17, 1326 (2015)
Q.Q. Li, Y.F. Kang, C.Y. Ren, G.P. Yang, Q. Liu, P. Liu, Y.Y. Wang, CrystEngComm 17, 775 (2015)
T. Alexander, P. Alexander, M. Stefan, W. Gerhard, M. Ingo, S. Thomas, Inorg. Chem. 53, 6346–6356 (2014)
G.M. Sheldrick, SHELXL–97, Program for the Refinement of the Crystal Structures, University of GÖttingen, Göttingen, (1997)
G.M. Sheldrick, SHELXTL Reference manual version 5.1 (Bruker AXS), Madison (1997)
H. W. Kuai, X. C. Cheng, X. H. Z. Zhu, Naturforsch 68b, 789–796 (2013)
T. Li, X.J. Hong, X. Liu, R. Chen, Q.G. Zhan, X. Xu, Y.P. Cai, CrystEngComm 16, 3883 (2014)
R. Mishra, M. Ahmad, M. RTripathi, Polyhedron 50, 22–30 (2013)
S. Mukherjee, D. Samanta, S.P. Mukherjee, Cryst. Growth Des. 13, 5335–5343 (2013)
R. Luo, H. Xu, H.X. Gu, X. Wang, Y. Xu, X. Shen, W.W. Bao, D.R. Zhu, CrystEngComm 16, 784–796 (2014)
D. Deya, S. Roya, R.N.D. Purkayastha, R. Pallepogu, P. McArdle, J. Mol. Struct. 1053, 127–133 (2013)
W.L. Liu, J.H. Yu, J.X. Jiang, L.M. Yuan, B. Xu, Q. Liu, B.T. Qu, G.Q. Zhang, C.G. Yan, CrystEngComm 13, 2764 (2011)
R. Mishra, M. Ahmad, M.R. Tripathi, Polyhedron 50, 22–30 (2013)
H. Erer, O.Z. Yeşilel, M. Arıcı, Cryst. Growth Des. 15, 3201–3211 (2015)
Z. Zhang, J.F. Ma, Y.Y. Liu, W.Q. Kan, J. Yang, Cryst. Growth Des. 13, 4338–4348 (2013)
Z.M. Ju, W. Yan, X.J. Gao, Z.Z. Shi, T. Wang, H.G. Zheng, Cryst. Growth Des. 16(5), 2496–2503 (2016)
J.X. Ma, X.F. Huang, X.Q. Song, S.W. Liu, Chem. Eur. J. 19, 3590–3595 (2013)
K. Jayaramulu, R.P. Narayanan, S.J. George, T.K. Maji, Inorg. Chem. 51, 10089–10091 (2012)
M.D. Allendorf, C.A. Bauer, R.K. Bhakta, R.J.T. Houk, Chem. Soc. Rev. 38, 1330–1352 (2009)
S.S. Nagarkar, B. Joarder, A.K. Chaudhari, S. Mukherjee, S.K. Ghosh, Angew. Chem. Int. Ed. 52, 2881–2885 (2013)
H. Xu, H.C. Hu, C.S. Cao, B. Zhao, Inorg. Chem. 54, 4585–4587 (2015)
G.P. Li, G. Liu, Y.Z. Li, L. Hou, Y.Y. Wang, Inorg. Chem. 55(8), 3952–3959 (2016)
S.K. Sahoo, D. Sharma, R.K. Bera, G. Crisponi, J.F. Callan, Chem. Soc. Rev. 41, 7195–7227 (2012)
Acknowledgements
This work was supported by the NSF of China (No. 21673173, 21272184, 21572177, 21371142, 21531007, 21103137 and J1210057), the NSF of Shaanxi Province of China (No. 2016JZ004 and 2015JZ003), the Xi’an City Science and Technology Project (No. CXY1511(3)), the Northwest University Science Foundation for Postgraduate Students (No. YZZ14052 and YZZ15040) and the Chinese National Innovation Experiment Program for University Students (No. 201510697004) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, YP., Kang, YF., Liang, XQ. et al. Two 1D Looped Coordination Polymers as Luminescent Probes for Highly Selective Sensing of Fe3+ Ions. J Inorg Organomet Polym 27, 1376–1382 (2017). https://doi.org/10.1007/s10904-017-0591-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0591-z