Abstract
Imbalance of zinc ion (Zn2+) in human body causes diseases like Alzheimer’s and Parkinson’s and therefore Zn2+ estimation in biological fluids has diagnostic values. Fluorescence “off-on” sensors have advantages of high sensitivity and in situ application over other sensors. A new fluorescent “off-on” Zn2+ sensor, N-benzoate-N′ salicylaldehyde ethynelediamine (L), has been synthesisied. In 1:1(v/v) CH3OH:PBS (PBS = phosphate buffer solution), L shows ca. 20 times enhancement in fluorescence intensity on interaction with Zn2+, due to snapping of photoinduced electron transfer (PET) process, which is selective over metal ions - Na+, K+, Ca2+, Ni2+, Cu2+, Cd2+, Hg2+ and Pb2+. These metal ions either individually or all together does not interfere the sensing ability of L towards Zn2+. A 1:1 interaction between L and Zn2+ ion with binding constant 104.25 has been established from spectroscopic data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the recognition and sensing of biologically and environmentally important metal ions by fluorescence technique has emerged as a significant goal in the field of chemical sensors [1, 2]. Among the various analytical methods that are available for the detection of cations, the techniques based on fluorescence sensors demonstrate substantial advantages in terms of sensitivity, selectivity, low cost, facile operation, response time, local observation and the widespread availability of equipment for analysis [3–5].
Zinc ion (Zn2+) is an important metal ion in biology and its imbalance in human body has been related to a number of diseases like Alzheimer’s and Parkinson’s [6–13]. For diagnosis of such diseases it is required to estimate Zn2+ in biological fluids. Fluorescent sensors have advantages of being cheap and applicable for in situ estimation over other methods. “off-on” type fluorescent sensors have extra advantage of high sensitivity. Photoinduced electron transfer (PET) is one of the prime principle behind fluorescent “off-on” sensors for Zn2+ [14–16]. In recent years, a number of fluorescent sensors for Zn2+ have been developed [14]. A photo switchable Zn2+ selective spiropyran-based sensor was developed by S. Giordani et al. [17]. Chen and co-workers reported a quinoline carboxaldehyde and phenylenediamine based ratiometric fluorescent sensor for Zn2+ [18]. P. Banerjee et al. also reported the selective fluorescent zinc sensing property by certain Schiff base compounds [19]. A dual fluorescent sensor based on calyx-4-arene for Zn2+ and Ni2+ was reported by C.P. Rao etal [20]. We have previously reported fluorescent Zn2+ sensors based on N, N, N, N-tetradentate macrocycle [21] and ferrocene derivative [22].
In this paper, we report a new compound (L) Scheme 1, synthesized by a two-step condensation reaction of benzoic acid, ethylenediamine and salicylaldehyde. In 1:1 (v/v) CH3OH:H2O, L was found to exhibit a fluorescent peak at λmax value 450 nm on excitation by 350 nm wavelength photons. L can detect Zn2+ by fluorescence “on” mode with an enhancement in fluorescence intensity of ca 20 times while metal ions Na+, K+, Ca2+, Ni2+ , Cu2+, Cd2+, Hg2+ and Pb2+ do not interfere.
Experimental
Materials and methods
All the chemicals were from Merck except salicylaldehyde and ethylene diamine (Loba Chemie). The metal salts were recrystallized from water (Millipore) before use. Metal salt solutions (10−2 M) were prepared in phosphate buffer solutions (PBS) at pH 7.0. Fluorescent spectra were recorded in a Hitachi 2500 spectrophotometer using quartz cuvette (1 cm path length). A 9.0 × 10−4 M solution of L in 1:1 (v/v) CH3OH: PBS, pH 7.0 was used in the experiments. The excitation wavelength was 350 nm, emission range was 400–600 nm (λmax at 450 nm) and excitation/emission slit was 5 nm.
UV/Visible spectra of L (10-4 M in 1:1 (v/v) CH3OH:PBS) were recorded in a Shimadzu UV 1800 spectrophotometer. 1H NMR and 13C NMR spectra were recorded in a Bruker Ultrashield 300 spectrometer. All NMR spectra were obtained in CDCl3 at room temperature and the chemical shifts are reported in δ values (ppm) relative to TMS.
Electrochemical measurements were carried out in a CHI 600B electrochemical analyser, consisting of a three-electrode assembly with a glassy carbon disc as the working electrode, Ag/AgCl (3 M NaCl) as the reference electrode and tetrabutylammonium perchlorate (TBAP, 0.01 M) as the supporting electrolyte. The working electrode was cleaned as reported [23] by polishing with 0.1 μM alumina slurry using a polishing kit (CHI), followed by sonication in distilled water for 5 min.
Synthesis of L
0.122 g (0.01 mol) of benzoic acid was dissolved in 10 mL of methanol and 0.067 g (0.01 M) of ethylenediamine was added to it. The mixture was refluxed for 1 h and the solvent was evaporated in rota-evaporator. The product, N-benzoateethylenediamine, thus obtained was dried and recrystallized from methanol. 0.164 g (0.01 M) of N-benzoateethylenediamine was taken in 10 mL methanol in a round bottom flask, 0.124 g (0.01 M) of salicylaldehyde was added and refluxed for 20 min. The solid compound (N-banzoate-N′ salicylaldehyde ethynelediamine, L) obtained was separated by filtration and dried in a dessicator.
-
FTIR (KBr): 3448 cm−1 (νN−H), 1638 cm−1 (νC=O), 2339 cm−1 (νC=N) and 3427 cm−1 (νN−H).
-
1 HNMR HNMR (CDCl3,TMS, δ ppm): 3.95 (−CH2-, ethylenediamine), 6.86–7.29 (C6H5-), 8.36 (−NH).
-
13 CNMR (CDCl3,TMS, δ ppm): 166 (−C = O), 160 (−N = C-), 132–116 (C6H5-), 59 & 31 (−NHCH2CH2N=).
Results and discussion
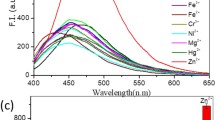
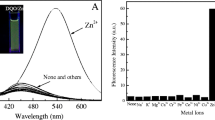
The fluorescence spectral changes of L on interaction with metal ions—Na+, K+, Ca2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+ and Pb2+ was investigated in 1:1 (v/v) CH3OH:PBS. It was observed that on excitation of L by 350 nm wavelength radiation, the emission band was obtained in the range 400–600 nm with the λmax value at 450 nm. However, on addition of different concentration of Zn2+ ion (0.99 × 10−4 M to 9.0 × 10−4 M), it was revealed that the intensity of the fluorescent peak at 450 nm increased remarkably with the increasing concentration of Zn2+ ion. The increase in the fluorescence intensity on addition of Zn2+ ions at the final added concentration was calculated to be approximately 20 times to the original one. Figure 1 depicts the fluorescence spectral changes of L upon addition of different concentration of Zn2+ (from 0.99 × 10−4 M to 9.0 × 10−4 M). Figure 2 depicts the plot of I/Io as a function of Zn2+ concentration, where I is the intensity at a given concentration of Zn2+ and Io is the intensity at zero concentration of Zn2+. The I/Io value increased linearly (R2 = 0.9561) to 20 till the concentration of Zn2+ became 9.0 × 10−4 M and remained constant thereafter.
Similarly, the fluorescence spectral change of L was investigated by carrying out similar titrations with metal ions—Na+, K+, Ca2+, Ni2+,Cu2+, Cd2+, Hg2+ and Pb2+. The results showed that although a slight increase in the fluorescence intensity up to ca. 2.5 times was observed on addition of Ca2+, Cd2+, Hg2+ and Pb2+ to L, the other metal ions Na+, K+, Cu2+ and Ni2+ quenched the fluorescence signal of L to a remarkable extent. Figure 3 is the bar diagram profile to show the effect of various metal ions, at 9.0 × 10−4 M concentration, on the fluorescent intensity of L. The diagram clearly portrays the sensitivity and selectivity of the sensor compound towards Zn2+.
In order to determine the number of Zn2+ bound to L and to determine the binding constant, log[(Io–I)/(I–Iα)] was plotted against log[Zn2+] (Fig. 4) [24, 25]. Here Io, I and Iα are is the fluorescence intensity of L in absence of Zn2+, in presence of an intermediate concentration of Zn2+ and at saturated concentration of Zn2+, respectively. The plot obtained was linear, with the slope and the X-axis intercept representing the number of Zn2+ bound and the log of binding constant (β) respectively. A least squares fitting of data (R2 = 0.971) yielded the slope to be 1.03, indicating the binding of one Zn2+ to L. The binding constant (β) was obtained to be 104.25.
The UV/Visible spectra of L in 1:1 (v/v) CH3OH:H2O at different added concentration of Zn2+ has been shown in Fig. 5. In absence of Zn2+, L showed peaks at λmax values 220 nm, 255 nm and 320 nm. Interaction with Zn2+ shifted the 220 nm peak to 230 nm with a shoulder at 235 nm, the 255 nm peak was shifted to 260 nm with a shoulder at 272 nm, the 320 nm peak significantly shifted to 354 nm with an enhancement in absorbance. In order to confirm the number of Zn2+ bound to L, obtained from fluorescence data, log[(Ao-As)/(As-Amax)] value was plotted against log[Zn2+] for the absorbance values of 354 nm peak (Fig. 4, inset). Here, Ao, As and Amax are the absorbances of L at zero, at an intermediate and at saturated concentration of Zn2+ respectively. The plot was found to be linear (R2 = 0.996) with slope 1.12 indicating that one Zn2+ binds to L and the binding constant (β) value was found to be 104.54. These values are in conformity to those obtained from fluorescent intensity calculations.
The significant change in the fluorescent intensity of L on interaction with Zn2+ may be explained on the basis of the disruption of the efficient thermodynamically favourable PET mechanism [26] initially prevailing in L. The Zn2+ likely binds to L via the two N-atoms of the ethylenediamine moiety. In L the PET process exists due to the transfer of electron density, originating at the lone pairs of electrons on N atoms of the receptor part (ethylenediamine) to the LUMO of the fluorophore part (salicylaldehyde). This results in the quenching of the fluorescence intensity of L. Both fluorescence and UV/Visible spectral titration indicate the binding of one Zn2+ to L. Due to the binding of Zn2+ to L the energy of the electrons originating at the N atoms are lowered and the PET process is hindered, as a result of which the fluorescence intensity is regenerated. We have already reported a fluorescent sensor for Pb2+, having structure similar to L, which is also based on PET [27] Fig. 6.
The Cyclic voltammogram of L (10−4 M) was recorded in 1:1 (v/v) CH3OH:PBS solution using glassy carbon (GC) disc as the working electrode and Ag/AgCl (3 M NaCl) as the reference electrode. An irreversible voltammogram was observed with a reduction peak potential at −0.010 V. Upon additions of different concentration of Zn2+ (0.99 × 10−4 M to 9.0 × 10−4 M) to the electrolytic medium, the reduction peak potential of L was shifted to −0.035 V (Fig. 6). Thus Zn2+ interaction results a 0.025 V shift in reduction potential of L towards the negative direction. No change in the cyclic voltammogram of L was observed when similar titrations were carried out with the other metal ions- Na+, K+, Ca2+, Ni2+,Cu2+, Cd2+, Hg2+ and Pb2+. From this electrochemical data, the selective interaction between L and Zn2+ could be further confirmed.
To summarize, in this paper we have presented a simple and cost effective new fluorescent sensor for Zn2+ over Na+, K+, Ca2+, Ni2+, Cu2+, Cd2+, Hg2+ and Pb2+. The fluorescence intensity enhancement is ca. 20 fold due to snapping of PET process. This fluorescent intensity enhancement is higher than that reported for many other sensors [28–32]. This sensor also shows electrochemical response towards Zn2+ and sensors showing both fluorescent and electrochemical response towards Zn2+ are not very common. A 1:1 complex formation between L and Zn2+ with binding constant value 104.25 is proved.
References
Formica M, Fusi V, Giorgi L, Micheloni M (2012) New chemosensors for metal ions in solution. Coord Chem Rev 256:170
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ion. Chem Soc Rev 37:1465
Wright AT, Anslyn EV (2006) Differential receptor arrays and assays for solution based molecular recognition. Chem Soc Rev 35:14
Liu J, Lu Y (2004) Colorimetric biosensors based on DNAzyme-assembled gold nano particles. J Fluoresc 14:343–354
Lee JH, Wang Z, Liu J, Lu Y (2008) Highly sensitive and selective colorimetric sensors for Uranyl (UO2 2+): Development and comparison of labeled and label-free DNAzyme-gold Nanoparticle systems. J Am Chem Soc 130:14217
Kaim W, Schwederski B (1994) Bioinorganic chemistry: Inorganic elements in the chemistry of life. An introduction and guide. Wiley, Chichester
Berg JM, Shi Y (1996) The galvanization of biology: A growing appreciation for the roles of zinc. Science 271:1081–1085
Czarnik AW (1993) Fluorescent chemosensors for ion and molecule detection. Am Chem Soc, Washington
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietery reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101:294–301
Cuajungco MP, Lees GJ (1997) Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis 4:137–169
Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD (2001) The role of zinc in caspase activation and apoptotic cell death. Bio Metals 14:315–330
Bush AI (2000) Metals and neuroscience. Curr Opin Chem Biol 4(2):184–191
Weiss JH, Sensi SL, Koh JY (2000) Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci 21:395–401
Jiang P, Guo Z (2004) Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord Chem Rev 248(1–2):205–229
Xue L, Liu C, Jiang H (2009) Highly sensitive and selective fluorescent sensor for distinguishing cadmium from zinc ions in aqueous media. Org Lett 11(7):1655–1658
Henary MM, Wu YG, Fahrni CJ (2004) Zinc(II)-selective ratiometric fluorescent sensors based on inhibition of excited state intramolecular proton transfer. Chem Eur J 10:3015
Natali M, Soldi L, Giordani S (2010) A photoswitchable Zn(II) selective spyropyrane-based sensor. Tetrahedron 66:7612–7617
Chen H, Wu Y, Cheng Y, Yang H, Li F, Yang P, Huang C (2007) A ratiometric fluorescent sensor for zinc(II) with high selectivity. Inorg Chem Commun 10:1413
Roy P, Dhara K, Manassero M, Banerjee P (2009) Synthesis, characterization and selective fluorescent zinc(II) sensing property of three Schiff-base compounds. Inorg Chim Acta 362:2927–2932
Joseph R, Ramanujam B, Pal H, Rao CP (2008) Lower rim 1,3-di-amide-derivative of calix[4]arene possessing bis-{N-(2,2′-dipyridylamide)} pendants: a dual fluorescence sensor for Zn2+ and Ni2+. Tetrahedron Lett 49:6257
Goswami P, Das DK (2012) N, N, N, N-tetradentate macrocyclic ligand based selective fluorescent sensor for zinc (II). J Fluoresc 22:1081–1085
Dutta K, Das DK (2012) 2,7-Diferrocenyl-3,6-diazaocta-2,6-diene: A highly selective dual fluorescent sensor for Zn2+ and Ag+ and electrochemical sensor for Zn2+. Indian J Chem Sect A 51A:816–820
Chandra S, Raizada S, Rani S (2008) Structural and spectral studies of palladium(II) and platinum(II) complexes derived from N, N, N, N-tetradentate macrocyclic ligands. Spectrochim Acta A 71:720–724
Valeur B (2001) Molecular fluorescence-principles and applications. Wiley-VCH, pp 339–346.
Nguyen DM, Wang X, H-Yang A, Rodriguez L, Bondar MV, Belfield KD (2010) Novel hydrophilic Bis(1,2,3-triazolyl)fluorenyl probe for in vitro zinc ion sensing. ACS Appl Mater Interfaces 2:2978–2981
Ashokkumar P, Ramakrishnan VT, Ramamurthy P (2011) Photoinduced electron transfer (PET) Based Zn2+ fluorescent probe: Transformation of turn-on sensors into ratiometric ones with dual emission in acetonitrile. J Phys Chem A 115:14292–14299
Das DK, Goswami P, Sarma S (2013) Salicylaldehyde phenyl hydrazone: A new highly selective lead(II) probe. J Fluoresc 23:508–503
Goswami S, Das AK, Aich K, Manna A, Maity S, Khanrab K, Bhattacharyya N (2013) Ratiometric and absolute water-soluble fluorescent tripodal zinc sensor and its application in killing human lung cancer cells. Analyst 138:4593
Saha UC, Chattopadhyay B, Dhara K, Mandal SK, Sarkar S, Khuda-Bukhsh AR, Mukherjee M, Helliwell M, Chattopadhyay P (2011) A highly selective fluorescent chemosensor for zinc ion and imaging application in living cells. Inorg Chem 50:1213–1219
Tang L, Zhao J, Cai M, Zhou P, Zhong K, Hou S, Bian Y (2013) An efficient sensor for relay recognition of Zn2+ and Cu2+ through fluorescence ‘off–on–off’ functionality. Tetrahedron Lett 54:6105–6109
Jia Jia G, Zhen-yuan LR-c, Huang Ming-hao X, Cai-shuang WY-f, Guo-wen X, Yun-sheng H (2011) Design and synthesis of fluorescent sensors for zinc ion derived from 2-aminobenzamide. Eur J Org Chem 4609–4615
Fan J, Peng X, Wu Y, Lu E, Hou J, Zhang H, Zhang R, Fu X (2005) A new fluorescent sensor for Zn2+. J Lumin 114:125–130
Acknowledgement
Authors thank DST, New Delhi for FIST and UGC, New Delhi for SAP programme to the department. PG thanks UGC for fellowship under RFSMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, D.K., Goswami, P. & Medhi, B. N-benzoate-N′ salicylaldehyde ethynelediamine: A new fluorescent sensor for Zn2+ ion by “off-on” mode. J Fluoresc 24, 689–693 (2014). https://doi.org/10.1007/s10895-013-1338-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1338-z