Abstract
A rapid, simple and highly sensitive second derivative synchronous fluorometric method has been developed for the simultaneous analysis of binary mixture of cinnarizine (CN) and domperidone (DOM). The method is based upon measurement of the native fluorescence of these drugs at Δλ = 80 nm in aqueous methanol (50% V/V). The different experimental parameters affecting the native fluorescence of the studied drugs were carefully studied and optimized. The fluorescence-concentration plots were rectilinear over the range of 0.1 to 1.3 μg mL−1 and 0.1–3.0 μg mL−1 for CN and DOM, respectively with lower detection limits of 0.017 and 5.77 × 10−3 μg mL−1 and quantification limits of 0.058 and 0.02 μg mL−1 for CN and DOM. The proposed method was successfully applied for the determination of the studied compounds in synthetic mixtures and in commercial tablets. The results obtained were in good agreement with those obtained with reference methods. The high sensitivity attained by the synchronous fluorometric method allowed the determination of CN in real and spiked human plasma. The mean % recoveries in case of spiked human plasma (n = 3) were 96.39 ± 1.18 while that in real human plasma (n = 3) was 104.67 ± 4.16.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
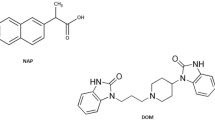
Cinnarizine (CN) is a calcium channel blocker, co-formulated with domperidone (DOM) in its pharmaceutical preparations. CN, 1-(Diphenylmethyl)-4-(3-phenyl-2-propenyl) piperazine (Fig. 1) is a piperazine derivative with antihistaminic, sedative, and calcium channel blocking activity. It is used for the symptomatic treatment of nausea and vertigo caused by Menier’s disease and other vestibular disorders. It is also used for the prevention and treatment of motion sickness [1, 2]. On the other hand, DOM, 5-Chloro-1-[1-[3-(2-oxo-1-benzimidazolinyl) propyl]-4-piperidyl]-2-benzimidazolinone (Fig. 1) is a dopamine antagonist used as an antiemetic for the short term treatment of nausea and vomiting of various aetiologies. It is also used for its prokinetic actions in disorders of gastro-intestinal motility [1, 2].
Several analytical methods have been reported for the determination of CN, either per se or in pharmaceutical preparations and biological fluids, these methods include non-aqueous titration [3], spectrophotometry [4–8], electroanalysis [9, 10], high-performance liquid chromatography (HPLC) [11–14] and GC [15].
Regarding DOM, many reports have been described for its determination, both in formulations and biological fluids. The reports include non-aqueous titration [3], spectrophotometry [16–19], fluorimetry [20], voltammetry [21], HPTLC [22] and HPLC [23–27].
CN and DOM are co-formulated in a binary mixture for the treatment of motion sickness. The combined dosage form of CN and DOM is more widely used and more effective in controlling the motion sickness disease than the individual drugs [28].
The determination of CN and DOM in the coformulated tablets has great significance for the control of clinical therapy. So, a simple, rapid, and sensitive analytical methods for separation and determination of CN and DOM in the coformulated tablets are required.
Four analytical methods have been reported for the simultaneous determination of this mixture, viz; derivative ratio spectrophotometry [29] and HPLC [30–32]. But the spectrophotometric method sensitivity was rather poor [29]. Also, the HPLC procedures were not sensitive and required a large amount of organic solvents and long analysis time [30–32]. Moreover, the spectrophotometric or HPLC methods did not enable the determination of CN in the biological fluids.
To overcome these problems, a new, sensitive, simple but useful second derivative synchronous fluorometric method was developed for the separation and determination of CN and DOM in their coformulated tablets.
In fluorometric methods, high sensitivity and selectivity are generally expected. However, problems of selectivity can occur in multicomponent analysis because of the overlap of the broadband spectra observed. Synchronous fluorescence spectroscopy (SFS) has been found to have several advantages such as simple spectra, high selectivity and low interference etc [33]. Because of its sharp, narrow spectrum, SFS serves as a very simple, effective method of obtaining data for quantitative determination in a single measurement [34].
SFS techniques are classified according to the scanning modes of monochromators into constant-wavelength, variety-angle and constant-energy. At present, constant wavelength method, that is to maintain a constant difference wavelength between the emission and excitation wavelengths, is used most extensively.
The combination of SFS and derivatives is more advantageous than differentiation of the conventional emission spectrum in terms of sensitivity, because the amplitude of the derivative signal is inversely proportional to the band width of the original spectrum [35, 36].
Recently, derivative synchronous fluorometry (DSF) technique has been utilized for the determination of different mixtures in their co-formulated dosage forms and biological fluids such as metacycline and oxytetracycline [37], aspirin with caffeine [38] or aspirin with salicylic acid [39], diflunisal and salicylic acid [40], carvedilol and ampicillin [41], oxytetracycline in medicated premixes and feeds [42] and mixtures of non steroidal anti-inflammatory drugs [43].
The aim of the present work was to develop a simple, sensitive and rapid method for the simultaneous determination of CN with DOM through second derivative synchronous fluorometry (SDSFS) based on their native fluorescence. Because of the high overlap of the emission spectra of CN and DOM, it was difficult to analyse and determine their contents by conventional fluorometry. Such problem was resolved by using SDSFS. The synchronous spectrum at constant Δλ = 80 nm between the emission and excitation wavelengths, has been selected as the optimum one to perform the determination. This method was applied to the simultaneous determination of CN with DOM in synthetic mixtures and in co-formulated pharmaceutical preparations. Also, the sensitivity of the synchronous fluorometric method enables the determination of CN in spiked and real human plasma.
To the best of our knowledge, neither conventional nor synchronous spectrofluorometric method has not been reported for the analysis of CN and DOM in binary mixtures up till now. This initiated the present study.
Experimental
Apparatus
The fluorescence spectra and measurements were recorded using a Perkin-Elmer UK model LS 45 luminescence spectrometer, equipped with a 150 W Xenon arc lamp, gratting excitation and emission monochromators for all measurements and a Perkin-Elmer recorder. Slit widths for both monochromators were set at 10 nm. A 1 cm quartz cell was used. Derivative spectra were evaluated using Fluorescence Data Manger (FLDM) software.
A pH Meter (Model pHS-3C, Shanghai Leici instruments Factory, China) was used for pH adjustment.
Materials and reagents
All reagents and solvents were of Analytical Reagent Grade.
-
CN and DOM pure samples were purchased from Sigma (St. Louis, Mo, USA ) and used as received.
-
Methanol (Merck, Darmstadt, Germany)
-
Borate buffer ( 0.2 M, pH 6–11)
-
Acetate buffer (0.2 M, pH 3.0–5.6)
-
Sodium hydroxide (BDH, UK), 0.1 M aqueous solution.
-
Plasma samples were obtained from Mansoura University Hospital, Mansoura, Egypt and were kept frozen until use after gentle thawing.
Standard solutions
Stock solutions of CN and DOM were prepared by dissolving 10.0 mg of each of the studied compound in 100.0 mL of methanol and further dilute with the same solvent as appropriate. The standard solutions were stable for 10 days when kept in the refrigerator.
General procedure
Aliquots of CN and DOM standard solutions covering the working concentration range cited in Table 1 were transferred into a series of 10 mL volumetric flasks. 2.0 mL of acetate buffer solution (pH 3.5) and 5 mL of methanol were added to each flask. The solutions were diluted to the mark with distilled water and mixed well. The synchronous fluorescence spectra of the solutions were recorded by scanning both monochromators at a constant wavelength difference Δλ = 80 nm with scan rate of 600 nm min−1 using 10 nm excitation and emission windows. The second derivative fluorescence spectra of CN and DOM were derived from the normal synchronous spectra using FLDM software. For best resolution and smoothing, number of points of 99 were used for deriving the second derivative spectra. The fluorescence intensities of the second derivative spectra were estimated at 247 nm and 305 nm for CN and DOM, respectively. A blank experiment was performed simultaneously. The corrected FI of the second derivative technique was plotted vs final concentration of the drug (μg mL−1) to get the calibration graph. Alternatively, the corresponding regression equations were derived.
Applications
Procedure for the synthetic mixture
Aliquots of CN with DOM standard solutions in different ratios of 4:3 were transferred into a series of 10 mL volumetric flasks. Two milliliters of acetate buffer solution (pH 3.5) and 5 mL of methanol were added. The solutions were diluted to the mark with distilled water and mixed well. The “general procedure” was then adopted. The corrected FI of the second derivative technique was plotted vs final concentration of the drug (μg mL−1) to get the calibration graphs. Alternatively, the corresponding regression equations were derived.
Procedure for the co-formulated preparation
Five milligrams of powdered tablets equivalent to 2.86 mg CN and 2.14 mg DOM for touristil tablets were transferred into small conical flask and extracted with 3 × 30 mL of methanol. The extract was filtered into 100 mL volumetric flask. The conical flask was washed with few milliliters of methanol. The washings were passed into the same volumetric flask and completed to the mark with the same solvent. Aliquots covering the working concentration range cited in Table 1 were transferred into 10 mL volumetric flasks. The general procedure was then applied and the nominal content of tablets was determined either from a previously plotted calibration graph or using the corresponding regression equation.
Procedure for spiked human plasma
One milliliter aliquots of human plasma spiked with increasing quantities of CN were transferred into separating funnels. The spiked plasma was extracted with 3 × 5 mL of chloroform. The organic layer was passed over anhydrous sodium sulphate and evaporated to dryness. The general procedure of synchronous fluorometric method was then applied. A blank experiment was carried out simultaneously. The nominal content of the drug was determined using the corresponding regression equation.
Procedure for real human plasma
As for the real human plasma, CN (25 mg/ tablet) was orally administered to healthy volunteer (male, 40 years old). Five milliliters of blood sample was withdrawn from the volunteer. After 2 h, 4 mL of 5% EDTA solution were added, and centrifuged at 3500 rpm for 15 min to get 3.5 mL of plasma. The general procedures of synchronous fluorometric method was then proceeded. The nominal content of CN in plasma was determined according to previous report [44] using the following equation:
Results and discussion
SDSF spectra of CN and DOM
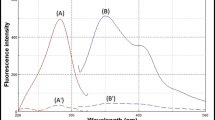
Both of CN with DOM exhibit native fluorescence with λmax at 315 nm and 324 nm, respectively after excitation at 280 nm (Fig. 2). It is clear that, the emission spectra of CN and DOM were greatly overlapped and analysis of such mixture is challenging. Such problem is aggravated if it is desired to determine those compounds in their co-formulated preparations and biological fluids.
It was necessary to record first, the normal synchronous spectra for CN and DOM to derive the second derivative synchronous spectra. Figure 3 shows the SF spectra of different concentrations of CN (maximum at 248 nm), and Fig. 4 illustrates the SF spectra of different concentrations of DOM (maxima at 233 and 282 nm). The measurement of the relative fluorescence of DOM was carried at 282 nm as this peak is more sensitive, with reproducible results and less overlapped with CN spectrum.
This encouraged us to perform SDSFS technique for the simultaneous determination of each of the studied drugs in its co-formulated preparation without prior extraction or separation step.
Fluorescence spectra of CN and DOM were separated entirely using SDSFS. Figure 5 shows that CN can be separated at 247 nm in presence of DOM. Figure 6 shows that DOM can be separated at 305 nm in presence of CN.
Optimization of experimental conditions
Different experimental parameters affecting the formation and stability of the fluorescence of the studied compounds were carefully studied and optimized. Such factors were changed individually while others were kept constant. These factors included Δλ selection, pH, type and volume of the diluting solvent, stability time and ionic strength.
Selection of optimum Δλ
The synchronous fluorescence spectra of CN with DOM were recorded using different Δλ. The optimum Δλ value is very important for performing synchronous fluorescence scanning technique concerning resolution, sensitivity and features. It can directly influence spectral shape, band width and signal value. For this reason a wide range of Δλ (20, 40, 60, 80, 100 and 120 nm) was examined. When Δλ is less than 80 nm, the spectra shape is irregular and the fluorescence intensity is very weak. When Δλ is more than 80 nm, poor separation of the two peaks was obtained. Therefore, Δλ of 80 was chosen as the optimal one for separation of mixture of CN with DOM, since it resulted in two distinct peaks with good shape and to minimize the spectral interference caused by each compound in the mixture.
Effect of pH
The influence of pH on the normal synchronous fluorescence intensity of the studied drugs was investigated using different types of buffers covering the whole pH range, such as acetate buffer solutions over the pH range of 3–5.6 and borate buffer from pH 6–11. The results showed that the relative fluorescence intensities of the studied compounds change with pH. as shown in Fig. 7. Variation of pH from 1.0 to 13.0 was investigated. It is emphasized that relative fluorescence intensity of CN and DOM is less affected by the pH change over the range 3.0 to 5.0. Therefore, acetate buffer of pH 3.5 was chosen as the optimal one throughout the study.
Effect of diluting solvent
Dilution with different solvents such as water, methanol, ethanol, isopropanol, dimethyl sulfoxide (DMSO) and dimethyl formamide was attempted. The results are shown in Figs. 8 and 9. Figure 8 illustrates that the fluorescence intensity of CN increased in methanol more than the other solvents. On the other hand, the fluorescence intensity of DOM was increased in DMSO more than methanol and their peaks were shifted in DMSO to 307 nm. The results are further investigated in Fig. 9. Since the aim of the work is to determine CN in presence of its co-formulated drugs, we must emphasize the effect of organic solvent on the relative fluorescence intensity of CN. So, methanol was selected as the best solvent for the dilution.
The effect of volume of methanol was also studied. Figures 8 and 9 showed that relative fluorescence intensity of CN and DOM have no great change over the range of 4–6 mL of methanol. So, 5 mL of methanol is selected as the optimum volume of diluting solvent for determination of CN with DOM.
Effect of time
The effect of time on the development and stability of the fluorescence intensity of the drugs was also studied. It was found that the fluorescence intensity was immediately developed instanteously and remained stable for more than 2 h.
Effect of ionic strength
The impact of ionic strength on the system was also studied using different concentrations of NaCl ranging from 5 × 10−3 M to 0.1 M. NaCl has been used as a counter ions to study ionic strength since sodium ions have higher adsorption over the negative charge on the molecule and chloride ions have the same effect. The results showed that there was no effect of NaCl concentration on the fluorescence intensity of the studied drugs.
Analytical performance
The fluorescence-concentration plots for the studied drugs by SDSFS were linear over the concentration range cited in Table 1.
Linear regression analysis of the data gave the following equations:
where F is fluorescence intensity, C is the concentration of the drug (μg mL−1) and r is correlation coefficient.
The limit of quantification (LOQ) was determined by establishing the lowest concentration that can be measured according to ICH Q2B recommendations [45], below which the calibration graph is non linear (LOQ = 10 σ/S where S is the slope and σ is the standard deviation of the intercept of regression line of the calibration curve). The limit of detection (LOD) was determined by evaluating the lowest concentration of the analyte that can be readily detected (LOD = 3.3 σ /S). The results of LOD and LOQ of CN with DOM and NIC by the proposed method are abridged in Table 1.
The proposed method was evaluated by studying the accuracy as percent relative error and precision as percent relative standard deviation. The results are abridged in Table 1.
Statistical analysis [46] of the results obtained by the proposed and the comparison methods [3, 29] using Student’s t-test and variance ratio F-test, shows no significant difference between the performance of the two methods regarding the accuracy and precision, respectively (Table 2).
Analysis of synthetic mixture sample
The proposed method was applied to the simultaneous determination of CN with DOM in synthetic mixtures containing different concentrations of both drugs in a ratio of 4:3 (Fig. 10). The relative fluorescence intensities of second derivative technique were measured for both drugs. The second derivative signal of CN was measured at 247.43 nm which is considered as zero crossing point for DOM and the second derivative signal for DOM was measured at 305.20 nm which is the zero crossing point for CN. The concentrations of both drugs in the synthetic mixture were calculated according to the linear regression equation of the calibration graphs. The results obtained regarding %RSD and % relative error were compared with that obtained using a reference method. The results indicates high accuracy of the proposed method as shown in Table 3.
Validation of the method
Linearity
The proposed method was tested for linearity, specificity, accuracy and precision. Linear regression equations were obtained. The regression plots showed a linear dependence of FI values on drug concentration over the range cited in Table 1. The small values of the figures point out to the low scattering of the points around the calibration curve and high accuracy and precision of the proposed method.
Accuracy and precision
The intra-day precision was evaluated through replicate analysis of plasma sample spiked with 0.04 μg mL−1 of CN. This concentration was analyzed on three successive times. The mean percentage recovery was 102.33 ± 2.47 at 248 nm. The repeatability and reproducibility are fairly good as indicated by the small values of standard deviations.
The inter-day precision was evaluated through replicate analysis plasma sample spiked with 0.04 μg mL−1 of CN on three successive days. The percentage recovery based on the average of three separate determinations was 103.83 ± 2.67 at 248 nm. The results obtained by the proposed method are abridged in Table 4.
The accuracy of the proposed method was evaluated by analyzing standard solutions of the studied drugs. Moreover the results were favorably compared with those obtained using a reference method [29]. The reference method include first derivative ratio spectrophotometric measurement for the mixture at 291 nm using Δλ of 4 and scaling factor of 10.
Robustness of the method
The robustness of the proposed method is demonstrated by the constancy of the fluorescence intensity with the deliberated changes in the experimental parameters such as pH, 3.5 ± 0.5 and change in the volume of methanol, 5 ± 1 mL for the studied drugs. These minor changes that may take place during the experimental operation didn’t greatly affect the fluorescence intensity of the mixture.
Pharmaceutical applications
The proposed method was applied to the determination of the studied drugs in their commercial tablets. The specificity of the method was investigated by observing any interference encountered from the common tablet excepients, such as talc (20 mg), lactose (15 mg), starch (15 mg), avisil (15 mg), gelatine (0.7 mg), and magnesium stearate (10 mg). These excepients did not interfere with the proposed method. The results of the proposed method were compared with those obtained using the comparison method [29]. Statistical analysis [46] of the results obtained using Student’s t-test and variance ratio F-test revealed no significant difference between the performance of the two methods regarding the accuracy and precision, respectively (Table 5).
Biological applications
The high sensitivity of the synchronous spectrofluorometric method allowed the determination of CN in biological fluids (Fig. 11). The method was further applied to the in-vitro and in-vivo determination of CN in spiked and real human plasma.
CN is absorbed from gastro-intestinal tract. Following oral ingestion of a single oral dose of 50 mg, CN gives a mean peak plasma concentration of 0.08 mg L−1 attained in about 2.3 h [47]. This value lies within the working concentration range of the proposed method. The mean percentage recovery of CN in spiked human plasma sample was 99.88 ± 1.31 at 248 nm (Table 6). The method involved extraction with chloroform. The extraction procedure described by Rosseel et al. [13] was adopted.
The within-day precision was evaluated through replicate analysis of plasma sample spiked with different concentrations of the drug (0.025–0.04 μg mL−1). The mean percentage recovery based on the average of three separate determinations was 99.88 ± 1.31 at 248 nm.. The results are abridged in table [6]. On the other hand, the % recovery of CN in real human plasma was 99.75 ± 3.50 at 248 nm.
Linear regression analysis of CN in spiked human plasma gave the following equations:
Conclusion
New simple and sensitive method was explored for the simultaneous determination of CN and DOM in binary mixture. The second derivative synchronous spectrofluorometric method, by virtue of its high sensitivity, could be applied to the analysis of both drugs in their co-formulated dosage forms; it was possible to measure as low as 0.025 μg mL−1 with good accuracy. Moreover, second derivative spectrofluorimetry technique enables the determination of CN in the presence of DOM by applying the zero-crossing technique in the spectra without prior separation steps. Moreover, the proposed method is time saving. Also, synchronous spectrofluorometric method was applied for the determination of CN in biological fluids.
References
Budavari S (1996) The Merck index, 12th edn. Merck, White House Station
Parfitt K (1999) Martindale, the complete drug reference, 32th edn. Pharmaceutical Press, London, pp 411–915
British Pharmacopoeia Commission (2003) British pharmacopeia, vols. I and II. Her Majesty’s Stationery Office, London (through electronic version)
Metwally FH, Elzeany EBA, Darwish HW (2005) New methods for determination of cinnarizine in mixture with piracetam by spectrodensitometry, spectrophotometry, and liquid chromatography. J AOAC Int 88:1666–1676
Abdine H, Belal F, Zoman N (2002) Simple spectrophotometric determination of cinnarizine in its dosage forms. Farmaco 57:267–271
El-Gindy A, Emara S, Hadad GM (2004) Determination of certain drugs in binary mixtures formulations by second derivative ratio spectrophotometry and LC. Farmaco 59:703–712
El-Gindy A, Emara S, Mostafa A (2004) Spectrophotometric and LC determination of two binary mixtures containing antihistamins. Farmaco 59:713–722
Xu BZ, Zhao FL, Tong SY (1999) Spectrophotometric determination of cinnarizine based on charge-transfer reaction. Guangpuxue-Yu-Guangpu-Fenxi 19:886–888
Hassan SSM, Abdel-Aziz RM, Abbas AB (1996) Potentiometric membrane sensors for the selective determination of cinnarizine in pharmaceutical preparations. Anal Chim Acta 321:47–52
Zeng YH, Sun HY (1993) Determination of cinnarizine and its adsorptive voltammetric behaviour. Fenxi-Huaxue 21:1185–1187
De Steene JCV, Mortier KA, Lambert WE (2006) Tackling matrix effects during development of a liquid chromatographic–electrospray ionisation tandem mass spectrometric analysis of nine basic pharmaceuticals in aqueous environmental samples. J Chromatogr A 1123:71–81
Hassan SSM, Elmosallamy MAF, Abbas AB (2002) LC and TLC determination of cinnarizine in pharmaceutical preparations and serum. J Pharm Biomed Anal 28:711–719
Rosseel MT, Lefebvre RA (1993) Sensitive determination of cinnarizine in human plasma by high-performance liquid chromatography and fluorescence detection. Chromatographia 36:356–358
Sane RT, Sahasrabudhe SP, Nayak VG, Ladage KD, Kothurkar RM, Nayak VG (1989) High-performance liquid-chromatographic determination of cinnarizine from pharmaceutical preparations. Indian Drugs 26:491–493
Xie XT, Wu GL, Liu CH (1993) Determination of cinnarizine in tablets by gas chromatography. Sepu 11:315–316
Amin AS, Ragab GH (2003) Spectrophotometric methods for the determination of anti-emetic drugs in bulk and in pharmaceutical preparations. Anal Sci 19:747–751
Rama-Mohan Y, Avadhanulu AB (1998) Extractive spectrophotometric determination of domperidone in its pharmaceutical dosage forms. Indian Drugs 35:754–756
Rao GR, Kini GR, Avadhanulu AB, Vatsa DK (1990) Spectrophotometric estimation of acebutolol hydrochloride and domperidone in their dosage forms. East Pharm 33:133–135
Al-Khamis KI, Hagga MEM, Al-Khamees HA (1990) Spectrophotometric Determination of Domperidone Using A Method. Anal Lett 23:451–460
Baeyens W, De-Moerloose P (1979) Fluorescence properties of domperidone and its determination in pharmaceutical preparations. Anal Chim Acta 110:261–270
Wahdan T, Abd El-Ghany N (2005) Determination of domperidone in tablet dosage form by anodic differential pulse voltammetry. Farmaco 60:830–833
Zarapkar SS, Salunkhe BB (1990) Determination of domperidone by high-performance thin-layer chromatography in pharmaceutical preparations. Indian Drugs 27:537–540
Ali MS, Ghori M, Khatri AR (2006) Stability indicating simultaneous determination of domperidone (DP), methylparaben (MP) and propylparaben by high performance liquid chromatography (HPLC). J Pharm Biomed Anal 41:358–365
Kobylinska M, Kobylinska K (2000) High-performance liquid chromatographic analysis for the determination of domperidone in human plasma. J Chromatogr B 744:207–212
Zavitsanos AP, MacDonald C, Bassoo E, Gopaul D (1999) Determination of domperidone in human serum and human breast milk by highperformance liquid chromatography-electrospray mass spectrometry. J Chromatogr B 730:9–24
Kanumula GV, Raman B (2000) Simultaneous determination of ranitidine hydrochloride and domperidone in pharmaceutical dosage by reverse-phase high-performance liquid chromatography. Indian Drugs 37:375–378
Smit MJ, Sutherland FCW, Hundt HKL, Swart KJ, Hundt AF, Els J (2002) Rapid and sensitive liquid chromatography-tandem mass spectrometry method for the quantitation of domperidone in human plasma. J Chromatogr A 949:65–70
Oosterveld WJ (1987) The combined effect of cinnarizine and domperidone on vestibular susceptibility. Aviat Space Environ Med 58:218–223
Salem MY, El-Bardicy MG, El-Tarras MF, El-Zanfally ES (2002) Simultaneous determination of domperidone maleate and cinnarizine in a binary mixture using derivative ratio spectrophotometry and classical least squares calibration. J Pharm Biomed Anal 30:21–33
Zarapkar SS, Bhandari NP, Halker UP (2000) Simultaneous estimation of cinnarizine and domperidone maleate in tablets by reversephase HPLC. Indian Drugs 37:295–298
Argekar AP, Shah SJ (1999) Simultaneous determination of cinnarizine and domepiridone maleate from tablet dosage form by reverse phase ion pair high performance liquid chromatography. J Pharm Biomed Anal 19:813–817
Argekar AP, Powar SG (1999) Simultaneous HPTLC determination of cinnarizine and domperidone maleate in formulations. J Planar Chromatogr Mod TLC 12:272–274
Chen GZ, Huang XZ, Xu JG, Zheng ZZ, Wang ZB (1990) The methods of fluorescence analysis, 2nd edn. Science, Beijing, p 112
Patra D, Mishra AK (2002) Recent developments in multi-component synchronous fluorescence scan analysis. Trends Anal Chem 21:787
Lloyd JBF (1971) Nat Phys Sci 231:64 http://www.chemistrymag.org/cji/2003/058065pe.htm. 2003 Vol.5 No.8 P.65.
Murillo Pulgarín JA, Alañón Molina A, Fernández López P (1998) Simultaneous determination of atenolol, propranolol, dipyridamole and amiloride by means of nonlinear variable-angle synchronous fluorescence spectrometry. Anal Chim Acta 370:9–18
Aodeng GW, Zhang Y, Fan HY (2006) Determination of metacycline in mixture sample by synchronous-derivative fluorimetry. Guang Pu Xue Yu Guang Pu Fen Xi 26:1530–1532
Karim MM, Jeon CW, Lee HS, Alam SM, Lee SH, Choi JH, Jin SO, Das AK (2006) Simultaneous determination of acetylsalicylic acid and caffeine in pharmaceutical formulation by first derivative synchronous fluorimetric method. J Fluoresc 16:713–721
Wei YF, Li XH, Ma DM (2005) Simultaneous determination of aspirin and salicyclic acid by synchronous fluorescence spectrometry. Guang Pu Xue Yu Guang Pu Fen Xi 25:588–590
Murillo Pulgarín JA, Alañón Molina A, Fernández López P, Sánchez-Ferrer Robles I (2007) Direct determination of closely overlapping drug mixtures of diflunisal and salicylic acid in serum by means of derivative matrix isopotential synchronous fluorescence spectrometry. Anal Chim Acta 583:55–62
Xiao Y, Wang HY, Han J (2005) Simultaneous determination of carvedilol and ampicillin sodium by synchronous fluorimetry. Acta A Mol Biomol Spectrosc 61:567–573
Fernandez-González R, Garcýa-Falcón MS, Simal-Gándara J (2002) Quantitative analysis for oxytetracycline in medicated premixes and feeds by second-derivative synchronous spectrofluorimetry. Anal Chim Acta 455:143–148
Toma’s PR, Carmen ML, Virginia T, Jose C (1998) Analysis of binary mixtures of flufenamic, meclofenamic and mefenamic acids by derivative synchronous fluorescence spectrometry Talanta 47:537–545
Sun Y, Nakashima MN, Takahashi M, Kuroda N, Nakashima K (2002) Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatography with fluorescence detection Biomed Chromatogr 16:319–326
US Department of Health and Human Services, Food and Drug Administration (2001) Guidance for industry bioanalytical method validation. Center for Drug Evaluation and Research, Rockville. http://www.fda.gov/eder/guidance/4252fnl.pdf (accessed September 1, 2004)
Miller JC, Miller JN (1993) Statistics for analytical chemistry, 3rd edn. Wiley, New York, p 115
Moffat AC, Osselton MD, Widdop B, Galichet LY (2004) Clark’s analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material, 3rd edn., vol II. The Pharmaceutical Press, London, pp 925, 1695
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walash, M.I., Belal, F., El-Enany, N. et al. Second-derivative Synchronous Fluorometric Method for the Simultaneous Determination of Cinnarizine and Domperidone in Pharmaceutical Preparations. Application to Biological Fluids. J Fluoresc 18, 61–74 (2008). https://doi.org/10.1007/s10895-007-0238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-007-0238-5