Abstract
Fe3O4@Ionic liquids β-cyclodextrin polymer(Fe3O4@ mono-6- deoxy-6- (1-ethyl- imidazolium)-β-cyclodextrin iodide polymer, ILs-β-CDCP) was prepared. A novel method based on Fe3O4@ILs-β-CDCP solid phase extraction coupled with fluorescence spectrophotometry for Rhodamine B (RhB) determination, was investigated. Results were shown that RhB was adsorbed on Fe3O4@ILs-β-CDCP and eluted with sodium dodecyl sulfate (SDS) (1.0%) rapidly. Different parameters, such as pH, adsorption time and volume, eluent volume and time were studied. This method introduced linearity for RhB between 0.01–9.00 μg/mL−1 , the limit of detection was 5.2 ng/mL−1 , correlation coefficient (R) was >0.9987 and the relative standard deviation (RSD) was 3.1% (n = 3, c = 4.00 μg/mL). The mechanism of adsorption of RhB on Fe3O4@ILs-β-CDCP was studied through the FTIR analysis and the inclusion constant of Fe3O4@ILs-β-CDCP-RhB. This method was applied successfully for determination of RhB in real samples with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhodamine B (RhB) a pigment, is widely applied as color material in textiles and printing industries [1]. RhB is dangerous for human , causes skin and respiratory tract irritation [16, 22, 23], and has been banned for used as color additive in food stuffs. Some merchants use it in food mono-factories [11] so that harming people health [2]. Therefore , improving an easy method for determination of RhB in various samples is important. Different methods were applied such as ultraviolet spectrophotometry [18] fluorescence spectrophotometry [4], capillary chromatography [8] and HPLC [19] for determination of RhB. Among these methods, fluorescence spectrophotometry has the advantages of lower-cost analysis, easier operation and better accuracy. Thus, direct separation through spectrophotometry is not easy due to the little amounts of RhB in real samples. so, in this work magnetic solid phase extraction (MSPE ) of (Fe3O4@ILs-β-CDCP) coupled with fluorescence spectrophotometry was introduced to determine (RhB) in food samples.
MSPE is a process depend on using magnetic absorbents for separation of various analytes from high amounts of sample. The magnetic absorbent was mixed with sample then analyte adsorbed by magnetic agents. The analyte-magnetic agents was separated from the sample through an external magnetic field after elution with the perfect eluent. Now a days , MSPE absorbent is exactly focused on Fe3O4 nanoparticles (NPs) with special functional groups. Various polymers were used to modify Fe3O4NPs [6, 13].
Ionic liquids (ILs) are a type of organic salts that had special physio-chemical properties like fair stability and hydrophobic properties [7, 9], IL/Fe3O4@ graphene were used as sorbents for MSPE nitrobenzene polymers in water extraction [3].
Ionic liquids coated MNPs were used as adsorbent in separation of aromatic hydrocarbons in water [10], separation of RhB in food using an ionic liquid ß-CDCP SPE -HPLC , determination of Allura Red in food by Ionic liquid ß-CDCP SPE -HPLC [15]. Ionic liquid coated Fe3O4@SiO2 nanoparticles were used accompanied with HPLC determination of RhB in food samples [5]. However , it was not mentioned for determination of RhB by Fe3O4@ ILs-β-CDCP.
In this study, MSPE agents were prepared, these agents have the property of the ionic liquids and (MNPs). Here MSPE adsorbent providing a fast and satisfactory preparation of sample , that applied for separation of high samples concentration in a few minutes. a novel method of MSPE coupled with fluorescence spectrophotometry for determination of (RhB) in food samples was introduced.
Experimental
Apparatus and Chemicals
Chemicals were offered through reagent grade, others mentioned. Standards solutions were brought from Reagent Corporation, Shanghai (China).
N,N-dimethylformamide, sodium hydroxide, hydrochloric acid, potassium iodide, methanol, acetonitrile, acetone, 1-ethylimidazole (Beijing Aipuxilong Biotechnology Co. Ltd., Beijing, China),
Centrifuge (Anke Scientific Instrument Factory, Shanghai), timing multifunctional oscillator (Guohua Co., Ltd., China),digital constant temperature water-bath (Guohua Co., Ltd., China) .
A F-4500 spectrophotometer (Hitachi, Japan) was used for all the fluorescence measurement, with excitation and emission slits at 5.0 nm, λex = 330 nm and 1-cm quartz cell.
Procedure
The synthetic procedure for ILs-β-CDCP was prepared as in literature [14, 26, 27].
Synthesis of Fe3O4@ ILs-β-CDCP
First (3.0 g) of ILs-β-CDCP were dissolved in 15 mL DMF and 30 mL in 70 °C in distilled water for 15 min, then 1.6 mL HDL under nitrogen. The mixture was put through stirring for 24 h. The product was gathered by filtering , and 200-300 mL of acetone was added and filtered and left under a continuous stirring for 24 h in 50 °C.
secondly (4.0 g) of ILs-β-CDCP was added to 10 g of Fe3O4. 7H2O + 10 g FeCl3. 6H2O + 200 mL deionized water and ammonia solution 25 mL in 50 °C under nitrogen for 30 min, temperature was increased to 90 °C, The mixture was washed with deionized water using magnet and dried in vacuum for 24 h in 50 °C to obtain Fe3O4@ ILs-β-CDCP.
Adsorption/Elution
At room temperature, the sample solution after treatment and the pH buffer were added into a tube, and then distilled water was added to 40.0 mL. 0.1 g Fe3O4@ ILs-β-CDCP were added into the tube, then solution was shaked for 15.0 min and centrifuged. 3.0 mL of Sodium dodecyl sulfate (SDS) (1%) was added into the used Fe3O4@ ILs-β-CDCP as eluent. The mixture was ultrasonically vibrated at room temperature. Supernatant solution was separated via A F-4500 fluorescence spectrophotometer.
Sample Preparation
An amount of 5 g of hot pepper, chili powder and Chinese prickly ash were sensitively weighed into the beakers respectively , eluted in 90.0 mL of ethanol. Then evaporated at 60 °C, finally diluted with deionized water in 100.0 mL flask and put in darkness to be detected.
Results and Discussion
Characterization of Fe3O4@ ILs-β-CDCP
Infrared Spectroscopy
Fig. 1 1, showed the FTIR spectrum of (a) ILs-β-CDCP, (b) Fe3O4@ILs-β-CDCP. Characterization data were as follows:
(A) ILs-β-CDCP (IR/KBr, cm−1): 1118,1326, 3127 cm−1. The peak of 1326 cm−1 due to C = C vibration; 3127 cm−1 peak was attributed to C-N vibration, which proved the ILs-β-CDCP synthesis .
(B) Fe3O4@ILs-β-CDCP (IR/KBr, cm−1): 1118,1326, 3127 cm−1. The peak of 1118 cm−1 were due to amide N-H absorption effect ; the peak of 1326 cm−1 correspond to the absorption of band I, II and III of amide; in addition, the peak of 3127 cm−1 was attributed to C = O vibration, which indicated HDI and ILs-β-CDCP reaction and confirmed Fe3O4@ ILs-β-CDCP formation.
Scanning Electron Microscopy
The microscopic morphological structures which applied by SEM to differentiate and compare the external features of Fe3O4ILs-β-CDCP. Fig. 1. 2, showed the SEM micrographs of β-CDCP (a), ILs-β-CDCP (b) and Fe3O4@ILs-β-CDCP(c). When (a) with (b) and(c) was compared , ILs-β-CDCP showed a spongy, fluffy porous and relatively coarse surface while Fe3O4@ILs-β-CDCP (C) showed a different surface similar to layer shape, and more bright with macro pores. The modification of Fe3O4 made the polymer’s surface change.
Characterization by X-Ray Diffraction
The XRD spectra of Fe3O4 , ILs-β-CDCP and Fe3O4@ILs-β-CDCP are shown in Fig. 1. 3. The four peaks appeared at 2 θ of Fe3O4 and ILs-β-CDCP 30.03°, 37.01°, 58.00°, 63.00°. Fe3O4@ILs-β-CDCP had the same peaks as Fe3O4, which proved that the crystal shape of Fe3O4 particles were not altered through modifying of Fe3O4@ ILs-β-CDCP.
Thermo Gravimetric Analysis
Thermo gravimetric analysis (TGA) revealed the weight loss process of the materials, which indicated the difference between the ILs-β-CDCP(a)and Fe3O4@ILs-β-CDCP (b). In this paper, TGA was conducted in a nitrogen atmosphere, and the heating rate employed was 5 °C min−1 all cases from 28 to 1000 °C (Fig. 1.4). The experimental results could be concluded that (1) the ILs-β-CDCP(a) showed a mass loss of about 1.5% after heating to 200–300 °C corresponding to the water content; (2) for the Fe3O4@ILs-β-CDCP(b), an additional weight loss of 5.4% was observed from 300 to 500 °C due to the decomposition of ILs. This observation suggested that the ILs had been located on the surface of β-CDCP.
Optimization of Adsorption
Effect of pH
The pH value is it not only affecting the existing RhB, but also can change the density of the negative charge on the surface of Fe3O4@ ILs-β-CDCP. Thus, it was necessary to investigate the pH value effect, the adsorption efficiency of RhB, was varied with the pH Fig. 2a. It could be concluded that the extraction efficiency of RhB by Fe3O4@ ILs-β-CDCP was above 91.0% when the pH was of 7.0. It reached the highest value 94.1% when pH was 8.0. This due to the fluorescence intensity of RhB
which was increased with pH increasing before 8.0.
Effect of Adsorption Temperature and Time
The extraction efficiency of RhB, on Fe3O4@ILs-β-CDCP at various temperatures (5.0–50.0 °C) were investigated in Fig. 2b. The efficiency of extraction of RhB, by Fe3O4@ILs-β-CDCP was higher than 90% from 25.0 °C to 50.0 °C. The process was carried out at 25.0 °C.
The extraction process was finished in 15.0 min, the adsorption efficiency of RhB remained almost stable (93.0%) Fig. 2c , so 15.0 min was adopted as the extraction time for (RhB).
Effect Sample Volume
The extraction efficiency of RhB, varied with the increase of sample amount, the amount of the sample increased from 10.0 to 90.0 mL. The efficiency of adsorption of (RhB), was above 94% from 10.0 to 80.0 mL and went down slightly when volume was higher than 80.0 mL. Fig. 2d. Therefore the volume allowed was 80.0 mL.
Adsorption Capacity
The capacity of adsorption is known as highest volume of RhB which adsorbed per gram of the(Fe3O4@ILs-β-CDCP). The capacity of adsorption of RhB, on Fe3O4@ILs-β-CDCP was studied through changing RhB concentration in aqueous samples before MSPE procedure. When the concentration of RhB was 60.0 μg mL−1, the adsorption of RhB reached the maximum. The capacity of adsorption for Fe3O4@ILs-β-CDCP was found to be 10.90 mg g−1.
Optimization of Elution
Selection of Eluent
Various eluents were studied in this work, their elution efficiency was ordered as follow: sodium dodecyl sulfonate (SDS) > cetyltrimethyl ammonium bromide (CTAB) > methanol > ethanol > NaOH (0.1 M) > HCl (0.1 M). So (SDS) was adopted.
Effect of Eluent Volume
Effect of (SDS)(1.0%) volume on the elution efficiency of RhB was studied. The results showed that it was over 94% when sodium dodecylsulfonate (SDS) was over than 3.0 mL. So the volume 3.0 mL was selected for (SDS). .
Effect of Elution Time
The elution efficiency was increasing along with time until it reached 94.2% at 15.0 min , then it did not changed and the elution process was finished thereafter. Elution time of 15.0 min was adopted for (RhB).
Reuse of Fe3O4@ ILs-β-CDCP
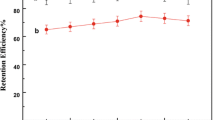
The Fe3O4@ ILs-β-CDCP MNPs were washed with 2.0 mL of ethanol tow times after each MSPE procedure. The reusability of Fe3O4@ ILs-β-CDCP was applied via efficiency of adsorption and elution Fig. 3. The Fe3O4@ ILs-β-CDCP material could be reused nine times with efficiency of adsorption efficiency higher than 84% for Rhodamine B.
Effect of Interferents
The effect of interferents which food samples may contain on separation of RhB in the availability of interferents was investigated. The limit of tolerance for different interferents was 400 for SO4 2− , Br− , 230 for Citrate and phenol, 90 for NO3 − ,18 for Zn2+, Cu2+,glucose, 17 for Safranin T, Allura Red, Congo red, 15 for Bright yellow, Sunset Yellow and 2 for Rhodamine 6G. The results showed that most of the foreign material in samples had no interference in RhB determination.
Analytical Performance
Under optimum conditions described above, The proposed method introduced wide linearity within the concentration range of 0.10–9.00 μg mL−1 with a correlation coefficient (R) >0.9987. Low detection limit 5.2 ng mL−1 was found.
Sample Analysis
This method was introduced to separate RhB in hot pepper, chili powder and Chinese prickly ash Table 1. RhB amount was 5.46 μg kg−1 in chili powder, the recovery rate of RhB was from 99.0% to 100.8%. and it was 4.33 μg kg−1 in hot pepper the recovery rate of RhB from 97.0% to 100.7%.
No RhB was detected in Chinese prickly ash, the recovery rate of (RhB) was from 100.5% to 100.2%.
Adsorption Mechanism of Fe3O4@ ILs-β-CDCP for Rhodamine B
The mechanism of adsorption of RhB on Fe3O4@ILs-β-CDCP was studied through the FTIR analysis and the inclusion constant.
FTIR Analysis
The FTIR spectrum for Fe3O4@ILs-β-CDCP and rhodamine B are important to study the mechanism of adsorption of rhodamine B on this polymer [24].
FTIR spectrum for rhodamine B, the Fe3O4@ILs-β-CDCP and Fe3O4@ ILs-β-CDCP - rhodamine B was studied (Fig. 4. 1). The intensity of the σ C=C peaks from 1118 to 1326 cm−1 in imidazole ring of Fe3O4@ ILs-β-CDCP -rhodamine B , are so high than those of the Fe3O4@ ILs-β-CDCP . The ILs bond interactions play a great role in the Fe3O4@ ILs-β-CDCP -rhodamine B inclusion [21]. The σ O-H peak (3127 cm−1) in the Fe3O4@ ILs-β-CDCP -rhodamine B increased, which composes that the hydroxyl part in rhodamine B is enclosed in the Fe3O4@ ILs-β-CDCP- rhodamine B.
Inclusion Constant
The inclusion constant K shows inclusion properties of host-guest molecules. The higher K was, the more stable inclusion complex was. K is calculated according to Benesi-Hildebrand- equation (double reciprocal plot) [20].
In this work, the inclusion constants of the monomers of two kinds of polymers (ILs-β-CDCP and Fe3O4@ILs-β-CDCP) and RhB were obtained .
The double reciprocal plots for them were studied (Fig. 4 2). The two of them showed a satisfactory linearity and correlation coefficients of 0.9827 for ILs-β-CDCP and 0.9838 for Fe3O4@ILs-β-CDCP. It was concluded that both ILs-β-CDCP and Fe3O4@ILs-β-CDCP with rhodamine B at the ratio of 1:1. The inclusion constant (K) of Fe3O4@ILs-β-CDCP- RhB inclusion complex was 1.18 × 104 L/mol, which was higher than 5.87 × 103 L/mol for ILs-β-CDCP- RhB. It indicated that the inclusion ability of Fe3O4@ILs-β-CDCP towards RhB was stronger than that of ILs-β-CDCP. This was due to the adsorption efficiency of Fe3O4@ILs-β-CDCP which was better than that of ILs-β-CDCP.
The inclusion constants of ILs-β-CDCP- rhodamine B and Fe3O4@ ILs-β-CDCP- rhodamine B inclusion complexes were studied at (pH = 4.0 and pH = 11.0) and results were displayed in (Fig. 4 3), the values of R2 were nearly 1 and it was form the inclusion complexes with rhodamine B at the ratio of 1:1.
On the these basis, it could be concluded that: (1) the inclusion constants of Fe3O4@ ILs-β-CDCP-rhodamine B were always greater than that of ILs-β-CDCP- rhodamine B ,which is reflected that the inclusion ability of Fe3O4@ ILs-β-CDCP towards rhodamine B was stronger than that of ILs-β-CDCP; (2) the inclusion constants of Fe3O4@ ILs-β-CDCP-rhodamine B were stable in different pH. It was consistent with the effect of pH on the adsorption efficiency and explained the adsorption efficiency was associated with the formation of inclusion complexes. These two all declared that the presence of ionic liquid enhanced the inclusion ability of Fe3O4@ILs-β-CDCP with rhodamine B and was conducive to adsorption of rhodamine B.
Comparison with Other Method
Table 2, listed the linear range and the limit of detection for separation of (RhB)in real samples obtained by the reported methods, such as HPLC, SPE-UV spectrophotometry, SPE-HPLC, MSPE-HPLC and HPLC–CL method. Compared with other reported methods, the method adopted in the present work obviously had a satisfactory linear range and limit of detection.
Conclusion
In this work, Fe3O4@ILs-β-CDCP was synthesized as magnetic solid phase extraction material to separate (RhB), in food samples. The proposed method for the analysis of Rhodamine B was proved to be satisfactory.
References
Alesso M, Bondioli G, Talio MC, Luconi MO, Fernandez LP (2012) Micelles mediated separation fluorimetric methodology for rhodamine B determination in condiments, snacks and candies. Food Chem 134:513–517
Baldev E, MubarakAli D, Ilavarasi A, Pandiaraj D, Sheik Syed Ishack KA, Thajuddin N (2013) Degradation of synthetic dye, rhodamine B to environmentally non-toxic products using microalgae. Colloid Surface B 105:207–214
Cao XJ, Shen LX, Ye XM, Zhang FF, Chen JY, Mo WM (2014) Ultrasound-assisted magnetic solid-phase extraction based ionic liquid-coated Fe3O4@graphene for the determination of nitrobenzene compounds in environmental water samples. Analyst 10:1–8
Chauhan VM, Hopper RH, Ali SZ, King EM, Udrea F, Oxley CH, Aylott JW (2014) Thermo-optical characterization of fluorescent rhodamine B based temperature-sensitive nanosensors using a CMOS MEMS micro-hotplate. Sensor Actuat B Chem 192:126–133
Chen JP, Zhu XS (2016) Magnetic solid phase extraction using ionic liquid-coated core–shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of rhodamine B in food samples. Food Chem 200:10–15
Chen HM, Deng CH, Zhang XM (2010) Synthesis of Fe3O4@SiO2@PMMAcore – shell–shell magnetic microspheres for highly efficient enrichment of pep-tides and proteins for MALDI-TOF MS analysis. Chem Int Ed 49:607–611
Dang GF, Ma XG, Zhou JP (2012) The preparation of ionic liquid load modified magnetic nanomaterials and used for the determination of trace cadmium ion in water samples. Chinese Journalof Instrumental Analysis 31:823–827
Desiderio C, Marra C, Fanali DS (1998) Quantitative analysis of synthetic dyes in lipstick by micellar electrokinetic capillary chromatography. Electrophoresis 19:1478–1483
Galan-Cano F, Alcudia-Leon MC, Lucena R, Cardenas S, Valcarcel M (2013) Ionic liquid coated magnetic nanoparticles for the gas chromatography/mass spectrometric determination of polycyclic aromatic hydrocarbons in waters. Chromatogr A 1300:134–140
He H, Yuan DH, Gao ZQ, Xiao DL, He H, Dai H, Peng J, Li NV (2014) Mixed hemimicelles solid-phase extraction based on ionic liquid-coated Fe3O4/SiO2 nanoparticles for the determination of flavonoids in bio-matrix samples coupled with high performance liquid chromatography. Chromatogr A 1324:78–85
Jain R, Mathur M, Sikarwar S, Mittal A (2007) Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. Environ Manag 85:956–964
Li WJ, Zhou X, Tong SS, Jia Q (2013) Poly(N-isopropylacrylamide-co-N,N '--methylene bisacrylamide) monolithic column embedded with gamma-alumina nanoparticles microextraction coupled with high-performance liquid chromatography for the determination of synthetic food dyes in soft drink samples. Talanta 105:386–392
Li XS, Xu LD, Zhu GT, Yuan BF, Feng YQ (2012) Zirconium arsenate-modified magnetic nanoparticles: preparation, characterization and application to the enrichment of phosphopeptides. Analyst 137:959–967
Mahlambi MM, Malefetse TJ, Mamba BB, Krause RW (2010) β-cyclodextrin-ionic liquid polyurethanes for the removal of organic pollutants and heavy metals from water: synthesis and characterization. Polym. Res 17:589–600
Qin XX, Zhu XS (2015) Determination of allura red in food by ionic liquid ß-cyclodextrin-cross-linked polymer solid phase extraction and high-performance liquid chromatography. Analytical Letter 48:189–199
Roberts ALK, Fletcher JM, Moore L, Byers S (2010) Trans-generational exposure to low levels of rhodamine B does not adversely affect litter size or liver function in murine mucopolysaccharidosis type IIIA. Mol Genet Metab 101:208–213
Soylak M, Unsal YE, Yilmaz E, Tuzen M (2011) Determination of rhodamine B in soft drink, waste water and lipstick samples after solid phase extraction. Food Chem Toxicol 49:1796–1799
Sun WJ, Li J, Mele G, Zhang ZQ, Zhang FX (2013) Enhanced photocatalytic degradation of rhodamine B by surface modification of ZnO with copper (II) porphyrin under both UV–Vis and visible light irradiation. Mol Catal A-Chem 366:84–91
Valverde RS, Perez IS, Franceschelli F, Galera MM, Gil Garcia MD (2007) Determination of photoirradiated tetracyclines in water by high-performance liquid chromatography with chemiluminescence detection based reaction of rhodamine B with cerium (IV). Chromatogr A 1167:85–94
Wan K, Li TF, Lu Q, Wang MJ, Lu P (2006) Effect of the pH on the β-cyclodextrins inclusion complex with methylene bule. Chem Res Appl 18:917–920
Ping WH, Zhu XS, Wang B (2014) An ionic liquid loaded β-cyclodextrin - cross linked polymer as the solid phase extraction material coupled with high-performance liquid chromatography for the determination of Rhodamine B in food. Analytical Letter 47:504–516
Xiao J, Gong S, Ledoux MS (2007) Caytaxin deficiency disrupts signaling pathways in cerebellar cortex. Neurosci 144:439–461
Xie C, Chang J, Hao XD, Yu JM, Liu HR, Sun X (2013) Mitochondrial-targeted prodrug cancer therapy using a rhodamine B labeled fluorinated docetaxel. Eur JPharm Biopha 85:541–549
Yang ZJ, Chai KG, Ji HB (2011) Selective inclusion and separation of cinnamaldehyde and benzaldehyde by insoluble b-cyclodextrin polymer. Sep Purif Technol 80:209–216
Zhang Q, Cui LH, Myint A, Lian M, Liu LJ (2005) Sensitive determination of phenolic compounds using high-performance liquid chromatography with cerium(IV) rhodamine 6G-phenolic compound chemiluminescence detection. Chromatogr A 1095(1–2):94–101
Zhou ZM, Li X, Chen XP, Hao XY (2010) Synthesis of ionic liquids functionalized β-cyclodextrin-bonded chiral stationary phases and their applications in high-performance liquid chromatography. Anal Chim Acta 678:208–214
Zhou N, Zhu XS (2013) Ionic liquids functionalized β-cyclodextrin polymer for separation/analysis magnolol. Pharmaceut Anal 4(4):242–249
Acknowledgements
Authors acknowledge financial supply of National Natural Science Foundation of China (21375117) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Natural Science Foundation of China (21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of Interest
Authors have a financial relationship with the organizations that sponsored the research. And the organizations are listed as follows:
(1) For Prof. Xia shi Zhu
Prof. Xia shi Zhu has received research grants from the National Natural Foundation of China(21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
(2) For Almojtaba Bakheet.
He is the current PhD students, declares that he has no conflict of interest.
Ethical Standards – Animal Rights and Informed Consent
The research proposed in this article does not contain any studies with human or animals subjects. There are no ethical issues with human or animal subjects in our studies.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Ahmed Bakheet, A., Zhu, X.s. Determination of Rhodamine B in Food Samples by Fe3O4@ Ionic Liquids-β-Cyclodextrin Cross Linked Polymer Solid Phase Extraction Coupled with Fluorescence Spectrophotometry. J Fluoresc 27, 1087–1094 (2017). https://doi.org/10.1007/s10895-017-2042-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2042-1