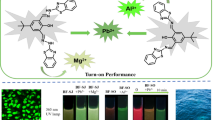
Abstract
A donor-acceptor Schiff-base fluorescent probe BKS with chelation enhanced fluorescence (CHEF) mechanism was designed and synthesized via benzophenone(Acceptor), salicylaldehyde and carbazole(Donor) for Al3+ detection, which exhibited typical aggregation-induced emission (AIE) characteristic. BKS probe could provide outstanding selectivity to Al3+ with a prominent fluorescence “turn-on” at 545 nm in a wide pH range from 2 to 11. By the Job’s plot, the binding stoichiometry ratio of probe BKS to Al3+ was determined 1:1. The proposed strategy offered a very low limit of detection at 1.486 µM in THF/H2O(V/V = 1:4, HEPBS = 10 mM, pH = 7.40), which was significantly lower than the standard of WHO (Huang et al., Microchem J 151:104195, 2019)–(Yongjie Ding et al., Spectrochim Acta Mol Biomol Spectrosc 167:59–65, 2021) guidelines for drinking water. BKS probe could provide a wider linear detection range of 50 to 500 µM. Furthermore, the probe could hardly be interfered by other examined metal ions. The analysis of Al3+ in real water samples with appropriate recovery (100.72 to 102.85) with a relative standard deviation less than 2.82% indicated the accuracy and precision of BKS probe and the great potential in the environmental monitoring of Al3+.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It’s well known that Aluminum (Al) is the third most abundant metallic element in the earth’s crust, and is commonly found in nature as oxides, fluoride, and silicide [1]. Aluminum compounds are widely used in aerospace, manufacturing, food processing, the production of computers, the manufacture of aircraft fuselage frames, food and food ingredients, clinical medicine, etc., which has brought great convenience to human society [2, 3]. Nevertheless, the trace level of Al3+ ions in the environment can affect the growth of roots, which can interact with cell wall, cytoplasm and plasma membrane in acid soil. Moreover, it is verified that aluminum can also affect human growth and development, impair people’s intelligence, and have impalpable relationship with Parkinson’s disease, gastrointestinal problems and osteal porosis [4, 5]. The limit of aluminum in drinking water is 0.2 ppm (to 7.41 µM) set by World Health Organization (WHO). Therefore, it is essential to develop a rapid and sensitive method for detecting aluminum.
Until now, some instrumental methods have been developed to detect lower concentrations of Al3+, such as atomic absorption (AAS) [6], Electrochemical luminescence and electrochemistry [7], inductively coupled plasma atomic emission spectroscopy technique (ICP-AES) [8] and inductively coupled plasma mass spectrometry (ICP-MS) [9, 10], while they commonly require time-consuming sample preparation and tedious operations, which are unsuitable for real-time analysis [11]. Therefore, in recent years, various fluorescent probes for Al3+ ion detection have been attracted considerable interests due to their advantages such as high selectivity, real-time detection, and versatility [12]. Compared with the fluorescent chemo-sensor with the aggregation-caused quenching (ACQ) effect, the probes with aggregation-induced emission (AIE) activity have been reported for turn-on detection of Al3+ ion by incorporating several receptor ligands such as Schiff base, pyridines, hydrazines, carboxylic acids, and sulfonate salts in different media [13,14,15,16], which is attributed to the effective suppression of the non-radiative transition [17]. Meanwhile, as the preference of Al3+ ion coordinating a sphere containing O and N as hard-base sites, Schiff base compounds exhibit higher fluorescence performance than solid state in organic solution and AIE medium, so they are often used in the designing of fluorescent probes [18].
In the present work, a novel D-A Schiff based fluorescent probe BKS with AIE effect was designed and synthesized with the reactants including 4-nitro-(4’-fluorine)-benzophenone, carbazole and salicylic aldehyde. The as-prepared fluorescent probe BKS exhibited fluorescence “turn-on” response to Al3+ in THF/H2O(V/V = 1:4, HEPBS = 10 mM, pH = 7.40) solution with high selectivity and sensitivity within a wide pH range of 2 to 11. In addition, the as-prepared probe was successfully employed in actual water samples for the detection of Al3+ with satisfactory recovery.
Experimental
Materials and Instruments
All chemicals and reagents were of analytical grade and used directly without further purification. 1H NMR and 13C NMR spectra were recorded on AVANCE III HD 400 NMR spectrometer (Brock, Switzerland). The infrared spectra were obtained through the American Nicolet AVATAR370 type infrared spectrometer. UV-visible absorption was measured with a UV-2600 spectrophotometer. The fluorescence measurements were performed using a Hitachi F-700 fluorescence spectrometer (Japan). The pH values were measured with a PHS-3 C (Shanghai Leici instruments Co., Ltd., China).
Synthesis and Characterization of the Probe (BKS)
The compound BKS was designed and synthesized as shown in Scheme 1. The compounds (1) to (3) were prepared according to the known method. The details were given in the Supplementary information (SI, Fig. S1).
Successively, a mixture of compound (3) (0.5 g, 1.38 mmoL) and salicylic aldehyde (0.46 g, 4.14 mmoL) was added in 150 mL single-port round-bottom flask with 20 mL toluene. When the mixture solution was heated to 50 ℃, acetic acid (1 mL) was added dropwise, the solution was continuously stirred and refluxed for 24 h at 110 ℃. Then the residue was obtained after evaporation of solvents. Finally, the yellow solid product of 0.43 g (yield 68.5%) was obtained after recrystallization from anhydrous ethanol. 1H NMR (400 MHz, Chloroform-d) δ 12.90 (s, 1 H), 8.71 (s, 1 H), 8.16 (d, J = 7.8 Hz, 2 H), 8.09 (d, J = 8.4 Hz, 2 H), 8.01 (d, J = 7.9 Hz, 2 H), 7.76 (d, J = 8.2 Hz, 2 H), 7.54 (d, J = 8.2 Hz, 2 H), 7.48–7.39 (m, 5 H), 7.34 (t, J = 7.4 Hz, 2 H), 7.05 (s, 3 H). (SI, Fig. S2). C NMR (101 MHz, Chloroform-d) δ 164.37, 161.32, 152.37, 141.71, 140.26, 136.05, 135.53, 134.00, 132.78, 131.82, 131.74, 126.36, 126.27, 123.87, 121.33, 120.67, 120.53, 119.42, 119.02, 117.48, 109.83. (SI, Fig. S3).
Fluorescence and UV-Vis Spectroscopic Studies
A stock solution of the probe BKS (20 µM) was prepared in THF. 2 mM of different metal ions (Al3+、Li+、Na+、K+、Ag+、Cu2+、Fe3+、Zn2+、Mn2+、Ca2+、Mg2+、Cd2+、Cr2+、Pb2+、Ba2+、Ga2+、La3+、Ni3+、In3+、Ce3+) from their chloride salts were separately prepared in HEPBS buffer solution(V/V = 1:4, HEPBS = 10 mM, pH = 7.40). In the UV-vis and fluorescence studies, the probe BKS and each metal ion used were 10 µM and 50 µM, respectively. The fluorescence titration experiments were conducted at 400 nm with emission slit of 10 nm, by adding the stock solutions of metal ions into the probe solution. Each measurement was performed in triplicate.
Analysis of Al3+ in Real Water Samples
All water samples were the surface water collected from a local river and lake belonging to the Dongting Lake area (located in Yueyang City, Hunan Province, China). Tap water sample was freshly collected from our laboratory. All water samples were tested after the addition of THF at the volume ratio of 1:4 and 10 µM BKS probe. Spiked samples were prepared as the same mention above, with the pH adjusted with 5 M NaOH. All results were expressed as mean values of at least three replicates in the experiments.
Results and Discussion
Aggregation-Induced Emission (AIE) Behaviors of BKS Probe
Contrast to the phenomenon of aggregation-caused quenching (ACQ), the AIE activity showed no emission in dilute solutions but strongly emitted in the aggregate-state and solid [19]. So for as-prepared BKS probe, the AIE property was firstly investigated. In our pre-experiments, fluorescence intensity of BKS probe was measured in six different pure organic solvents, respectively. The results revealed that the BKS probe was highly soluble and the strongest fluorescence emission intensity in THF (SI, Fig. S4). Therefore, the mixture of THF/H2O with different content of water (% volume, fw) was chosen as test solutions in the follow-up experiments. As shown in Fig. S5, compared with that of the probe in THF, the UV absorption of probe BKS gradually red shifted with the increase of water content and displayed the maximum shift of 30 nm at 95% fw. Then the typical test in mixed solution of THF/H2O were executed and the fluorescence emission spectra in Fig. 1a. BKS probe (2 × 10− 5 M) initially exhibited quite weak luminescence. From fw=70%, the fluorescence intensity rapidly increased and achieved the maximum at 95% fw, as 6.4 times as that of the probe in THF. Figure 1b presents the relationship curve of I/I0 versus fw, where I0 represents the fluorescence intensity in a pure tetrahydrofuran solution, and I represents the fluorescence intensity in THF/H2O mixtures with various volume ratios. This curve demonstrates that the probe BKS has typical AIE properties. Moreover, the optical photo in Fig. 1c obviously illustrated BKS probe possessed AIE effect. The results above were attributed to the reduced solubility and the aggregation of the probe BKS with the increased water content, which led to the transformation of the D-A configuration of probe BKS from spatial torsion to planarized configuration, thus increasing the effective conjugate length. Moreover, in the aggregate state, due to the formation of intramolecular hydrogen bonds according to the hydroxyl group and N atoms, the rotation or vibration of the benzene ring was restricted (RIR) [20, 21], which blocks the non-radiative decay and favors the radiative transition, resulting in enhancing fluorescence.
Fluorescent Response of BKS to Al3+
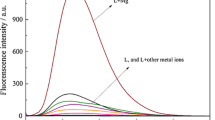
The selectivity for AIE-active probes is very important because it is closely related to the efficiency of these chemical probe in itself. At first, the metal ion response performance of BKS (10 µM) was investigated by UV-visible absorbance and fluorescence spectra. As shown in Fig. S6, the UV absorption spectrum of the probe BKS had no obvious change with adding other metal ions, while a new absorption band was formed at 410 nm with addition of Al3+ ion. Herein, the red shift of absorption peak could be deducted that there might be the production of a new BKS-Al3+ complex. In addition, all cations tested except Al3+ (in Fig. 2a) had weak fluorescence intensity at 545 nm, which was due to the isomization of the C = N bond by excited state intramolectional proton transfer (ESIPT), resulting in weak fluorescence of the probe [22]. As we all know, Al3+ is recognized as a hard acid and prefers to coordinate hard-base donors (N & O) [23]. The O/N-rich Schiff based fluorescent probes have rich hard alkaline centers and can provide binding sites for hard acid Al according to HSAB [24]. The aluminum ion binding through O donor site and the C = N moiety of BKS restricted C = N isomerization [25].
Therefore, chelated enhanced fluorescence (CHEF) can enhance the fluorescence of the probe. In the THF/H2O solution of BKS, Al3+ had a strong fluorescence emission at 545 nm due to enhanced fluorescence by chelation, which may be the addition of Al3+ made the probe form a rigid structure, leading to the destruction of ESIPT characteristics [26].
The addition of Al3+ caused the coordination of non-bonding electrons in C = N atoms, thus inhibiting the isomerization of C = N and enhancing the fluorescence of the probe. The alignment of Al3+ and probe BKS inhibited the rotation of the C = N bond, thus inhibiting the ESIPT effect and enhancing the fluorescence of the probe, resulting in chelation-enhanced fluorescence effect (CHEF) [27].
The probe selectively and sensitively recognizes Al3+ and inhibits the isomerization of the C = N bond by CHEF, enhancing the PL emission of the probe. The aluminum ion is a hard metal that can be coordinated with the C = N and O sites on the probe BKS, showing strong affinity. Therefore, BKS can selectively and sensitively recognize Al3+, which is a promising turn on-fluorescence sensor (Scheme 2).
Meanwhile, the optical photo obtained under a 365 nm UV light directly certified the high fluorescence response of probe BKS to Al3+ in Fig. 2b. Only where Al3+ ions are present will the bright white fluorescence appear, while other ions produced no visible fluorescence, except Cu2 + and Fe3+ solutions getting dark by completely quenching. These results demonstrated that BKS has an excellent selectivity to Al3+ over other interested metal ions.
Competition Studies
To further verify the anti-disturbance from other coexistent metal ions, the competition experiments for Al3+ were carried out by measuring the fluorescence intensity at 545 nm after adding other competitive metal ions, respectively. The fluorescence emission spectrum was measured at an excitation wavelength of 400 nm in the presence of aluminum ions and other ions. As depicted in Fig. 3, the presence of other ions had no obvious influence on the determination of Al3+. These results illustrated that the probe could detect Al3+ with high selectivity.
Study on Fluorescence Titration and Detection Limit (LOD) of Aluminum Ion
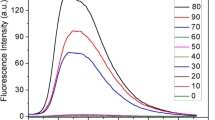
To check the sensitivity of sensor probe BKS for Al3+ ion, the fluorescence titration experiments were investigated in THF/H2O(v/v = 1:4, HEPBS = 10 mM, pH = 7.40) solution. Probe BKS exhibited a strong emission peak at 545 nm with excitation at 400 nm. With the increase of Al3+ (50 to 500 µM) concentration, the fluorescence intensity of the probe gradually increased at 545 nm. When the amount of Al3+ was over 500 µM, the fluorescence intensity was not in the linear range (Fig. 4a). The fluorescence intensity of the probe BKS-Al3+ at 545 nm had a good linear relationship with the concentration of Al3+ (R2 > 0.99, Fig. 4b). The results indicated that detection of BKS-Al3+ can be used for quantitative analysis of Al3+. With the increasing of Al3+ ion concentration, the fluorescence intensity gradually increased, with a slight blue shift from 545 nm to 544 nm. Based on the equation LOD = 3σ/k [28, 29], the detection limit of probe BKS-Al3+ for Al3+ was calculated to be 1.486 µM, which lower than the standard of WHO guidelines for drinking water [30, 31], indicating that probe BKS had a high sensitivity in identifying Al3+.
pH Effect on BKS with Al3+
The influence of pH on the probe BKS and BKS-Al3+ systems was investigated. As shown in Fig. 5, the fluorescence spectrum of the probe BKS did not show any significant change in the pH range from 4.0 to 9.0. After the addition of Al3+ ions, a prominent fluorescence “turn-on” at 545 nm in a wide pH range of 2 to 11, indicating that the change in pH has little effect on the probe and that the BKS can sensitively detect Al3+ ions over a wide range of pH.
Response Time of Probe BKS to Al3+
The interaction time between the probe BKS and Al3+ was investigated. As shown in Fig. 6, the fluorescence response strength of the probe BKS to the aluminum ion increased linearly with time within 0 to 20 s after the addition of the aluminum ion, and a stable fluorescence intensity can be reached within 20 s. After the addition of Al3+, the fluorescence intensity of the probe BKS remained constant after 5 min of continuous irradiation, indicating that the BKS is stable enough to detect Al3+.
Reversibility Study
It is well known that the good reversibility is also a significant characterization for a fluorescent probe. The reversibility of BKS to Al3+ was determined by reversible experiment. As shown in Fig. 7, the fluorescence intensity was restored after EDTA was added to the BKS-Al3+ system, and it was enhanced again after Al3+. When Al3+ and EDTA were added alternately, the fluorescence “on-off” response was clearly visible and repeated well (three times). This proved that the probe BKS had good reversibility.
Binding Mechanism of Probe BKS with Al3+
To determine the coordination stoichiometry between probe and Al3+ (BKS-Al3+), Job’s plot analysis was performed using continuous variational method [32, 33]. Finally, their absorption spectra were verified. The highest absorbance intensity was reached at a 0.52 mol fraction (Fig. 8a). The results indicated that BKS-Al3+ formed the complex with a binding ratio of 1:1. According to the modified Benesi-Hildebrand equation as followed (Fig. 8b) [34], the association constant (Ka) of probe BKS with Al3+ was calculated to be 0.477 × 103 M− 1.
To accurately master the combination detail of BKS with Al3+, FT-IR spectra were carried out in the absence and presence of Al3+ respectively [35, 36] (Fig. 9). It can be seen that -OH stretching vibration peak of the probe BKS appeared at 3051 cm− 1. However, BKS binding to Al3+ did not have a stretching vibration peak of -OH at 3051 cm− 1, but showed new stretching vibration peak at 3450 cm− 1and 3345 cm− 1, which might be attributed to the disappearance of the -OH peak due to the combination of Al3+ with BKS. Meanwhile, it can be seen that compared with the infrared spectrum of BKS, the -N = CH- stretching vibration peak of the BKS binding to Al3+ were moved from 1600 cm− 1 to 1586 cm− 1, which might be attributed to the displacement of the -N = CH- peak due to the combination of Al3+ with BKS. The intensity were increased, and the intensity of the stretching vibration peak of -OH at 3450 cm− 1 and 3345 cm− 1 were increased.
For further insight, an NMR study on BKS and its corresponding Al3+ complex was carried out in deuterated DMSO [37, 38](Fig. 10). In the spectrum of BKS, the phenolic -OH signal (H1) was clearly visible at 12.70 ppm. The peak for the imine proton (H2) appeared at 9.07 ppm. In the spectra of the BKS-Al3+ complex, the -OH (H1) peak disappeared, and the aromatic protons (H3) linked to hydroxyl group had a slight to higher field, indicating that phenol O atom coordinated with Al3+ ion through deprotonated oxygen atom. The movement of imine protons (H2) towards the lower field indicated that the imine N participates in the coordination of Al3+ ions.
The findings of this study were compared with the key features of other metal ion detection sensors published in the literature (Table S1).
Applications
To understand and evaluate the application potential of probe BKS in the detection of Al3+ ions in ambient water, different water samples were collected from Dongting lake water, laboratory tap water and drinking water and were filtered for further testing (Table 1). Fluorescence intensity of probe BKS was significantly enhanced at 545 nm (λex = 400 nm). The recoveries of Al3+ in Dongting lake water were 100.72–100.98%, 100.89–101.08% in laboratory tap water and 101.25–102.85% in laboratory drinking water. Therefore, probe BKS had high selectivity and specificity and can be used for monitoring Al3+ in all kinds of water environment.
Conclusion
A donor-acceptor Schiff base chemosensor comprising carbazole, benzophenone and salicylic aldehyde was developed for the selective recognition of aluminum ions in THF/H2O system. Simple and inexpensive fluorescent probe exhibited a turn-on fluorescence response toward Al3+ at micromolar range over other metal ions. The Al3+ ion and probe coordination hindered the rotation of C = N isomerization, thus inhibiting the ESIPT effect. The CHEF effect predominated in the system to enhance the fluorescence of the probe. The change in fluorescence intensity was such that the synthesized Schiff base exhibited ‘‘turn-on” mode of high sensitivity towards Al3+ ions. The limit of detection (LOD) of BKS and the binding constant (Ka) was found to be 1.486 µM and 0.477 × 103 M− 1. The results of Job’s plot and 1H NMR confirmed that the complexation ratio of BKS to Al3+ was 1:1. Finally, the probe was used to detect Al3+ ions in environmental water samples, and it was found that it could be used to monitor Al3+ in various water environments.
Data Availability
No datasets were generated or analysed during the current study. All data provided in this paper are included in this published article and supplementary information.
References
Yang Y-Y, Ma P-Y, Xue J-H, Yang D-D, Shi Y-S, Zhao X, Qi Ma (2024) A highly selective fluorescent probe based on multi-binding site hydrazone chemosensor for Al3+ detection. Microchem J 200:110495. https://doi.org/10.1016/j.microc.2024.110495
Shaochen Sun T, Li Y, Zhu F, Tao L, Wang S, Wang X, Zhang T, Zhang G, Li (2024) Novel pyrazine Schiff-base derivative functionalized water-soluble polymer as a highly selective fluorescent chemosensor for Al3+ ions in pure aqueous solution. Tetrahedron 162(3):134121. https://doi.org/10.1016/j.tet.2024.134121
Anamika Hoque MS, Islam S, Khan B, Datta E, Zangrando GK, Kole, Md Akhtarul Alam (2023) Crystallographic elucidation of an aluminium-bound amido Schiff base chemosensor: a selective turn-on fluorescent chemosensor for Al3+ ions. Dalton Trans 52:10145–10154. https://doi.org/10.1039/D3DT01512B
Shivani Sharma, Chayawan A, Jayaraman J Debnath (2022) 2-Hydroxy-naphthalene hydrazone based dual-functional chemosensor for ultrasensitive colorimetric detection of Cu2+ and highly selective fluorescence sensing and bioimaging of Al3+. J Photochem Photobiol A 437:114408. https://doi.org/10.1016/j.jphotochem.2022.114408
Feyza Kolcu İsmet Kaya (2022) Carbazole-based Schiff base: a sensitive fluorescent ‘turn-on’ chemosensor for recognition of Al(III) ions in aqueous-alcohol media. Arab J Chem 15(7):103935. https://doi.org/10.1016/j.arabjc.2022.103935
Rangasamy Manjunath P Kannan (2018) Highly selective rhodamine-based fluorescence turn-on chemosensor for Al3+ ion. Opt Mater 79:38–44. https://doi.org/10.1016/j.optmat.2018.03.021
Sikandar Khan M, Muhammad AW, Kamran, Hamed M, Al-Saidi SS, Alharthi JS, Algethami Affiliation (2023) An ultrasensitive colorimetric and fluorescent turn-on chemosensor based on Schiff base for the detection of Cu2+ in the aqueous medium. Environ Monit Assess 195:633. https://doi.org/10.1007/s10661-023-11260-3
Zhao X, Li Y, Guo L (2022) A highly selective and high-contrast Colorimetric Off-OnChemosensor for Cu2+ based on Boron-Dipyrromethene (BODIPY) derivatives. Chin J Org Chem 42(11):3757–3765. https://doi.org/10.6023/cjoc202204026
Anshori JA, Ismalah D, Abror AF, Zainuddin A, Hidayat IW, Yusuf M, Maharani R, Ace Tatang Hidayat (2022) A new highly selective off-on typical chemosensor of Al3+, 1-((Z)-((E)-(3,5-dichloro-2-hydroxybenzylidene)hydrazono)methyl) naphthalene-2-ol, an experimental and in silico study. RSC Adv 12:2972–2979. https://doi.org/10.1039/D1RA08232A
Patitapaban Mohanty PP, Dash S, Mishra R, Bhaskaran, Bigyan Ranjan Jali (2024) Thiourea Functionalised receptor for selective detection of Mercury ions and its application in serum sample. J Fluoresc 17:03740. https://doi.org/10.1007/s10895-02403740-7
He Tian X, Qiao Z-L, Zhang C-Z, Xie Q-Z, Li J-Y, Xu (2019) A high performance 2-hydroxynaphthalene Schiff base fluorescent chemosensor for Al(3+) and its applications in imaging of living cells and zebrafish in vivo. Spectrochim Acta A Mol Biomol Spectrosc 207: 31 – 8.10. https://doi.org/1016/j.saa.2018.08.063
Jinchuan Yang K, Wang H, Xu W, Yan Q, Jin D Cui (2017) Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: a review. Talanta 202:96–110. https://doi.org/10.1016/j.talanta.2019.04.054
Meng, Gao, Ben Zhong Tang (2017) Fluorescent sensors based on Aggregation-Induced Emission: recent advances and perspectives. ACS Sens 2(10):1382–1399. https://doi.org/10.1021/acssensors.7b00551
Ju Mei NLC, Leung, Ryan TK, Kwok JWY, Lam, Ben Zhong Tang (2015) Aggregation-Induced Emission: together we Shine. United We Soar! Chem Reviews 115:21, 11718–11940. https://doi.org/10.1021/acs.chemrev.5b00263
Saravanan Enbanathan S, Munusamy D, Jothi, Selin Manoj Kumar (2024) An AIE dynamic a highly selective and expeditious benzothiazole-pyrazine based colorimetric chemosensor for Ni2+and fluorogenic chemosensor for Cu2+ and Al3+ detection. J Mol Liq 404:124949. https://doi.org/10.1016/j.molliq.2024.124949
Yaxun Wu F, Ni Z, Chen W, Yang Y, Xiang S, Gong X, Cao C Yang (2023) Aggregation-dependent thermally activated delayed fluorescence emitters: AIE or ACQ? Adv Opt Mater 11(12):202300186. https://doi.org/10.1002/adom.202300186
Li Q, Li Z (2017) The strong Light-Emission materials in the aggregated state: what happens from a single molecule to the collective group. Adv Sci 4(7):1600484. https://doi.org/10.1002/advs.201600484
Ankush Gupta N, Kumar (2016) A review of mechanisms for fluorescent ‘‘turn-on’’ probes to detect Al3+ ions. RSC Adv 6(108):106413–106434. https://doi.org/10.1039/c6ra23682k
Han Jia N, Xu Y, Nagai M, Doi T, Sawada T, Serizawa S, Ando S, Habuchi, Tsuyoshi Michinobu (2023) Controlling AIE and ACQ properties of conjugated carbazole-tetraphenylethene copolymers by ethynylene spacer. Polym Chem 14:2510–2519. https://doi.org/10.1039/D3PY00168G
Mengyao Xv Y, Liang Y, Chi Y, Pan B, Yang (2023) Theoretical study of the solid-state effect on red hot excitons combined with aggregation induced emission molecule. Chem Phys 573(1):111980. https://doi.org/10.1016/j.chemphys.2023.111980
Kaixin Yang H, Tang Y, Gao JL, Zhang M, Qin J, Li W, Lu S, He Y (2023) An AIE-active orange-emitting cationic iridium(III) complex for latent fingerprints detection via a simple powder dusting method. J Lumin 257:119721. https://doi.org/10.1016/j.jlumin.2023.119721
Gu B, Huang L, Su W, Duan X, Li H (2017) A benzothiazole-based fluorescent probe for distinguishing and bioimaging of Hg2+ and Cu2+. Anal Chim Acta 954:97–104. https://doi.org/10.1016/j.aca.2016.11.044
Milan Shyamal P, Mazumdar S, Maity GP, Sahoo G, Salgado-Morán A Misra (2016) Pyrene Scaffold as Real-Time fluorescent turn-on Chemosensor for Selective Detection of Trace-Level Al(III) and its Aggregation-Induced Emission Enhancement. J Phys Chem A 120(2):210–220. https://doi.org/10.1021/acs.jpca.5b09107
Jaya Malini M Sayed (2021) 1′-hydroxy-2′-acetonaphthone: a simple fluorescence turn-on signaling probe with high selectivity and sensitivity for Al3+ in pure water. J Photochem Photobiol A 418:113431. https://doi.org/10.1016/j.jphotochem.2021.113431
Zhiyong Xing J, Wang J, Huang X, Chen Z, Zong C, Fan G, Huang (2022) A significant fluorescence Turn-On probe for the Recognition of Al3+ and its application. Molecules 27(8):27082569. https://doi.org/10.3390/molecules27082569
Zohreh Salarvand M, Amirnasr S Meghdadi (2019) Colorimetric and fluorescent sensing of Al3+ by a new 2-hydroxynaphthalen based Schiff base Off-On chemosensor. J Lumin 207:78–84. https://doi.org/10.1016/j.jlumin.2018.10.115
Kumar V, Kumar A, Diwan U, Shweta, Ramesh SK, Srivastava KK, Upadhyay (2015) Salicylideneimines as efficient dual channel emissive probes for Al3+: harnessing ESIPT and ICT processes. Sens Actuators B 207:650–657. https://doi.org/10.1016/j.snb.2014.10.068
Yuanhao Liao S, Wang Y, Song Z, Shi G, Chen X, Nan H, Feng W, He (2022) A novel bifunctional fluorescent probe for selectively sensing Hg2+ or ClO– and its application in living cell imaging. J Photochem Photobiol A 434(1):114216. https://doi.org/10.1016/j.jphotochem.2022.114216
Junqiang Leng X, Lan S, Liu W, Jia W, Cheng J, Cheng Z Liu (2022) Synthesis and bioimaging of a BODIPY-based fluorescence quenching probe for Fe3+. RSC Adv 12:21332–21339. https://doi.org/10.1039/D2RA00818A
Meng-Xia Huang, Jia-Ping Lai, Hui Sun, Wei-Zhen Wu (2019) A simple, highly selective and ultra-sensitive “off-on-off” fluorescent chemosensor for successive detection of aluminum ion and phosphate in water samples. Microchemical J 151:104195. https://doi.org/10.1016/j.microc.2019.104195
Yongjie Ding, Chunxiang Zhao, Pengcheng Zhang, Yahong Chen, Weiwu Song, Guanglu Liu, Zengchen Liu, Lin Yun, Ruiqi Han (2021) A novel quinoline derivative as dual chemosensor for selective sensing of Al3+ by fluorescent and Fe2+ by colorimetric methods. Spectrochim Acta A Mol Biomol Spectrosc 167: 59–65. https://doi.org/10.1016/j.molstruc.2021.129965
Yuntong Huang W, Chen M, Dong N, Li L, Chen L, Ling Q, Xu M, Lin (2023) A novel fluorescence probe for the recognition of Cd2+ and its application. Spectrochim Acta Part A Mol Biomol Spectrosc 301(15):122979. https://doi.org/10.1016/j.saa.2023.122979
Mohanasundaram Ranjani V Keerthana (2023) Multifaceted chiral probe 2,3-Dihydro-4-hydroxy-chromene Schiff Base in detecting Cu2+ ions, l-Histidine, and Indazole: Spectroscopic Investigation and Confocal and live cell imaging. ACS Appl Bio Mater 6(6):2358–2369. https://doi.org/10.1021/acsabm.3c00200
Zhang X, Shen L-Y, Zhang Q-L, Yang X-J, Huang Y-L, Redshaw C, Xu H (2021) A simple turn-off Schiff Base fluorescent sensor for copper (II) Ion and its application in Water Analysis. Molecules 26(5):26051233. https://doi.org/10.3390/molecules26051233
Li C, Sun Q, Qiang Zhao (2020) Highly selective ratiometric fluorescent probes for the detection of Fe3+ and its application in living cells. Spectrochim Acta Part A Mol Biomol Spectrosc 228(5):117720. https://doi.org/10.1016/j.saa.2019.117720
Qi Wu LH, Feng JB Chao (2021) Shaomin Shuang. Ratiometric sensing of Zn2+ with a new benzothiazole-based fluorescent sensor and living cell imaging. Analyst 146:43–48. https://doi.org/10.1039/d1an00749a
Song H-M, Zhao L-X, Zhang S-Q, Ye T, Fu Y, Fei Ye (2021) Design, synthesis, structure-activity relationship, Molecular Docking, and Herbicidal evaluation of 2-Cinnamoyl-3-Hydroxycyclohex-2-en-1-one derivatives as Novel 4-Hydroxyphenylpyruvate dioxygenase inhibitors. J Agric Food Chem 69:43: 12621–12633. https://doi.org/10.1021/acs.jafc.1c04621
Wang Z-W, Zhao Li‐Xia, Ma P, Ye T, Fu Y, Fei Ye (2021) Fragments recombination, design, synthesis, safener activity and CoMFA model of novel substituted dichloroacetylphenyl sulfonamide derivatives. Pest Manag Sci 77:4: 1724–1738. https://doi.org/10.1002/ps.6193
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Chenyan Lv carried out all experiments and wrote the main manuscript text, while Bowen Hu and Yong Tao did supervision and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Informed consent obtained from all individual participants included in the study.
Consent for Publication
The authors affirm that present study does not contain any human research data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, C., Hu, B. & Tao, Y. A Novel AIE-Active Salicylaldehyde-Schiff Base Probe with Carbazole Group for Al3+ Detection in Aqueous Solution. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03859-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03859-7