Abstract
The study aimed to explore with consanguineous couples in Australia the acceptability and perceived utility of whole exome reproductive carrier screening for autosomal recessive and X-linked recessive conditions. Semi-structured interviews with 21 consanguineous couples were conducted prior to the offer of screening. Interviews were coded, and thematic analysis was informed by an inductive approach. Three major themes were identified: experiences and attitudes of Australian consanguineous couples, childhood genetic conditions and beliefs, and the perceived utility of genomic screening. All but one couple had previously sought genetic advice, and a large majority of couples were aware of childhood conditions within their family or community. Thirteen couples perceived consanguinity as increasing the risk of having affected children. Nine spoke of premarital screening programs routinely conducted in their countries of origin. All supported the concept and availability of genomic reproductive carrier screening. Hypothetically, if found to be carriers of a severe childhood disorder, 13 couples reported they would test a pregnancy, and 12 of whom would consider termination of pregnancy or pre-implantation genetic diagnosis. Four couples would not test a pregnancy and two were unsure. A majority of couples would communicate potential at-risk status to family members, although there were some caveats. Fourteen couples chose to have exome screening and reported that they would utilize the results with the goal of preventing childhood conditions. Of these couples, nine (64%) had an affected child but were aware that testing may reveal they were at risk for a child with a different condition and five (71%) without an affected child. While from diverse ethnic and backgrounds, all couples practiced a religion and all but one couple were recruited from the same clinical genetics unit, with a likely higher genetic literacy and bias towards accepting genetic testing. However, the choice made by all couples was reportedly made with consideration of their personal values, their current family situation, and exome testing issues, including fear of incidental findings and concerns about test reliability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consanguinity, a union between genetically related individuals, is common on a global scale, with the majority being first cousin unions, equivalent to a coefficient of inbreeding (F) of 0.0625 (Bennett et al. 2002; Hamamy et al. 2011). An estimated 10.4% of the global population is in, or the product of, a second cousin relationship or closer (Bittles and Black 2010). The incidence of consanguineous marriage has substantial geographical, ethnic, religious, and cultural variability, with population frequencies of 20–50%+ in many regions of North Africa, the Middle East, and South and West Asia (Hamamy et al. 2011; Fareed and Afzal 2017; Bittles and Black 2015). Across Australia, it has been estimated that < 1% of marriages are consanguineous (Bittles 2008). Nevertheless, as in other Western countries (Posch et al. 2012), migration patterns in Australia have resulted in many endogamous communities where consanguineous is traditional and remains favored (Nelson et al. 1997; Port et al. 2005; de Costa 1988).
Autosomal recessive (AR) and X-linked recessive (XLR) conditions contribute substantially to childhood morbidity and mortality. An individual’s carrier status is most frequently identified through the birth of an affected child. Due to inheritance by descent, the likelihood of consanguineous partners being carriers for the same AR condition is higher than that of unrelated partners (Fareed and Afzal 2017; Bittles 2001). In absolute terms, this appears to increase the chance of having a child with a major congenital anomaly from 1–3 to 2–7% (Hamamy et al. 2011; Bittles and Black 2010; Zlotogora and Shalev 2010; Bittles 2012; Becker et al. 2015).
The goal of genome-based reproductive carrier screening is to identify carrier couples prior to having an affected child. Ideally, but not necessarily, the screening would be utilized prior to a first pregnancy. In certain populations, targeted reproductive carrier screening programs already exist for conditions based on population frequency and ancestry (Khorasani et al. 2008; Al-Farsi et al. 2014; Verdonk et al. 2018). High uptake rates of such programs correlate with familiarity of the condition being screened, community elder and religious leader supports, financial subsidization of testing, and basic genetic literacy (Khorasani et al. 2008; Cowan 2009). In several countries, genetic and/or hematological screening of prospective couples is a mandated pre-marital requirement (Cowan 2009; Fallah et al. 2009; Al-Farsi et al. 2014). Depending on the socio-cultural and legal environment, reproductive options may be available to carrier couples (Khorasani et al. 2008; Fallah et al. 2009; Al-Farsi et al. 2014; Hamamy 2012; Zlotogora and Shalev 2014; Henneman et al. 2016).
Despite the demonstrated demand for reproductive carrier screening (Verdonk et al. (2018), until recently, only a handful of the approximately 1700 known AR conditions (Sulem et al. 2015) were amenable to screening (Hamamy 2012; Zlotogora and Shalev 2014). With technical developments leading to shortened turn-around times, lowered costs, and improved accuracy of genomic testing, expanded reproductive carrier screening utilizing exome testing has become a reality (Sallevelt et al. 2017). Regardless of ancestry, this approach can identify couples as being at risk for most known AR conditions, thus providing substantially improved reproductive risk information (Sallevelt et al. 2017).
Genomic testing itself is not without risk. Incidental findings, the possibility of mis-assignment of variant pathogenicity and the emotional and societal impacts of being identified as a carrier couple are all considerations (van El et al. 2013). While Verdonk et al. (2018) explored Dutch Moroccan and Turkish consanguineously married women’s perspectives on reproductive carrier screening and reproductive choices, this is the first study of which we are aware that explores the acceptability and perceived utility of reproductive carrier genomic screening in consanguineous couples.
Subjects and Methods
Recruitment
Couples were recruited from the clinical genetics unit at Liverpool Hospital which provides services to an ethnically diverse population group, and a major tertiary referral hospital, the Sydney Children’s Hospital. Couples were eligible if related at least as second cousins (F ≥ 0.0156), and they were planning further children. A current pregnancy was an exclusion criterion although having a previous pregnancy or child was not. Health interpreters were used as required, and documentation was translated into Arabic. The study was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee (reference no 14/026).
Procedure
Consent was obtained for interview and audiotaping. Demographic data were collected at the start of the interview.
Face-to-face interviews with couples were conducted by the second author, KB-S (a Genetic Counsellor), and/or the last author, EK (a Clinical Geneticist). None of the couples were known to KB-S or EK from previous clinical encounters. A semi-structured interview guide was used to explore their experience of being a consanguineous couple in the Australian community, beliefs about health conditions in children, awareness of genetic conditions and risks associated with consanguinity, and views on genetic testing for carrier status and in pregnancy (Supplementary Material: Interview Schedule for Consanguineous couples). Iterative interviewing enabled data saturation, and all interviews were transcribed verbatim.
Data Analysis
Thematic analysis using an inductive approach was performed through interview transcription, generation of initial codes, refinement of codes, searching for themes, reviewing themes and defining and naming final themes (Braun and Clarke 2006).
Analysis began during data collection, allowing the interview schedule to be expanded and adjusted according to previous interview data. The first author (SJ-T) familiarized herself with the data by reading transcripts several times and making notes, informing the development of a preliminary coding framework. The coding tree was reviewed by KB-S and refined, with any discrepancies in coding discussed and resolved, to arrive at an agreed-upon set of codes. Three de-identified transcripts were independently coded by these authors, informing the final coding framework. Resultant coder reliability was at least 85%. All transcripts were then coded line-by-line and encoded data managed in Microsoft Excel to generate themes with supporting quotes.
Results
Demographics
All but one of the 21 couples interviewed were recruited from Liverpool Hospital; all were interviewed as a couple. Of these, 14 proceeded with genomic testing (Kirk et al. 2018). A further couple, who could not take part in an interview due to a timing issue, was also elected to undergo genomic testing.
Median ages were 27 years for females (range 20–39 years) and 34 years for males (25–46 years) and the average duration of marriage was 8 years. The median number of children per couple interviewed was two, with a total of 37 children born to the 21 interviewed couples (range 0–4 children per couple; 3 children were deceased). Five couples had experienced fetal death in utero at > 20 weeks’ gestation. Thirteen interviews were conducted in English; interpreters facilitated seven interviews in Arabic and one in Urdu. Further demographic information is listed in Table 1.
Themes
Three major themes were identified.
Theme 1: experiences and attitudes of Australian consanguineous couples
Sixteen couples reported consanguinity as being common or viewed as normal within their own community. Two couples had married against the accepted norm of their culture. Reported reasons for marriage were love (n = 4), familiarity prior to marriage (n = 4), or arranged marriage (n = 3). The remaining ten couples did not elaborate on their reasons for marriage.
Experience in Australia
Several couples reported surprise at learning after immigrating that consanguineous marriage was uncommon in Australia. Most treated their consanguinity as private information, choosing to disclose it to either select friends outside of their community or to no one. Where consanguinity was revealed, no overtly negative comments about being in a consanguineous relationship were reported, either from the general Australian community or health professionals.
Even the non-Muslims they accept it. They don’t comment on it in front of us. I don’t know, when I leave they say something? (laughs) They might say it’s weird. I don’t know. From what I understand, mostly from TV, they say that it is weird to marry your cousin, or maybe have a relationship with your cousin. For us, that’s normal, that’s the culture. C012
Concerns about potential for stigma were reported by six couples. One of these feared adverse reactions not only towards themselves, but towards their children.
I didn’t want [to disclose consanguinity]… if I did have healthy children, I didn’t want them to be stigmatized. Even if they were normal, people would think that there was something wrong with them because we are related. And because we’ve had problems in the past. So I wanted to protect our future children potentially. C002
Genetic Information Accessed in the Past
All but one couple had sought genetic advice previously, with some reporting specific reasons, e.g., child affected by a health problem known or suspected to have a genetic cause (n = 10) and for reproductive advice specifically because they were consanguineous (n = 5). Doctors, particularly geneticists, were the reported preferred information source.
Blood Mixing Test
Nine couples originating from Lebanon, Syria, Iran, or Iraq spoke of having had “the test,” a “blood test,” or “the injection” prior to marriage. However, no couple was certain about which conditions were investigated. One couple suspected this included blood group and thalassemia screening; several believed that “everything” was tested for; three reported they believed that a normal test guaranteed healthy children; and three had heard of an injection or medicine that could ensure healthy children.
And we make a small test in our country before we married. They make us our blood, it say, we already have same blood, it doesn’t have a problem. So we sure the baby it will be come out healthy. Otherwise no test find a problem. When the baby come out we find a surprise. C013
[Discussing premarital thalassemia screening in Iran] So if the result is that their blood is not compatible, and they love each other, they marry anyway and then they take pills for five months, for example, that will make their blood more compatible. Maybe one year or two year tablets. C010
Risk Perception
Thirteen couples rated their likelihood of having a child with a genetic condition as higher than the non-consanguineous population. Eight of these couples quantified the increased risk, either correctly at 2–6% per pregnancy (n = 2) or as high risks, ranging from 20–30 to 50–70% (n = 6). Three couples did not think that being consanguineous increased their chance of having a child with a genetic condition.
I don’t think there’s a difference between marrying, like, someone that is your cousin or not your cousin, because then you’ve got to think, there’s people out there that don’t marry from family and from different nationalities and you know, they have problems with their kids. C003
The remainder was unsure as to how consanguinity affected risks.
Back in Iraq, even talking about getting married, you know, family members getting married, that can cause problems with kids. There was no one talked about it. I mean ..... in this country we know more about the risks of getting married to a family member.
Interviewer: How high do you think the risk is? What is the extra risk that comes along with that?
Interviewee: We don’t know. C006
Approximately half of the couples expressed confusion about the role of consanguinity as a potential cause of childhood conditions. Five spoke of non-related couples having affected children, and six couples spoke of consanguineous couples having “all healthy” children.
Well... (pauses), as I see others marrying their cousins and I see others not marrying their cousins and they’re having the similar issue that I’m going through. So what is the truth? … I don’t know. Sometimes I have mixed feelings, sometimes I see that it maybe because my husband and I are cousins. But I see other people, that they have in their family, that they’re not related, and they end up having children that are sick. So, I don’t know. It’s just confusing to me. C009
One couple spoke of the impact of endogamy.
As a Mandaean community our blood is completely pure and goes back ever since history to the Mandaeans. Because no new blood has been introduced into the Mandaean people, no interference, it is pure Mandaean blood.
Interviewer: So do you think that reduces the risks?
Interviewee male: On the contrary. It is more on the increase. C004
Prenatal Events
Two couples had experience with prenatal testing: recurrence of a lethal autosomal recessive condition and aneuploidy. Under medical advice for teratogen exposure, one couple had opted for termination of pregnancy (TOP).
Disclosure
Four of five couples who had children with an established genetic diagnosis (three monogenic; two chromosomal) reported they had told family members of the diagnosis; two of these couples said it was because of the relatives’ increased risk and another couple disclosed the information to their family for support.
One couple, with two children affected by the same rare condition, reportedly did not disclose to their family that it was genetic in origin. The same couple informed friends of the genetic diagnosis, but they did not disclose their consanguinity.
Theme 2: childhood genetic conditions and beliefs
Experience
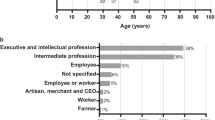
All but one couple had experience of genetic or potentially genetic conditions affecting children: their own children (n = 14), extended family (n = 3), or the wider community (n = 3) (Fig. 1). The remaining couple knew of genetic conditions affecting children but had no direct experience of their own. The spectrum of conditions ranged from perinatally lethal congenital anomalies to mild intellectual disability.
Explanatory Models
As an explanation for the birth of an affected child, four couples proposed the effects of war as a source of stress, either during pregnancy or secondary to religious persecution, subsequent refugee status, and isolation from family and other support systems. The idea of chemicals affecting a developing fetus was advanced by three couples, due to pollution, unclean water, or chemicals released by bombs. Maternal illness in pregnancy and at the time of delivery, as well as potentially teratogenic medications, or conversely not taking antenatal supplements, also was proposed.
My wife when she is pregnant, when the war come she have big shock. Big, big shock. 33 days of scare [war].
Interviewer: It must have been terrifying.
Interviewee female: Yeah it was terrifying. I was in my last [trimester].
Interviewee male: When he born [he] was in stress, like his hands like that, remember? C014
Three couples cited chance, or fate, as a cause of childhood conditions. All of the interviewed couples practiced a religion, and six couples proposed God’s will and being challenged by God as a factor in childhood conditions. However, none expressed disbelief in genetics as a contributory cause.
Mostly if the parents are related, we say it is because they are related. But in other situations, where there is no relations between the parents, we say God have allowed it. C010
Beliefs of Others
Many couples reported it was difficult to comment on how family and friends would explain childhood conditions. The influence of war, stress, chemicals and pollution, fate, and God’s decree were again raised. Poverty, poor hygiene, and chance were suggested. Family and friends were thought less likely to propose consanguinity as a cause than the couples themselves.
Interviewer: So the people in Iraq think perhaps war caused this?
Interviewee: Yes, because too many baby born like this. They say like this, ok, ‘they been there during bomb time’. C001
Even with a confirmed genetic diagnosis, one couple encountered disbelief from their family that the cause was genetic, as this was the first occurrence within the family.
Future Generations
About half of the couples expressed religious, cultural, or personal objections to marriages where there were multiple loops of consanguinity, speaking in terms of blood being “too close” or “too thick.” Six couples would discourage their own children from being in a consanguineous relationship.
Theme 3: perceived utility of genomic reproductive carrier screening
Clinical Utility
While six couples predicted a carrier result would make them sad or anxious, all perceived the utility of carrier testing and supported its availability. Twenty of the couples spoke about how they would hypothetically use carrier status knowledge in a pregnancy, and 13 predicted they would test a pregnancy. Of these, 11 would consider termination of an affected pregnancy (Fig. 2). One couple reported that they would accept pregnancy testing for information only, and another did not discuss whether they would consider termination.
Hypothetical views of use in pregnancy if identified to be a carrier couple. The four couples who would not test in an established pregnancy includes one couple for whom this was not felt to be relevant as they were undergoing in vitro fertilization for infertility and would opt for pre-implantation genetic diagnosis (asterisk). The figure does not include the one discordant couple in which the female would undergo prenatal testing and consider termination and the male would not
With my first baby she suffered a lot. It was very hard to watch a baby die. I mean, it [termination] is not better, but I feel that it is easier…
I don’t want to have children that are going to suffer. I will do everything in my power to get all the testing possible, to know as much as we can, to prevent anything happening to our future children. I don’t think that I would want to take any chances. C002
I’m thinking it all depends how far it’s gone. Like, if it’s only early stages and there’s no harm, like nothing’s done, yeah maybe. But if it’s gone beyond, what do you do, do you kill a child? Do you know what I mean? And in Australia, they help, they’re very good in helping disability [sic] kids and that. You know the government here’s very good. So you know, like, you’re not on your own.… They help you out a lot. I don’t know, in the heat of the moment you don’t know. You have to be (pauses), you have to experience it. C019
Two of the couples opposed to termination said it was prohibited by their religion (Mandaean, Islam).
No we wouldn’t do that [terminate]. If we start it we finish it… that’s why we’re here to do the test. If we have this problem, because of the [sic] related, and if so, should we keep trying or not? C012
Whatever God’s going to throw at us, we’re just going to have to take it. C003
Of six other couples, four would not test a pregnancy, of whom three reported the decision was due to risk of miscarriage and two were uncertain if they would undergo prenatal testing.
Additionally, one couple that was already undergoing in vitro fertilization (IVF) for infertility did not view prenatal testing as relevant for them, while another couple was discordant: the female partner would test and consider TOP, and the male would not test or stop a pregnancy (Fig. 2).
Due to the iterative nature of the interview, the issue of pre-implantation genetic diagnosis (PGD) was discussed with only 11 of the 21 couples. Of these, six viewed PGD as a potential option, including the one couple already undergoing IVF.
[In regard to PGD] I would be grateful. I would thank God for that. C006
[In regard to PGD] Yeah but, at the end of the day everyone says how hard it is to have a kid with a disability. .... It’s not like they will look down on us for doing that. C014
Conversely, five couples, four of whom were open to testing in an established pregnancy, would not use PGD, citing the expense and that it was “unnatural.”
Communication of Potential At-Risk Status
Thirteen couples reported that if they had genomic testing and were found to be carriers, they would disclose their potential at-risk status to their families. Reasons included advising family members of their own potential carrier status, for emotional support, and to assist with pregnancy decision-making. Four of these couples emphasized that there was no shame in being a carrier.
I think they will take it positively… They will understand what it is. No parents would like to put their kids in danger. So prevention is better than cure. C015
[I would tell] The whole family… I would tell people if they asked, I’m not ashamed, if that’s what you mean. C018
One couple would disclose their risk status only to family members who were also in consanguineous relationships. Two couples were male/female discordant regarding communication patterns. One couple would not feel comfortable sharing their hypothetical carrier status. Reasons for non-disclosure were that relatives already had healthy children, the family would not understand, or that it would not alter pregnancy decisions for others.
Genomic Testing Considerations
In relation to genomic testing, two couples raised concerns regarding the time taken for the return of results of genomic reproductive carrier screening; two asked whether a genetic diagnosis would allow a future baby to be cured; one articulated fear of incidental findings; one raised concerns over accuracy; and another raised privacy concerns. Three couples suggested that the ideal timing of testing would be prior to marriage or first pregnancy.
Interest
Fourteen of the interviewed couples elected to proceed with genomic screening, together with another couple who were not formally interviewed. The decision of whether or not to proceed with screening does not appear to have been influenced by having an affected child. Thus, of the 14 couples who chose to have screening, 9/14 (64%) were those with an affected child and 5/7 (71%) were without an affected child. Those with an affected child were aware that testing may reveal they were at risk for a child with a different condition.
Of the seven couples who declined screening, three would not have used the information to inform a pregnancy (no TOP; no PGD), two would have used the information to inform a pregnancy but had decided after enrolling in the study that their family was complete, one declined due to fear of incidental findings, and one declined because of concerns about test reliability.
Only one couple who reported that they were confident that they would not test in a pregnancy or undergo PGD, accepted the offer of testing. There was no religious or geographical relationship to interest in testing.
Discussion
The largely neutral experience of being a consanguineous couple in Australia reported by the study group may reflect limited disclosure to the wider Australian community, with couples viewing this as private information. A fear of perceived stigma has been reported previously, and studies support the idea that consanguinity can be viewed as a taboo topic within Australia and other Western countries (Bittles 2003; Bishop et al. 2008). Despite this concern, no actual experiences of stigma were reported by couples in our cohort.
Another factor influencing the Australian experience may be that while all of the male subjects were overseas-born, in five couples, the wives were born in Australia. This is in marked contrast to countries in which stigma has been reported, for example, in the UK where a substantial majority of Pakistani transnational unions involve a male UK-born resident marrying a Pakistan-born relative (Charsley et al. 2012).
Although almost all couples had previously interacted with genetics services, only two couples could accurately recount the risk figures they had been given with respect to consanguinity. Over a third of couples who commented on risk offered markedly high estimates for this risk, perhaps mistaking the figures quoted for the chance of recurrence of an AR condition. While confusion surrounding these figures would not be surprising in the case of those with affected children, incorrect recall of counseled genetic risks has been well described (Michie et al. 2005). The difficulty reconciling an increased risk of AR conditions due to consanguinity with the couples’ personal experiences of many consanguineous couples having exclusively healthy children, and affected children being born to non-consanguineous couples, has previously been reported (Al-Gazali et al. 2006).
The nine couples who spoke of a “blood mixing” test originated from Lebanon, Syria, Iran, and Iraq and were probably referring to premarital screening programs that exist for a range of conditions, predominantly hemoglobinopathies, in many Middle Eastern countries (Al-Gazali et al. 2006; Raz et al. 2003; Gharaibeh and Mater 2009; Al-Allawi et al. 2013; Nariman et al. 2016). Their uncertainty about details of the test is consistent with other studies from the Middle East which found that most people screened were unaware of the type of testing they had undergone (Al-Aama et al. 2008; Nariman et al. 2016).
Genetics as a contributing factor for conditions affecting children of consanguineous parents was accepted by all couples in this cohort, perhaps reflecting previous genetic counseling. However, other multifactorial contributing explanatory beliefs for childhood conditions were proposed, with many couples reflecting on stress or chance as possible causes. Three couples from Iraq and one couple from Lebanon spoke extensively of war and its aftermath as a cause of childhood conditions. Causes for acquired condition in children, such as infectious disease, were also volunteered.
The six couples who spoke of God’s will as a factor in childhood condition were of Muslim, Mandaean, or Chaldean Christian faiths, five of whom cited God’s will in conjunction with other factors. Contrary to other studies, none of the couples offered a belief that severe childhood conditions resulted from supernatural or divine punishment for parental transgressions (Panter-Brick 1991; el-Shazly et al. 2010).
The goals and implications of genomic testing, as well as limitations and risks including incidental findings, were rapidly understood by most participants. Several expressed gratitude for the availability of such testing. Almost two thirds of couples, across all of the religious backgrounds of those interviewed, would use information of their carrier status in making reproductive choices. Couples acknowledged that the decision to terminate a pregnancy would be difficult but spoke of wanting to prevent suffering, consistent with similar studies (Neter et al. 2005; Hans and Kimberly 2014).
While some caveats were expressed regarding the extent of communication and disclosure of carrier status within the family, in general, the participants supported openness. This is in contrast with a study amongst consanguineous British Pakistani families, amongst whom carrier status was poorly communicated due to concerns of stigma and discrimination (Shaw and Hurst 2009).
Limitations
This is a small study with a number of potential limitations. Although the couples were of diverse ethnic backgrounds, bias may have been introduced by the fact that all practiced a religion and all but one couple were recruited from the same clinical genetics unit, with a likely higher level of genetic literacy and bias towards the acceptance of genetic testing. Therefore, the relatively high hypothetical acceptability of prenatal testing and consideration of TOP expressed by these participants cannot be generalized to the broader Australian consanguineous community or to other countries, although it is in keeping with previous studies in consanguineous populations (Panter-Brick 1991; Neter et al. 2005; Fallah et al. 2009; Al-Allawi et al. 2013; Verdonk et al. 2018). A bias may also have been introduced by including parents with affected children, since other studies have demonstrated that parents of severely affected children are more likely to undergo prenatal testing and consider TOP for a second affected child (Ahmed et al. 2008; Cowan 2009; Jafri et al. 2015). However, in Cyprus, which has a β-thalassemia carrier rate of ~ 15%, the implementation of prenatal screening restored reproductive carrier confidence amongst couples and resulted in an increase in birth numbers (Cowan 2009). Finally, the self-selection of those responding to the invitation to participate may have also introduced a bias in regard to the openness to genomic testing.
Practice Implications
Verdonk et al. (2018) noted that any offer of reproductive carrier screening needs to be framed by choice. Importantly, the choice made by all couples in this study was reportedly with consideration of their values, their current family situation, and exome testing issues, including fear of incidental findings and concerns about test reliability. This underscores the importance of pre-test information to address limitations in genetic literacy, perhaps not present in this cohort, time to consider all of the personal and familial implications of choosing to test, and provision of decision support.
The increasing availability and decreasing costs of genomic screening provide the opportunity to offer reproductive carrier screening to those at higher risk of having children with AR or XLR conditions, including consanguineous couples. In considering the implementation of such screening with this group, two key questions need to be considered. Firstly, is an effective screen possible using available technology? This is answered, in the affirmative, by the paper by Kirk et al. (2018) which reported on the results of the 15 consanguineous couples from this cohort who were screened for AR and XLR conditions using the TruSight One panel of 4813 genes associated with human disease (Kirk et al. 2018). The authors concluded that reproductive carrier screening of consanguineous couples using genomic sequencing is practicable, and it is likely to detect many more at-risk couples than any targeted panel could achieve. This couple-based approach greatly reduces the associated analysis and counseling burden.
Secondly, will screening have clinical utility in that population? In other words, will it be acceptable to the target population, and will those who are identified as being at-risk use the information to inform their reproductive decision-making? In this study, we have addressed these issues, also in the affirmative. People from diverse cultural and faith-based backgrounds had varying understanding of the reproductive carrier implications of consanguinity, but given the choice, most would access screening and would take action based on the results. Nevertheless, although the study interviews are informative, the findings should not be extrapolated to all consanguineous couples in Australia.
Future Research
Many of the couples interviewed expressed the view that being in a consanguineous relationship was “normal” and common in their country of origin. Exploration of the views of consanguineous couples in such countries on genomic reproductive carrier screening would be informative to contrast with the views expressed in this study.
Three couples suggested that the ideal timing of testing would be prior to marriage or first pregnancy, consistent with recommendations by the women interviewed in the study reported by Verdonk et al. (2018). However, the inherent challenges in reaching such participants and enabling choice are compounded when the screening is targeting couples. The exploration of views of the Australian consanguineous couples documented here may inform further studies of truly preconception couples, defined as pre-first pregnancy, if these challenges can be overcome.
Conclusion
The concept and implications of genomic screening discussed during the interview were understood by the couples in this study. Their resulting decision to undertake testing was also consistent with an intention to apply potential carrier status to inform a pregnancy. While the decision was independent of whether the couple already had an affected child, they were perhaps influenced by their previous experience with the genetics service.
The results of the sequencing of the consanguineous couples from the interview cohort who chose to have screening nevertheless suggests that the principle of offering a screening program to this target group has merit (Kirk et al. 2018). Where this is implemented, the present findings may lay the foundation for exploring perceptions of exome sequencing with couples who have not had children and are truly preconception and have had no contact with genetics services. As in this study, important questions will need to be explored for any offer of screening that is underpinned by choice: (Bennett et al. 2002) consideration of the pre-existing beliefs and attitudes of couples in consanguineous relationships regarding consanguinity; (Hamamy et al. 2011) its medical, personal, and societal consequences; and (Bittles and Black 2010) the likely clinical utility of such a test.
References
Ahmed, S., Hewison, J., Green, J. M., Cuckle, H. S., Hirst, J., & Thornton, J. G. (2008). Decisions about testing and termination of pregnancy for different fetal conditions: a qualitative study of European White and Pakistani mothers of affected children. Journal of Genetic Counseling, 17(6), 560–572.
Al-Aama, J. Y., Al-Nabulsi, B. K., Alyousef, M. A., Asiri, N. A., & Al-Blewi, S. M. (2008). Knowledge regarding the national premarital screening program among university students in western Saudi Arabia. Saudi Medical Journal, 29(11), 1649–1653.
Al-Allawi, N. A., Jalal, S. D., Ahmed, N. H., Faraj, A. H., Shalli, A., & Hamamy, H. (2013). The first five years of a preventive programme for haemoglobinopathies in Northeastern Iraq. Journal of Medical Screening, 20(4), 171–176.
Al-Farsi, O. A., Al-Farsi, Y. M., Gupta, I., Ouhtit, A., Al-Farsi, K. S., & Al-Adawi, S. (2014). A study on knowledge, attitude, and practice towards premarital carrier screening among adults attending primary healthcare centers in a region in Oman. BMC Public Health, 14, 38.
Al-Gazali, L., Hamamy, H., & Al-Arrayad, S. (2006). Genetic conditions in the Arab world. BMJ, 333(7573), 831–834.
Becker, R., Keller, T., Wegner, R. D., Neitzel, H., Stumm, M., Knoll, U., et al. (2015). Consanguinity and pregnancy outcomes in a multi-ethnic, metropolitan European population. Prenatal Diagnosis, 35(1), 81–89.
Bennett, R., Motulsky, A., Bittles, A., Hudgins, L., Uhrich, S., Lochner, D., et al. (2002). Genetic counseling and screening of consanguineous couples and their offspring: recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 11(2), 97–119.
Bishop, M., Metcalfe, S., & Gaff, C. (2008). The missing element: consanguinity as a component of genetic risk assessment. Genetics in Medicine, 10(8), 612–620.
Bittles, A. (2001). Consanguinity and its relevance to clinical genetics. Clinical Genetics, 60(2), 89–98.
Bittles, A. H. (2003). The bases of western attitudes to consanguineous marriage. Developmental Medicine and Child Neurology, 45(2), 135–138.
Bittles, A. H. (2008). A community genetics perspective on consanguineous marriage. Journal of Community Genetics, 11(6), 324–330.
Bittles, A. H. (2012). Consanguinity in context. Cambridge: Cambridge University Press.
Bittles, A. H., & Black, M. L. (2010). Evolution in health and medicine Sackler colloquium: Consanguinity, human evolution, and complex diseases. Proceedings of the National Academy of Sciences of the United States of America, 107(Suppl 1), 1779–1786.
Bittles A. H., & Black M. L. (2015). Global patterns and tables of consanguinity. Retrieved from http://consang.net
Braun, V., & Clarke, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101.
Charsley, K., Storer-Church, B., Benson, M., & Van Hear, N. (2012). Marriage-related migration to the UK. International Migration Review, 46(4), 861–890.
Cowan, R. S. (2009). Moving up the slippery slope: mandated genetic screening on Cyprus. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 151C(1), 95–103.
de Costa, C. (1988). Pregnancy outcomes in Lebanese-born women in western Sydney. The Medical Journal of Australia, 149(9), 457–460.
el-Shazly, M., Bakry, R., Tohamy, A., Ali, W. M., Elbakry, S., Brown, S. E., et al. (2010). Attitudes toward children with clefts in rural Muslim and Hindu societies. Annals of Plastic Surgery, 64(6), 780–783.
Fallah, M. S., Samavat, A., & Zeinali, S. (2009). Iranian national program for the prevention of thalassemia and prenatal diagnosis: mandatory premarital screening and legal medical abortion. Prenatal Diagnosis, 29(13), 1285–1286.
Fareed, M., & Afzal, M. (2017). Genetics of consanguinity and inbreeding in health and disease. Annals of Human Biology, 44(2), 99–107.
Gharaibeh, H., & Mater, F. K. (2009). Young Syrian adults’ knowledge, perceptions and attitudes to premarital testing. International Nursing Review, 56(4), 450–455.
Hamamy, H. (2012). Consanguineous marriages: preconception consultation in primary health care settings. Journal of Community Genetics, 3(3), 185–192.
Hamamy, H., Antonarakis, S. E., Cavalli-Sforza, L. L., Temtamy, S., Romeo, G., Kate, L. P., et al. (2011). Consanguineous marriages, pearls and perils: Geneva International Consanguinity Workshop Report. Genetics in Medicine, 13(9), 841–847.
Hans, J. D., & Kimberly, C. (2014). Abortion attitudes in context: a multidimensional vignette approach. Social Science Research, 48, 145–156.
Henneman, L., Borry, P., Chokoshvili, D., Cornel, M. C., van El, C. G., Forzano, F., et al. (2016). Responsible implementation of expanded carrier screening. European Journal of Human Genetics, 24(6), e1–e12.
Jafri, H., Hewison, J., Sheridan, E., & Ahmed, S. (2015). Acceptability of prenatal testing and termination of pregnancy in Pakistan. Journal of Community Genetics, 6(1), 29–37.
Khorasani, G., Kosaryan, M., Vahidshahi, K., Shakeri, S., & Nasehi, M. M. (2008). Results of the national program for prevention of beta-thalassemia major in the Iranian Province of Mazandaran. Hemoglobin, 32(3), 263–271.
Kirk, E., Barlow-Stewart, K., Selvanathan, A., Josephi-Taylor, S., Worgan, L., Rajagopalan, S. et al. (2018). Beyond the panel: preconception screening in consanguineous couples using the TruSight One “Clinical Exome”. Genetics in Medicine. Accepted 19 May 2018.
Michie, S., Lester, K., Pinto, J., & Marteau, T. M. (2005). Communicating risk information in genetic counseling: an observational study. Health Education & Behavior, 32(5), 589–598.
Nariman, A., Sobhan, M. R., Savaei, M., Aref-Eshghi, E., Nourinejad, R., Manoochehri, M., et al. (2016). The genetic background of Southern Iranian couples before marriage. Balkan Journal of Medical Genetics: BJMG, 19(2), 71–74.
Nelson, J., Smith, M., & Bittles, A. H. (1997). Consanguineous marriage and its clinical consequences in migrants to Australia. Clinical Genetics, 52(3), 142–146.
Neter, E., Wolowelsky, Y., & Borochowitz, Z. U. (2005). Attitudes of Israeli Muslims at risk of genetic conditions towards pregnancy termination. Journal of Community Genetics, 8(2), 88–93.
Panter-Brick, C. (1991). Parental responses to consanguinity and genetic disease in Saudi Arabia. Social Science & Medicine, 33(11), 1295–1302.
Port, K. E., Mountain, H., Nelson, J., & Bittles, A. H. (2005). Changing profile of couples seeking genetic counseling for consanguinity in Australia. American Journal of Medical Genetics. Part A, 132A(2), 159–163.
Posch, A., Springer, S., Langer, M., Blaicher, W., Streubel, B., & Schmid, M. (2012). Prenatal genetic counseling and consanguinity. Prenatal Diagnosis, 32(12), 1133–1138.
Raz, A. E., Atar, M., Rodnay, M., Shoham-Vardi, I., & Carmi, R. (2003). Between acculturation and ambivalence: knowledge of genetics and attitudes towards genetic testing in a consanguineous Bedouin community. Journal of Community Genetics, 6(2), 88–95.
Sallevelt, S., de Koning, B., Szklarczyk, R., Paulussen, A. D. C., de Die-Smulders, C. E. M., & Smeets, H. J. M. (2017). A comprehensive strategy for exome-based preconception carrier screening. Genetics in Medicine, 219(5), 583–592.
Shaw, A., & Hurst, J. A. (2009). ‘I don’t see any point in telling them’: attitudes to sharing genetic information in the family and carrier testing of relatives among British Pakistani adults referred to a genetics clinic. Ethnicity & Health, 14(2), 205–224.
Sulem, P., Helgason, H., Oddson, A., Stefansson, H., Gudjonsson, S. A., Zink, f., et al. (2015). Identification of a large set of rare complete human knockouts. Nature Genetics, 47(5), 448–452.
van El, C. G., Cornel, M. C., Borry, P., Hastings, R. J., Fellman, F., Hodgson, S. V., et al. (2013). Whole-genome sequencing in health care. Recommendations of the European Society of Human Genetics. European Journal of Human Genetics, 21(Suppl 1), S1–S5.
Verdonk, P., Metselaar, S., Storms, O., & Bartels, E. (2018). Reproductive choices: a qualitative study of Dutch Moroccan and Turkish consanguineously married women’s perspectives on preconception carrier screening. BMC Women’s Health, 18, 79–89.
Zlotogora, J., & Shalev, S. A. (2010). The consequences of consanguinity on the rates of malformations and major medical conditions at birth and in early childhood in inbred populations. American Journal of Medical Genetics. Part A, 2152A(8), 2023–2028.
Zlotogora, J., & Shalev, S. A. (2014). A long-term follow up of premarital counseling in the Israeli Arab population. Journal of Community Genetics, 5(4), 377–381.
Acknowledgements
We thank the participants in this study.
Funding
This study was funded by a grant from the Apex Foundation for Research into Intellectual Disability. Bettina Meiser was supported by a National Health and Medical Research Council (NHMRC) of Australia Senior Research Fellowship Level B (ID 1078523).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to:
• The conception, design, analysis, and interpretation of data for the work
• Drafting the work or revising it critically for important intellectual content
• Final approval of the version to be published
• All agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
The study was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee (reference no 14/026).
Conflict of Interest
Sarah Josephi-Taylor, Arthavan Selvanathan, Tony Roscioli, Lisa Worgan, Sulekha Rajagopalan, Alison Colley, and Edwin P Kirk declare that they have no conflict of interest.
Bettina Meiser has a remunerated consultant role with the company Astrazeneca with respect to an unrelated project.
Kristine Barlow-Stewart has a remunerated consultant role with the company Sonic Genetics in regard to writing patient information.
Alan Bittles has acted as a consultant on consanguinity for Merck, Sharp, and Dohme.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (Bittles and Black 2015).
Animal Studies
No animal studies were carried out by the authors for this article.
Electronic Supplementary Material
ESM 1
(DOC 60 kb)
Rights and permissions
About this article
Cite this article
Josephi-Taylor, S., Barlow-Stewart, K., Selvanathan, A. et al. User Acceptability of Whole Exome Reproductive Carrier Testing for Consanguineous Couples in Australia. J Genet Counsel (2018). https://doi.org/10.1007/s10897-018-0298-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10897-018-0298-5