Abstract
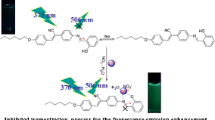
The recognition ability of N-Furfurylsalicylaldimine (HL) toward various cations (Pb2+, Hg2+, Ba2+, Cd2+, Ag+, Zn2+, Cu2+, Ni2+, Co2+, K+, Sr2+, and Na+) has been studied by UV–Vis and fluorescence spectroscopy. The compound showed highly selective fluorescence signaling behaviour for Zn2+ ions in methanol-water medium based on CHEF process and is capable of distinguishing Zn2+ from Cd2+ ion. From single crystal X-ray analysis it is revealed that a Zn2+ ion binds two ligand molecules through imine nitrogen and phenolate oxygen atom.

N-Furfurylsalicylaldimine as a selective sensing of Zn2+ ion through CHEF process. The x-ray structure of the receptor-Zn(II) complex shows 2:1 stochiometry
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much attention has been focused in recent years on the development of new chemical sensors for the recognition of heavy metal ions as well as anionic species [1]. Metal ions play a vital role in a wide range of chemical and biological processes [2], yet some of them are potentially toxic. It is indeed important to detect metal ions for applications in waste management and environmental toxicology [2]. Although zinc is only moderately abundant in nature, ranking twenty third of the elements in the Earth’s crust, it is, however, a omnipresent and indispensable element in the human body and the second most abundant transition metal after iron [3]. In total, the adult human body contains 2–3 g of zinc [4]. For a long period of time, zinc has been known as an essential trace element, acting as a structural component of many protein scaffolds (e.g., carbonic anhydrase and zinc finger protein) [3, 5]. A considerable amount of zinc at a concentration of several milli-molar is accumulated in the presynaptic neurons of the brain and subsequently released when the neurons are active [6]. Thus, the detection and separation of zinc in a wide range of concentrations from nano-to the milli-molar scale is of immense interest and significance [7]. Furthermore, the greatest and most important challenge for the detection of zinc appears from the interference of other transition metal ions, in particular cadmium ion. Even though a number of techniques like atomic absorption spectroscopy, inductively-coupled plasma mass spectrometry and inductively coupled plasma atomic emission spectroscopy are available for the detection of these metal ions in food samples, they require tedious procedures and are also very expensive [8]. Optical signals based on changes in absorbance or fluorescence is the most frequently applied technique because of the simplicity of the experimental methods. A great number of fluorescent sensors have been designed to detect different kinds of metal ions. For the fluorescent detection of ions; fluorescence enhancement “turn-on” is preferable to fluorescence quenching “turn-off”, because the former reduces the chance of false positive data by other fluorescent quenchers existing in samples. [9] Design of a fluorescent probe is generally based on intramolecular charge transfer (ICT) [10], photoinduced electron transfer (PET) [11], chelation-enhanced fluorescence (CHEF) [12], metal − ligand charge transfer (MLCT) [13], excimer/exciplex formation [14], imine isomerisation [15], intermolecular hydrogen bonding [16], excited-state intramolecular proton transfer [17], displacement approach [18], and fluorescence resonance energy transfer (FRET) [19]. In CHEF process, the electron pair is shared by coordination to a cation. As a result, the redox potential of the receptor is raised so that the highest occupied molecular orbital (HOMO) of the receptor becomes lower in energy than that of the fluorophore [20].
In recent time several chemosenors for zinc ions are reported in the literature [21–23]. Hsieh et al. reported A simple Schiff base type fluorescent receptor which exhibits an ‘off–on-type’ mode with high selectivity in the presence of Zn2+ ion through the formation of a rigid structure [24]. Another Schiff’s base chemosensor is reported by Li et al. which displays specific recognition to Zn2+, and especially avoids the interference of Cd2+ when tested against a range of physiological and environmentally relevant metal ions [25]. Recently Kim et al. reported a simple receptor which could simultaneously detect three biologically important metal ions (Zn2+ Fe2+ and Cu2+) in aqueous solution. The sensor could function as a “turn-on” fluorescence receptor only to Zn2+ ions and could be successfully applied to the detection of intracellular Zn2+ ion [26].
In this paper, we present N-Furfurylsalicylaldimine (HL) as a highly selective fluorescent sensor for Zn2+ ion in CH3OH-H2O mixture by chelation-enhanced fluorescence (CHEF). The fluorescence properties and high selectivity of the ligand for Zn2+ ion over other possible competitive cations were investigated in detail. The X-ray single crystal structure of the Zn(II) complex with the ligand is reported.
Experimental
Materials and Physical Measurements
The ligand N-Furfurylsalicylaldimine is prepared as per the literature method [27]. Furfurylamine (Sigma Aldrich), salisaldehyde (Merck, India) and zinc acetate dihydrate (Merck, India) have been used as received. Solution of HL is prepared in methanol medium. Metal salts such as nitrate of Na+, Ag+, Ca2+, Sr2+, Ba2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Cd2+ and chloride salt of Hg2+ were purchased from Merck, India and have been used as received. Metal salt solutions were prepared in a phosphate buffer (0.1 M) with KH2PO4 (0.1 M) and Na2HPO4 (0.1 M) solution (pH 7; MeOH:H2O = 9:1, v/v). Buffer capsules were purchased from Merck India. Absorption and fluorescence spectra are recorded in Shimadzu 1601 spectrophotometer and Hitachi F-7000 spectrofluorimeter respectively.
Determination of the Binding Constant
The binding constant was calculated based on the titration curve of the HL with metal ions. Binding constant was determined by a nonlinear least squares fit of the data with the following equation as referenced elsewhere [30].
Where ‘x’ is (I-Io)/(Imax-Io), ‘y’ is the concentration of metal ion, ‘a’ is the binding constant, and ‘b’ is the concentration of sample. Here (I-I0)/(Imax-I0) is the fluorescence intensity ratio.
Determination of Fluorescence Quantum Yield
Here, the quantum yield φ was measured by using the following equation,
Where, ‘x’ and ‘s’ indicate the unknown and standard solution respectively, φ = quantum yield,
F = area under the emission curve, A = absorbance at the excitation wave length, n = index of refraction of the solvent. Here φ measurements were performed using anthracene in ethanol as standard [φ = 0.27] (error ~ 10 %) [31].
Synthesis of [Zn(L)2]
Zinc(II) acetate.dihydrate (0.2915 g, 1 mmol) was dissolved in 10 ml methanol in a round-bottom flask. Methanolic solution (10 ml) of HL (0.2010 g, 1 mmol) was added drop-wise with constant stirring for about 20 min at room temperature. After that the whole reaction mixture was refluxed for 1 h to get a greenish-yellow solution. The solution was cooled to room temperature and filtered. The greenish-yellow filtrate was kept in air at room temperature for crystallization. After 2 weeks, pale yellow block shaped single crystals of diffraction quality were obtained on slow evaporation of the filtrate. Yield 70 %.
X-Ray Crystallography
Data collections of 1 was carried out at 120(2) K on an Oxford Diffraction Gemini Ultra diffractometer. Cell refinement, indexing and scaling of the data sets were done with CrysAlisPro package, Version 1.171.35.10 [32]. The structures were solved by using the olex2.solve solution program [33] using the charge flipping algorithm and refined by the full matrix least-squares method based on F2 with all observed reflections [34]. The crystallographic details are listed in table 1.
Results and Discussion
Emission Studies
At first we have investigated the fluorescence properties of the ligand HL and its fluorescence response to various metal cations in methanol-water solvent. The ligand itself shows negligible fluorescence at 443 nm upon excitation at 330 nm, however, fluorescence enhancement is observed when Zn2+ ion is added to the methanolic solution of the ligand. The native fluorescence of the ligand is minimal, but when Zn2+ is added incrementally, fluorescence increases and reaches a maximum on addition of 300 micro litres Zn2+ solution (concentration, 30 μM) at 443 nm (Fig. 1). The interaction of the ligand with Zn2+ ion resulted in fluorescence enhancement, which is attributed to a chelation-enhanced fluorescence (CHEF) effect. We believe that the zinc ion is effectively acting as an electrophile which attacks the electron-rich hydroxyl oxygen atom of the HL to form a rigid metal complex. The quantum yield of ligand HL remarkably changed from 0.003 to 0.321 on the formation of the complex with Zn2+ metal ion, which indicates 107 folds enhancement in quantum yield.
Ion selectivity is an important property of the fluorescence probes. Thus, we evaluated the fluorescent response of HL with different metal ions, including Ag+, Na+, Ca2+, Ba2+, Sr2+, Pb2+, Hg2+, Co2+, Ni2+, Cu2+ and Cd2+ in buffer solution and found the perfect selectivity for Zn2+ with a considerable signal output (Fig. 2). It is worth noting, that the ligand distinguishes Zn2+ from Cd2+, which might cause interference since both metal cations have very similar properties [28].
The existence of other transition metal ions may affect the detection ability of HL. The Zn2+ selectivity and the fluorescence behaviour of HL remain uninterrupted with the presence of many other metal ions. Compared with some available Zn2+ sensors, which exhibit some enhancement of the fluorescence for Cd2+ [28], the ligand HL has a selective response to Zn2+ without the interference of Cd2+ in the buffered solution, whereas, the existence of copper obviously quenched the fluorescence intensity [28, 29]. (Fig. 3)
We have studied the binding ability of HL towards Zn2+ by fluorescence titration. A Job’s plot indicates the 1:2 coordination stoichiometry of Zn2+ with HL (Fig. 4), exhibiting a binding constant a = 4.7 × 1010 M−2, (R 2 = 0.9783) (Fig. 5) from the theoretical nonlinear square fit of experimental data to a 1:2 binding model. The obtained data indicate a typical CHEF effect, originating from the coordination of the de-protonated ligand (L−) through the imine nitrogen and de-protonated phenolate oxygen atom (Scheme 1). The detection limit of the HL towards Zn2+ was found to be about 3.2 μM based on fluorescence titration experiments.
X-ray Crystallography
The crystal structure of the Zn2+ complex of the HL shows 1:2 binding and a distorted tetrahedral geometry around Zn2+ is identified. As seen in Fig. 6, an imine nitrogen atom (N9) from the receptor is at the apex of the tetrahedral zinc (Zn11) and the phenolate oxygen atom (O1) of the same receptor molecule is also coordinated to Zn(II). An imine nitrogen (N91) and a phenolate oxygen (O11) from another receptor moleculecompletes tetrahedral coordination. Thus the two receptor molecules are chelated with zinc atom. The N-Zn-N and O-Zn-O bond angles are 121.29° and 123.04° respectively. The bond lengths and the bond angles are listed in Table 2.
UV–Vis Spectroscopic Studies
We have also studied the change in absorption behaviour of the ligand upon binding with Zn2+ ion. As the concentration of Zn2+ was increased, the intensity of the absorption bands produced by the Zn2+-free ligand at 317 and 256 nm gradually decreased, accompanied by new absorption bands at 238, 272 and 363 nm produced by the Zn2+-bound ligand appearing at longer wavelength with gradual increase in the intensity (Fig. 7). The spectra obtained during the stepwise addition showed four isosbestic points indicates a clean conversion of HL into its corresponding Zn2+ complex. In addition Cu2+, Co2+ and Ni2+ show increased absorption bands at 238, 272 and 363 nm but the intensity is less than Zn2+ indicates that the ligand is sensitive for Zn2+ which is shown in bar graph representation (Fig. 8).
Conclusion
In this paper we have studied a sensor for Zn2+ ion. The thorough investigation of the fluorescence sensing and binding properties of the ligand HL displays high selectivity luminescent probe for Zn2+. The increase in emission in the presence of Zn2+ ion is accounted for by the formation of a rigid metal-ligand complex. The X-ray crystal structure reveals that the Zn(II) complex is mononuclear with distorted tetrahedral geometry. An 107-fold increase in quantum yield is identified upon coordination of HL with Zn(II) ion is attributed as Zn2+-selective chelation-enhanced fluorescence response.
References
ThomasIII SW, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386
Szacilowski K, Macyk W, Matuszek AD, Brindell M, Stochel G (2005) Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev 105:2647–2694
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Frederickson CJ, Koh J-H, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Neurosci 6:449–462
Berg JM, Shi YG (1996) The galvanization of biology: a growing appreciation for the roles of zinc. Science 271:1081–1085
Assaf SY, Chung SH (1984) Release of endogenous Zn2+ from brain tissue during activity. Nature 308:734–736
Outten CE, O’Halloran TV (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492
Spichiger-Keller US (1998) Chemical sensors and biosensors for medical and biological applications. Wiley-VCH, Weinheim
Nolan EM, Lippard SJ (2008) Tools and tactics for the optical detection of mercuric ion. Chem Rev 108:3443–3480
Xu Z, Xiao Y, Qian X, Cui J, Cui D (2005) Ratiometric and selective fluorescent sensor for CuII based on internal charge transfer (ICT). Org Lett 7:889–892
Gunnlaugsson T, Davis AP, O’Brien JE, Glynn (2002) M fluorescent sensing of pyrophosphate and Bis-carboxylates with charge neutral PET chemosensors. Org Lett 4:2449–2452
Lim NC, Schuster JV, Porto MC, Tanudra MA, Yao L, Freake HC, Bruckner C (2005) Coumarin-based chemosensors for Zinc(II): toward the determination of the design algorithm for CHEF-type and ratiometric probes. Inorg Chem 44:2018–2030
Beer PD (1998) Transition-metal receptor systems for the selective recognition and sensing of anionic guest species. Acc Chem Res 31:71–80
Nishizawa S, Kato Y, Teramae N (1999) Fluorescence sensing of anions via intramolecular excimer formation in a pyrophosphate-induced self-assembly of a pyrene-functionalized guanidinium receptor. J Am Chem Soc 121:9463–9464
Wu J-S, Liu W-M, Zhuang X-Q, Wang F, Wang P-F, Tao S-L, Zhang X-H, Wu S-K, Lee S-T (2007) Fluorescence turn on of coumarinderivatives by metal cations: a new signaling mechanism based on C=N isomerization. Org Lett 9:33–36
Sahana A, Banerjee A, Guha S, Lohar S, Chattopadhyay A, Mukhopadhyay SK, Das D (2012) Highly selective organic fluorescent probe for azide ion: formation of a “molecular ring”. Analyst.137:1544-1546.
Peng X, Wu Y, Fan J, Tian M, Han KJ (2005) Colorimetric and ratiometric fluorescence sensing of fluoride: tuning selectivity in proton transfer. J Org Chem 70:10524–10531
Das S, Guha S, Banerjee A, Lohar S, Sahana A, Das D (2011) 2-(2-Pyridyl) benzimidazole based Co(II) complex as an efficientfluorescent probe for trace level determination of aspartic and glutamic acid in aqueous solution: A displacement approach. Org Biomol Chem 9:7097–7104
Serin JM, Brousmiche DW, Frechet JMJ (2002) A fret-based ultraviolet to near-infrared frequency converter. J Am Chem Soc 124:11848–11849
Majzoub AE, Cadiou C, Olivier ID, Tinant B, Chuburu F (2011) Cyclam-methylbenzimidazole: a Selective OFF-ON Fluorescent Sensor for Zinc. Inorg Chem 50: 4029-4038 and references there in
Song EJ, You JKGR, Park GJ, Kim YKS-J, Kim C, Harrison RG (2013) A single molecule that acts as a fluorescence sensor for zinc and cadmium and a colorimetric sensor for cobalt. Dalton Trans 42:15514–15520
Ganguly A, Paul BK, Ghosh S, Kar S, Guchhait N (2013) Selective fluorescence sensing of Cu(II) and Zn(II) using a new Schiff base-derived model compound: naked eye detection and spectral deciphering of the mechanism of sensory action. Analyst 138:6532–6541
Xiao F, Shen J, Qu J, Jing S, Zhu D-R (2013) A fluorescent sensor with mixed N/O/Se donor atoms for probing Zn(II) ion. Inorg Chem Commun 35:69–71
Hsieh WH, Wanb C-F, Liao D-J, Wua A-T (2012) A turn-on Schiff base fluorescence sensor for zinc ion. Tetrahedron Lett 53:5848–5851
Li L, Dang Y-Q, Li H-W, Wang B, Wu Y (2010) Fluorescent chemosensor based on Schiff base for selective detection of zinc(II) in aqueous solution. Tetrahedron Lett 51:618–621
Kim KB, Kim H, Song EJ, Kim S, Noh I, Kim CA (2013) cap-type Schiff base acting as a fluorescence sensor for zinc(II) and a colorimetric sensor for iron(II), copper(II), and zinc(II) in aqueous media. Dalton Trans 42:16569–16577
Bhagwat UA, Mukhedkar VA, Mukhedkar AJ (1980) Study of ligand isomeric complexes of N-Furfurylsalicylaldimine. J Chem Soc Dalton Trans:2319
Hanaoka K, Kikuchi K, Kojima H, Urano Y, Nagano T (2004) Development of a Zinc ion-selective luminescent lanthanidechemosensor for biological applications. J Am Chem Soc 126:12470–12471
Baek NY, Heo CH, Lim CS, Masanta G, Cho BR, Kim HM (2012) A highly sensitive two-photon fluorescent probe for mitochondrial zinc ions in living tissue. Chem Commun 48:4546–4548
(a) Kubo Y, Kato M, Y Misawa, Tokita S (2004) A fluorescence-active 1,3-bis(isothiouronium)-derived naphthalene exhibiting versatile binding modes toward oxo anions in aqueous MeCN solution: new methodology for sensing oxoanions. Tetrahedron Lett 45:3769-3772. (b) Yang MH, Thirupathi P, Lee KH (2011) Selective and Sensitive Ratiometric Detection of Hg(II) Ions Using a Simple Amino Acid Based Sensor. Org Lett 13:5028-5031
Morris JV, Mahaney MA, Huber JR (1976) Fluorescence quantum yield determinations. 9, l0-diphenylanthracene as a reference standard in different solvents. J Phys Chem 80(9):969–974
Aligent (ed) (2010) CrysAlis PRO. Agilent Technologies Ltd, Yarnton
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Acknowledgments
This project was financially supported by CSIR, New Delhi, India [No. 01(2536)/11/EMR-II]. S.K.J. is thankful to UGC for providing research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary data
CCDC 945929 contains the supplementary crystallographic data for 1. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336 033; or e-mail: deposit@ccdc.cam.ac.uk. (DOCX 535 kb)
Rights and permissions
About this article
Cite this article
Jana, S.K., Bera, M., Puschmann, H. et al. Sensing of Zn2+ion by N-Furfurylsalicylaldimine Based on CHEF Process†. J Fluoresc 24, 1245–1251 (2014). https://doi.org/10.1007/s10895-014-1407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1407-y