Abstract
The host-guest inclusion complexes of sulphamethoxazole (SMTX) and sulphaguanidine (SGN) with β-cyclodextrin, in aqueous solutions, have been investigated. A 1:1 stoichiometry of the complexes was established, the association constants were calculated by different methods, and the influence of several chemical variables on the complexation processes were established. According to the results obtained, a spectrofluorimetric method for the determination of these sulphonamides has been proposed. The individual and binary mixtures of both sulphonamides have been determined in human urine samples, at representative therapeutic ranges, by application of a first-order multivariate calibration partial least squares (PLS-1) model. The calibration set was designed with 9 samples, containing different concentrations of the two sulphonamides, and 8 blank urine samples, with the aim of modelling the variability of the background. The concentration ranges for the sulphonamides were up to 0.5 μg mL−1 for SMTX and 1.0 μg mL−1 for SGN. Figures of merit as selectivity, analytical sensitivity and limit of detection (LOD) were also calculated. The proposed procedure was validated by comparing the obtained results with a HPLC method, with satisfactory results for the assayed method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sulphonamides belong to the group of antibacterial drugs, which are used for human and animal therapy, to cure infectious diseases of digestive and respiratory systems, affections of the skin (in the form of ointments) and for prevention or therapy of coccidiosis of small domestic animals [1]. Quality control of sulphonamide formulations and their quick systematic monitoring in body fluids are important analytical tasks. A number of articles have been published concerning the determination of sulphonamides by different analytical methods.
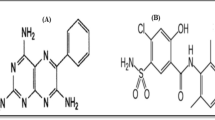
Sulphamethoxazole (SMTX), chemically N1-(5-methyl-3-isoxazolyl) sulphanilamide (Scheme 1), is widely used as an antibacterial, mainly in combination with trimethroprim. This is a well-recognized preparation, as the combination of a sulphonamide with an inhibitor of the dihydropholate reductase, increases the bacteriostatic effect of the sulphonamide, as is blocking the metabolic pathways of the microorganisms at two different points. Sulphaguanidine (SGN), chemically 4-Amino-n-(aminoiminomethyl)benzenesulfonamide, is an antimicrobial agent that is used to treat enteric infections.
Different methods have been described for determining both sulphonamides, but, in general, they are based on separation methods using different detection types. Its determination in urine has been made by micellar liquid chromatography [2] and by supported liquid membrane with high pressure liquid chromatography-electrospray mass spectrometry detection [3].
The fluorescence characteristics of different sulphonamides have been studied [4], and proposed for the determination of several of these compounds. Thus, sulphanilamide can be determined by reaction with homophthaldehyde [5]. The analysis of sulphadiazine [6], sulphafurazol and sulphanilamide [7] has been performed in foods and pharmaceuticals, using the fluorescamine reaction. The reaction of 9-cloro-acridine with sulphonamides produces a fluorescence quenching which allows the determination of sulphonamides [8]. Sulphacetamide, sulphaguanidine and sulphametazine were determined in milk and pharmaceutical preparations by photochemically-induced fluorimetry [9]. Fluorescence has also been used as HPLC detection for determining sulphacetamide and sulphaguanidine in milk and eggs [10], and, recently, fluorescence after pre-column derivatization with fluorescamine has been applied as detection technique [11–15].
On the other hand, it is known that cyclodextrins (CDs) have the property of forming inclusion complexes with guest molecules that have suitable characteristics of polarity and dimension [16–20]. The inclusion complex formation in the CD systems is favoured by substitution of the high-enthalpy water molecules located inside the CD cavity, with an appropriate guest molecule of low polarity. An overview of the non-chromatographic analytical uses of CDs has been presented by Szente and Szejtli [20]. As the complexation process implies an interaction producing a protection of the included especies, the CDs have been used in the pharmaceutical industry to encapsulate drugs that are photosensitives. There are a few reports dealing with the analytical determination of sulphonamides by using cyclodextrins; and they employ, in general, a capillary electrophoresis technique. In the case of sulphamethoxazole, Okamoto et al. [21] use dimethyl-β-cyclodextrin as modifier of mobile phase in micellar electrokinetic chromatography; in another paper [22] β-cyclodextrin is used in capillary electrophoresis. There is only one fluorimetric application [23], where Muñoz de la Peña et al. make fluorescence measurements in pharmaceutical preparations of the inclusion complex of SMTX in β-cyclodextrin. In the case of SGN, there are not described analytical determinations by using cyclodextrins.
In the last decade, multivariate techniques have been incorporated in analytical methodologies. In particular, full-spectrum multivariate calibration methods offer advantages of speed of determination as the separation steps can be avoided, and different multivariate algorithms have been applied to the analysis of drugs using spectroscopic and voltammetric signals [24–26].
Partial least squares (PLS) is a factor analysis based method first applied to chemical analysis by Wold et al. [27], and widely used in analysis during the last decade. By multivariate analysis, it is often possible to reduce interference problems. The most serious limitation of direct multicomponent analysis is that correct spectral data must be known for all relevant phenomena affecting the sample spectra. However, indirect multivariate analysis is based on statistical analysis of empirical data, which frequently allows correct prediction even in the presence of unidentified interferences, provided that a sufficiently heterogeneous sample set is available for calibration. Because of this, an adequate design of calibration experiments is required when applying these methods.
The objectives of this paper are: a) to develop an easy analytical method for determining SGN and SMTX, by using fluorescence measurements; and which could serves as an alternative to conventional separation methods; b) to apply the proposed method to a complex biological sample, as urine, developing the analytical method in aqueous solution, and then, making possible an easy treatment of real samples; c) to study the possibility of increasing the fluorescence signal in aqueous medium by using cyclodextrins and, at the same time, to increase the selectivity of the proposed method; d) to evaluate the possibility of application of a chemometric method based on partial least squares (PLS) calibration, for the resolution of the sulphonamides mixture, by applying no prior separation steps. As far as we know, there are no reports about the simultaneous fluorimetric determination of SMTX and SGN.
Experimental
Apparatus and reagents
Fluorescence spectral measurements were performed on a Fluorescence Spectrophotometer Varian Model Cary Eclipse. The instrument consists of two Czerny-Turner monochromators (excitation and emission), a Xenon light source, a range of fixed width selectable slits, selectable filters, attenuators and two photomultiplier tubes as detectors. The fluorimeter is connected to a PC microcomputer via an IEE serial interface. The Cary Eclipse Version 1.0 software was used for data acquisition, data interpretation and graphical display. All measurements were performed in 10 mm quartz cells at 20°C, by use of a thermostatic cell holder and a Selecta Model Frigiterm thermostatic bath.
All solvents used were of analytical reagent quality. Sulphamethoxazole and sulphaguanidine were purchased from Sigma. Standard solutions of each compound (5.10−4 M) were prepared by dilution in deionized water. Stock solutions of α-cyclodextrin (α-CD), β-cyclodextrin (β-CD), γ-cyclodextrin (γ-CD), (2-hydroxy)-propyl-β cyclodextrin (HP-β-CD) and heptakis-(2,6–di-O-methyl)-β-cyclodextrin (DIMEB), of concentration 1.5 10−2 M, were prepared in desionized water. Sodium acetate/acetic acid 1 M (pH = 4.6, 5.0 and 5.5) buffer solutions were prepared from analytical reagents.
Software
The program MVC1 [28] written in MATLAB 6.0 [29] was used for PLS-1 calibration. A home-made converter, running in MATLAB, was used to transform the bidimensional fomat spectral recorded files, to the two columns ASCII format accepted by the MVC1 program.
Validation of the proposed method
The proposed fluorimetric method for the determination of SGN and SMTX in urine was validated by comparison with a method based on HPLC separation [30], with UV-Vis detection, slightly modified by us. We have developed a gradient method for the analysis of these two analytes in urine, which allowed to separate the first peak (SGN) from the front and from the other urine components, and decreasing the time of measurement for the second peak (SMTX).
The chromatographic studies were performed on a Hewlett-Packard Mod 1100 LC instrument, equipped with degasser, quaternary pump, manual six-way injection valve, containing a 20 μL loop, Diode Array spectrophotometer detector and the CHEMSTATION software package to control the instrument, data acquisition and data analysis. An analytical column Nova-Pak C18 (150×3.9 mm, Waters Millipore) was used. The flow-rate was 1.0 mL min−1 and the injection volume, 20 μL. A wavelength of 260 nm was selected for monitoring SGN and SMTX.
The mobile phase was formed by a mixture of solvent A (pH 3.0 aqueous citric-citrate buffer 10 mmol/L) and solvent B, acetonitrile.The mobile phase composition was 94% A and 6% of B, during the three first minutes; and 60% A and 40% of B in the four remaining minutes.
Stock solutions containing 100 μg mL−1 of SGN and SMTX were prepared in water. Working solutions of different concentrations were prepared by dilution of the stock solutions and each sample was injected three times. Linear calibration graphs for area and height against the SGN and SMTX concentration were obtained between 0.1 and 1.0 μg mL−1.
For the analysis of urine samples, in a 10.0 mL flask, 0.1 mL of urine were spiked with the analytes and, after 5 minutes, completion to the mark was achieved with deionized water. All solutions were filtered through a 0.45 μm filter (Millisolve Kit, Millipore) before the injections. The resolutions of the two peaks was obtained in a time of 7 minutes.
Characteristics of the sulphonamide:cd inclusion complexes
Stoichiometry of the inclusion complex
The stoichiometry of the complexes with β-CD was established by the methods of Benesi-Hildebrand [31] and Scathard [32]. The following equilibrium can be considered:
In the Benesi-Hildebrand's method, in the case of a 1:1 complex, the following equation is applicable:
where [β-CD]0 denotes the initial β-CD concentration, F0 denotes the fluorescence intensity of SULPHA molecules in the absence of β-CD, F∞ denotes the fluorescence intensity when all of the SULPHA are essentially complexed with β-CD, and F is the observed fluorescence at each β-CD concentration tested.
If the stoichiometry is 1:1, the representation of 1/(F-F0) versus 1/[β-CD]0 should give a linear plot.
In the Scatchards method, in the case of a 1:1 complex, the relationship between the observed fluorescence intensity enhancement (F-F0) and the β-CD concentration is given by:
If the assumption of a 1:1 stoichiometry for the complex is applied, plotting (F-Fo) /[β-CD]0 versus (F-F0) should give a straight line.
Association constant of the inclusion complex
In the case of Benesi-Hildebrand method, the association constant may be determined by dividing the intercept by the slope of the straight line, while in the case of the Scatchard method, it is given by the slope of the straight line. However, non-linear least-squares regression analysis (NLR) [31], an alternative and more appropriate approach than the graphical methods, has been used. The initial parameter estimates needed for this method were obtained from the linear plots, and the NLR analysis of the data was performed by an iterative Marquardt-type process, by using the following equation:
AM1 calculations
Ground-state geometry optimization of the proposed structures for the inclusion complexes was performed with the AM1 method contained in the CHEM 3D package, version ultra 8.00, on a Pentium PC microcomputer. The molecular mechanics method was used to obtain the initial structures. Afterward, the energy was minimized by the AM1 method.
Partial least square theory
Partial least squares is a multivariate calibration model which involves a two-step procedure: (1) calibration, where the relation between spectra and reference component concentrations is established from a set of standard samples and (2) prediction, in which the calibration results are employed to estimate the component concentrations in unknown samples [33]. In the PLS-1 method, all model parameters are optimized for the determination of each analyte at a time. During the model training step, the calibration data are decomposed by an iterative algorithm, which correlates the data with the calibration concentrations [34]. This provides a set of regression coefficients to be applied to a new sample. Before calibration, however, the optimum number of latent variables, or factors, should be selected in order to avoid overfitting. This has been performed by applying the cross-validation method and the Haaland and Thomas statistical criterium [34]. The estimation of analytical figures of merit, such as sensitivity (SEN), analytical sensitivity (γ−1) and selectivity (SEL), usually employed for method comparasion, could be calculated [35].
Results and discussion
Absorption spectra. Influence of pH
To study the characteristics of the complexes of inclusion of the sulphonamides, the absorption spectra of both, in the absence and in the presence of 9×10−3 M β-cyclodextrin, were first recorded (Fig. 1). The figure shows absorption maxima for the 2×10−5 M aqueous solutions of SMTX and SGN at 266 and 260 nm, showing molar absorptivity coefficients of 17600 and 17000 M−1 cm−1, respectively. When recording the absorption spectra in the presence of β-cyclodextrin, small bathocromic shifts of 4 and 2 nm, for the SMTX and SGN inclusion complexes, respectively, are produced, and the absorbance is slightly increasing, giving rise to molar absorptivity coefficients of 22100 and 20500 M−1 cm−1, respectively. These facts are indicatives of the processes of inclusion in the presence of the cyclodextrin for both sulphonamides.
The influence of pH for the sulphonamides, both, in aqueous solution and in β-cyclodextrin solution, were also studied. The results are shown in Figs. 2 and 3, for SGN and SMTX, respectively. Several authors have reported pK values for the sulphonamides of interest. For sulfamethoxazole, values of pK1 of 1.4, corresponding to the primary amine group on the aromatic ring, [3] and pK2, corresponding to the nitrogen attached to the sulphur atom of 5.6 [36–38], have been reported.
For sulphaguanidine, values of pK1 of 2.75 [39, 40], 2.7 [4], and 3.3 [3] have been reported . The pK values reported in the references are consistent with the results presented in Figs. 2 and 3. These figures are also showing that the inclusion processes of the sulphonamides in the cyclodextrin cavity are not affecting the acid-base properties of the sulphonamides. This fact may be explained taking into account that the changes in the spectra with pH, are due to the proton exchange between the monocationic nitrogen atom of the p-amino benzene group and the neutral molecule, and this part of the molecule is protruding outside of the cavity.
Fluorimetric study of the inclusion complexes of sulphamethoxazole and sulphaguanidine with β-CD
Sulphamethoxazole shows a weak fluorescence emission in aqueous solution while sulphaguanidine shows stronger native fluorescence. The excitation and emission spectra were not corrected. In aqueous solution, the excitation spectrum shows maxima located at 274 and 264 nm, for SMTX and SGN, respectively, and the emission spectrum shows a maximum for both sulphamides at 340 nm (Fig. 4).
The excitation and emission spectra were not corrected. (A) Excitation and emission spectra, in aqueous solution, at pH 5.0, of SGN (—), β-cyclodextrin (...) and SGN: β-cyclodextrin (----). (B) SMTX (—), β-cyclodextrin (...) and SMTX: β-cyclodextrin (----). [SGN] = 4.7×10−6 M, [SMTX] = 4.0×10−6 M, and [β-CD] = 9×10−3 M. Excitation and emission slits of 10 and 5 nm, respectively, were used for the spectra recording
Although α-, β-, γ-, HP-β-CDs and DIMEB were investigated, only β-CD, HP-β-CD and DIMEB produced changes on the fluorescence spectra of SMTX and SGN. β-CD was selected because produces the higher fluorescence signal and the smaller blank signal. As may be appreciated, the changes in the fluorescence signals of these compounds in the presence of β-CD, compared with the fluorescence in its absence, are significant, but the wavelengths do not change. It has been observed that the intensity of fluorescence increases when increasing the concentration of β-CD. A value of β-CD concentration of 9.10−3 M was selected for the experiments in order to guarantee the quantitative complexation of the complexes.
Study of the influence of pH
A study of the influence of pH in the fluorescence spectra of both, SMTX:β-CD and SGN:β-CD systems, has been carried out. The results obtained are presented in Fig. 5. As can be appreciated, the fluorescence is maximum and practically constant for pH values between 3.0 and 5.0 for SMTX, and between 4.5 and 6.5 for SGN, at the excitation and emission wavelengths chosen. For values of pH higher than these, the fluorescence decreases.
Influence of pH on the fluorescence intensity of (A) SMTX:β-CD (λex = 264 nm; λem = 340 nm) and (B) SGN: β-CD complexes (λex = 274 nm; λem = 340 nm). [SGN] = 4.7×10−6 M, [SMTX] = 4.0×10−6 M, and [β-CD] = 9×10−3 M. Excitation and emission slits of 10 and 5 nm, respectively, were used for the spectra recording
Influence of the buffer concentration and order of addition of reagents
Several buffer solutions were studied with the aim of proposing a method for the determination of SMTX and SGN in presence of β-CD. Sodium acetate/acetic acid buffer (pH 4.6, 5.0 and 5.5) solutions were tested and a pH 5.0 was chosen as appropriate. A 0.4 M concentration was chosen as the optimum, as the dependence of the fluorescence intensity with the buffer concentration is not appreciable.
The order of addition of the reagents has no influence on the complexation, and the inclusion process is attained immediately.
Analytical parameters
Under the optimum operating conditions selected, the calibration graphs, obtained by plotting the fluorescence intensity versus SMTX or SGN concentration, were linear in the ranges 0.09–0.5 μg mL−1 and 0.07–1.0 μg mL−1, respectively, with Pearsons correlation coefficients of 0.999. Limits of detection, LOD Clayton [41] of 0.05 and 0.04 μg mL−1, respectively, were established.
Stoichiometry of the inclusion complexes
The straight line obtained when, in accordance with the Benesi-Hildebrands method, the values of 1/(F-F0) are plotted against 1/[β-CD]0, supports the existence of a 1:1 complex (Figs. 6a and 6b).
Applying the Scatchard's method, the representation of (F-F0)/[β-CD]0 versus [F-F0], is also giving a linear plot (Figs. 6c and 6d), confirming the 1:1 stoichiometry.
Association constant of the inclusion complexes
Once the stoichiometry of the system is known, the association constant may also be calculated, by application of the methods previously described. By the Benesi-Hildebrands method, the association constant is determined by dividing the intercept by the slope of the straight line obtained in the double-reciprocal plot. In the case of the Scatchard method, the association constant is given by the slope of the straight line. However, the linear transformations used in these graphical methods do not properly weigh the data [31]. The double-reciprocal plots tend to place more emphasis on lower concentration values in comparison to higher ones. As a result, the value of the slope is very dependent upon the ordinate value corresponding to the point having the smaller cyclodextrin concentration. Therefore, more adequate estimation can be made by using non-linear least-squares regression analysis (NLR) [31]. The formation constant calculated from the linear method may be used, however, as a parameter estimate in the NLR method. With the equation described in the experimental section, the experimental data can be directly fitted.
The values calculated for the constants of formation of the complexes, by means of the non-linear regression methods, are 361 and 414 M−1, for SGN:β-CD and SMTX: β-CD, respectively.
Characterizing the inclusion complexes
With the purpose of further characterizing the inclusion complex, semiempirical MO calculations using the AM1 program were performed. This program is commonly used to study geometric and thermodynamic properties of organic molecules, especially when hydrogen bonding occurs [42]. Several initial modes of inclusion were probed and optimized by energy minimization. The complex structure leading to the minimum heat of formation shows the aniline moiety located inside of the β-CD cavity. This fact is not surprising, since the most probable mode of binding in the CD inclusion complexes involves the insertion of the less polar part of the molecule into the cavity, while the more polar groups are exposed to the bulk solvent outside the opening of the cavity. The optimized structure of the complexes, obtained by energy minimization, is displayed in Fig. 7.
Interference studies
The possibility of other sulphonamides interference was investigated. For that, the fluorimetric signal of a mixture of SMTX and SGN, at 0.5 ppm, was compared with the fluorimetric signals of this mixture in the presence of different concentrations of the others (0.5; 1; 1.5 ppm). The sulphonamides assayed were sulfphathiazole, sulphadimethoxine, sulphamethazine, sulphamerazine, sulphaquinoxaline, sulphadiazine, sulphanilamide and sulphacetamide, in ratios of 1:1, 2:1 and 3:1, interference: analyte.
At the studied ratios, only interference from sulphanilamide was found. It is interesting to note that some of the sulphonamides could form inclusion complexes with ß-CD, but our experimental results show that, under the selected chemical conditions of pH and ß-CD concentration, only the competing effect of sulphanilamide is interfering. It is necessary to take into account that the association constants of the possible inclusion complexes should be different, depending of the included sulphonamide, and that some of the possible inclusion complexes formed can be weak enough and show not interference effect at the assayed maximum 3:1 ratio.
Simultaneous determination of sulphamethoxazole and sulphaguanidine
With the aim of performing the analysis of the two sulphonamides, a chemometric approach based in partial least-squares was evaluated. For training the PLS-1 model, a calibration set, according with a central composite design was constructed with 9 calibration samples, containing between 0 and 0.5 μg mL−1 for SMTX, and between 0 and 1.0 μg mL−1 for SGN. The component ratios were selected considering the linear calibration ranges (previously established from univariate exploratory experiments for each analyte). All calibration samples were prepared in triplicate and measured in random order. As the emission spectra of both sulphonamides are practically identical, we decided to employ the excitation spectra to construct the matrix. The excitation spectra were registered between 240 and 290 nm, using 340 nm as the emission wavelength.
Optimization of the PLS model
In order to determine the correct number of loading vectors to be used for the modelling of the data, a cross-validation calculation, for all the samples in the training set, was performed to calculate the PRESS (prediction residual error sum of squares). A number of two factors was optimized for both analytes. On the other hand, determination coefficients R2 of 0.986 and 0.982 were calculated for SGN and SMTX, respectively
Figures of merit
The program MVC1 allows obtaining the parameters of quality after applying the PLS-1 model. In this way, the parameters of sensitivity, analytical sensitivity and selectivity were obtained for both analytes. These parameters are shown in Table 1.
The PLS-1 model was applied to several data set of problems. The samples analyzed are composed by binary mixtures of variable amounts of the components randomly selected. Also, individual determination of each component was performed.
Simultaneous determination of sulphonamides in human urine
The proposed chemometric method has been applied to human urine samples from healthy patients, fortified with the two sulphonamides of interest. The dilution factor applied to the urine sample was fixed according to the amounts of these drugs excreted in the urine, under habitual therapy.
A portion of 0.1 mL of untreated freshly urine was spiked with the one or both drugs. With the aim of generating the possible interactions between analytes and the urine matrix, the samples were maintained at ambient temperature during 10 minutes; the necessary amount of β-CD aqueous solution was added to get a final concentration of 9 10−3 M; the pH was fixed to 5.0 by adding sodium acetate/acetic acid buffer and then diluted to 10 mL with ultrapure water. Six urine samples prepared as above described were analyzed, in the established conditions, by using the PLS optimized model. In all cases, high recovery values were obtained for SMTX and adequate values for SGN.
The evident interference generated by the urine matrix was considered, and a new calibration matrix composed by standard samples in presence of urine, according with a central composite design, was constructed. With the aim of modulating the variability of the urine samples background, several samples of urine blanks were included in the calibration matrix. A total of 8 urine blanks of different individuals were added to the model. From the statistical parameters obtained when applying the PLS model, for each component, in presence of urine, a number of three factors was established as optimum for both analytes.
The optimized PLS model in presence of urine was tested in the analysis of ten urine samples; each level of concentration was prepared in triplicate way. Sulphamethoxazole is present in numerous pharmaceutical preparations in combination with trimethoprim in a 5:1 SMTX:TMP ratio. The determination of SMTX in urine in the presence of TMP has been also performed; the resuls obtained lead to the conclusion that TMP does not produce interference in the concentration levels studied.
The concentrations of each analyte present in human urine and those values obtained by using the proposed method, are summarized in Table 2.
At the excitation wavelengths chosen, some interference from the biological matrix should be expected. However, a 1:100 dilution has been performed prior to the determination. A typical analysed sample contains 20 μg mL−1 of the drug. As 0.1 mL of urine is diluted to 10 mL, a 1:100 dilution is performed, and the measured concentration of the drug in the volumetric flask is 0.02 μg mL−1, that it is under the range of the calibration graphs (Linear ranges of 0.09–0.5 μg mL−1 and 0.07–1.0 μg mL−1 for SMTX and SMG, respectively).
These drugs are usually administered orally in pharmaceutical preparations in ranges of concentration of 250–500 mg L−1, being its adsorption of around 70–90%. The principal route of elimination it is urinary and around 20% is excreted, after metabolizing at the liver. This means that the urinary concentration should be between 17.5–35 μg mL−1, that are the ranges selected to test the procedure.
Also, it is necessary to take into account that, as it is discussed above, a total of 8 blank urine samples free from sulphonamides, were used as part of the calibration matrix, and modelled by the partial least squares calibration procedure, with the aim of modulating the variability of the urine samples, and eliminating the background urine interference.
There was no significant difference between the results obtained by using the HPLC technique and the multivariate calibration method. All predictions are seen to be reasonable for samples of the complexity of human urine. Comparison of the predicted concentrations and recoveries provided shows a good predictive ability towards the test set of spiked urine samples, and confirms the potentiality of the presently studied partial-least squares (PLS) calibration, for the analysis of complex biological samples.
Conclusions
On the basis of spectrofluorimetric measurements, the complex formation between SMTX, SGN and β-CD was studied. The stoichiometry and formation constants for the inclusion complexes were evaluated, and structural models were proposed. In these models, it can be observed that the aniline group and a part of the sulphanilic chain of the molecules are included in the cavity, through the open extreme of the structure of the cyclodextrin.
The proposed chemometric PLS model allows the simultaneous determination of SMTX and SGN, and the individual determination of these sulphonamides. Quantification of both sulphonamides in untreated urine has been performed, by using a calibration set composed by 9 standard spiked urine samples, and 8 blank urine samples obtained from different persons. The proposed procedure was validated by comparing the obtained results with a HPLC methods, with satisfactory results for the assayed method.
References
Marek J et al (1998) Farmakoterapie vnitrnich nemoci (Pharmacotherapy of Internal Diseases). Grada Publishing, Prague, pp. 159
Esteve-Romero J, Carda-Broch S, Gil-Agusti M, Capella-Peiro ME, Bose D (2005) Trends Anal Chem 24:75–91
Msagati TAM, Nindi MM (2004) Talanta 64:87–100
Bridges JW, Gifford LA, Hayes WP, Miller JN, Thorburn Burns D (1974) Anal Chem 46:1010–1017
Sakano T, Amano T (1976) Yakugaku Zasshi 96:1114–1121
Sterling JM, Haney WG (1974) J Pharm Sci 63:1448–1450
Arthur J, de Silva F, Strojny N (1975) Anal Chem 47:714–718
Steward JT, Wilkin RE (1972) J Pharm Sci 61:432–433
Sánchez Peña M, Salinas F, Mahedero MC, Aaron JJ (1992) J Pharm Biomed Anal 10:805–808
Petz M (1987) J Chromatogr 423:217–225
Schwaiger I, Schuch R (2000) Dtsch Lebebsm-Rundsch 96:93
Posyniak A, Sniegocki T, Zmudzki J (2002) Bull Vet Inst Pulawy 46:111
Martel AC, Zeggan S (2003) J Liq Chromatogr Rel Technol 26:953
Pang GF, Cao YZ, Fan CL, Zhang JJ, Li XM, Li ZY, Jia GQ (2003) Anal Bioanal Chem 376:534
Maudens E, Zhang G-F, Lambert WE (2004) J Chromatogr 1047:85–92
Szejtli J (1982) Cyclodextrins and their Inclusion Complexes. Akademiai Kiado, Budapest
Scypinski S, Love JLC (1984) Anal Chem 56:331–336
Muñoz de la Peña A, Ndou TT, Zung JB, Greene KL, Live DH, Warner IM (1991) J Am Chem Soc 113:1572–1577
Muñoz de la Peña A, Durán Merás I, Salinas F, Warner IM, Ndou TT (1991) Anal Chim Acta 255:351–336
Szente L, Szejtli J (1998) Analyst 123:735–741
Okamoto H, Uetake A, Tamaya R, Nakajima T, Sagara K, Ito Y (2000) J Chromatogr A 888:299–308
Bio. Rad Application Note (1999) 1575–45 2
Muñoz de la Pena A, Salinas F, Duran-Meras I, Dolores Moreno M (1994) Anal Lett 27:1893
Forina M, Casolino MC, De la Pezuela Martínez C (1998) J Pharma Biomed Anal 18:21–33
Salmain M, Varenne A, Vessieres A, Jaouen G (1998) Appl Spectrosc 52:1383–1390
Espinosa-Mansilla A, Acedo-Valenzuela MI, Muñoz de la Peña A, Salinas F, Cañada-Cañada F (2001) Anal Chim Acta 427:129–136
Wold S, Martens H, Wold H (1983) In: Rube A, Kagstrom B (eds.) The Multivariate Calibration Problem in Chemistry Solved by PLS: Matrix Pencils (in the series, lecture notes in mathematics) Springer, Heidelber, pp. 286
Goicoechea HC, Iñon FA, Olivieri AC (2004) Chem Intell Lab Syst 73:189–197
MATLAB 6.0 (1999) The Math Works Inc., Natick, Massachusetts, USA
Caballero RD, Carda-Broch S, García-Álvarez-Coque MC (2001) Anal Letters 34:1189–1203
Connors KA (1987) Binding Constants: The Measurement of Molecular Complex Stability. John Wiley and Sons, New York
Scatchard G (1949) Ann NY Acad Sci 51:660–672
Martens H, Naes T (1989) Multivariate Calibration. Willey, Chichester
Haaland DM, Thomas EV (1998) Anal Chem 60:1193–1202
Ferré J, Faber NM (2003) Chemom Intell Lab Syst 69:123–136
Muñoz de la Peña A, Salinas F, Durán-Merás I, Moreno MD (1994) Anal Letters 27:1893–1906
Wormser GP (1978) NY State J Med 78:1915
Berzas Nevado JJ, Lemus Gallego JM, Castañeda Peñalvo G (xxxx) Fresenius J Anal Chem 342:723
Drayton CJ (1990) Comprehensive Medicinal Chemistry. Pergamon, Oxford, pp. 6
Caballero RD, Torres-Lapasió JR, Baeza-Baeza JJ, García-Alvarez-Coque MC (2001) J Liq Chrom Rel Technol 24:117–131
Clayton CA, Hines JW, Elkins PD (1987) Anal Chem 59:2506–2514
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) J Am Chem Soc 107:3902–3909
Acknowledgements
Financial support provided by the Consejería de Infraestructuras y Desarrollo Tecnológico de la Junta de Extremadura and Fondos Feder (Project 2PR04A007) is greatly acknowledged. Diego Bohoyo Gil is grateful to the Consejería de Educación, Ciencia y Tecnología de la Junta de Extremadura for a fellowship (DOE 21/06/01). The authors are grateful to Diego Airado Rodríguez for his help in the validation of the proposed procedure by the liquid chromatographic method.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diez, N.M., de la Peña, A.M., García, M.C.M. et al. Fluorimetric Determination of Sulphaguanidine and Sulphamethoxazole by Host-Guest Complexation in β-Cyclodextrin and Partial Least Squares Calibration. J Fluoresc 17, 309–318 (2007). https://doi.org/10.1007/s10895-007-0174-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-007-0174-4