Abstract
Plant-feeding insects use visual and olfactory cues (shape, color, plant volatiles) for host location, but the relative importance of different cues and interactions with non-host-plant volatiles in ecosystems of varying plant biodiversity is unclear for most species. We studied invasive bark beetles and wood borers associated with pine trees to characterize interactions among color, host and non-host volatiles, by employing traps that mimic tree trunks. Cross-vane flight intercept traps (black, green, red, white, yellow, clear) and black funnel traps were used with and without attractants (α-pinene + ethanol), repellents (non-host green leaf volatiles, ‘GLV’), and attractant/repellent combinations in four pine forests in New Zealand. We trapped 274,594 Hylurgus ligniperda, 7842 Hylastes ater, and 16,301 Arhopalus ferus. Trap color, attractant, and color × attractant effects were highly significant. Overall, black and red traps had the highest catches, irrespective of the presence of attractants. Alpha-pinene plus ethanol increased trap catch of H. ligniperda 200-fold but only 6-fold for H. ater and 2-fold for A. ferus. Green leaf volatiles had a substantial repellent effect on trap catch of H. ligniperda but less on H. ater and A. ferus. Attack by H. ligniperda was halved when logs were treated with GLV, and a similar effect was observed when logs were placed among broadleaved understory shrubs emitting GLV. Overall, H. ligniperda was most strongly affected by the olfactory cues used, whereas H. ater and A. ferus were more strongly affected by visual cues. Collectively, the results support the semiochemical diversity hypothesis, indicating that non-host plant volatiles from diverse plant communities or artificial dispensers can contribute to resistance against herbivores by partly disrupting host location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Detecting and finding host plants is critically important for host-specific plant-feeding insects (Bernays and Chapman 1994). Plant volatiles are composed of a wide range of secondary metabolites characteristic of particular plant species (e.g., Baldwin 2010; Pichersky et al. 2006), which can be used by insects as olfactory signals for host recognition and location (Bruce and Pickett 2011). Non-host volatiles also are important during host searching; for example, volatiles from broadleaved trees can act as non-host cues for conifer-infesting insects (Huber and Borden 2001a; Jactel et al. 2011; Zhang and Schlyter 2004). In addition to olfactory cues, insects use visual cues such as host color and shape for host finding (e.g., Doering and Chittka 2007; Goyer et al. 2004) and for discrimination of host and non-host plants (e.g., Campbell and Borden 2009). However, the relative importance of olfactory and visual cues for host plant location (i.e., the process of locating host plants), and to what extent these cues are used synergistically, is largely unknown for most insect taxa (Hanks et al. 2012).
Interactions between olfactory and visual host and non-host cues are likely to play an important role in the dynamics of food webs. Impoverished plant communities such as plantation forests, often grown as ‘monocultures’, may be at an increased risk of insect outbreaks (Jactel and Brockerhoff 2007). The lack of non-host volatiles that could disrupt host location processes may contribute to this. According to the semiochemical diversity hypothesis (Zhang and Schlyter 2003), mixed plant volatiles from diverse plant communities can disrupt olfactory host location by several mechanisms including chemical disguise of host plants, masking insect attractants, and repelling effects (Jactel et al. 2011; Jactel and Brockerhoff 2007; Unsicker et al. 2009; Zhang and Schlyter 2004).
Bark beetles (Curculionidae: Scolytinae) and longhorn beetles (Cerambycidae) are among the best-studied forest insects because they can cause substantial tree damage and mortality, and play important roles in forest stand renewal, decomposition of woody plants, and forest food webs (Drever et al. 2009; Edburg et al. 2012; Kurz et al. 2008). The ability of adults to find suitable host material may be a limiting factor (Knížek and Beaver 2004), and selection for primary attraction and efficient searching mechanisms to find suitable host material are, therefore, important for individual fitness (De Jong and Sabelis 1988; Tunset et al. 1993). Many bark beetles and longhorn beetles associated with coniferous trees find host material by using host-specific volatiles for orientation and host recognition (Brockerhoff et al. 2006b; Byers and Zhang 2012; Pureswaran and Borden 2005). The occurrence of α-pinene, a common conifer monoterpene, and ethanol, a decomposition product of phloem and sapwood tissues, is characteristic of conifers that are dying or have died recently (Kelsey 1994). These compounds are important attractants or components of attractant blends especially for beetles colonizing conifer bark and wood (Hanks et al. 2012; Miller 2006; Miller and Rabaglia 2009; Schroeder and Lindelöw 1989).
Bark beetles can be broadly categorized as primary (species that usually attack live trees), secondary (species attacking trees that are weakened or died recently), or saprophytic (species breeding exclusively in dead trees) (Paine et al. 1997). Among the latter, especially, are numerous species that are successful invaders (Brockerhoff et al. 2006a, 2014), probably because they are prevalent in transport pathways such as wood packaging materials used in international trade, and because they may encounter fewer obstacles to establishment as they are not required to overcome defenses of live trees (Liebhold and Tobin 2008). Examples of this group are the pine bark beetles Hylastes ater (Paykull) and Hylurgus ligniperda (F.) (Curculionidae: Scolytinae), which have invaded two and four continents, respectively (Brockerhoff et al. 2006a). The pine longhorn beetle Arhopalus ferus (Mulsant) (Cerambycidae), has similar characteristics and is another successful invader that exploits trees that died recently (Brockerhoff and Hosking 2001). These three species often are highly abundant in pine forest regions in New Zealand (Brockerhoff et al. 2006b, Chase et al. 2017 ), particularly following tree harvesting, which provides pulses of deadwood resources for breeding. Although these particular species do not damage live trees, they can cause quarantine issues, may vector fungi colonizing wood (McCarthy et al. 2012, 2013), and H. ater may damage seedlings (Sopow et al. 2015). There is no indication of pheromone use in these species, but previous research has confirmed that pine-specific host volatiles play a role in host location and selection (Brockerhoff et al. 2006b; Suckling et al. 2001). However, for these and other saprophytic bark beetles and longhorn beetles, there is little information about the role of visual cues and interactions between olfactory and visual cues during host location, and whether non-host volatiles can interfere with host location processes as suggested by the semiochemical diversity hypothesis (Zhang and Schlyter 2003). Few studies on these topics have focussed on saprophytic species, despite their considerable species richness and their importance in biogeochemical cycles and forest food webs.
The underlying aims of the current study were to investigate the mechanisms that are thought to explain the semiochemical diversity hypothesis and its potential application. We conducted a suite of experiments to determine the relative importance of olfactory cues, visual cues, and their interactions in host finding of H. ater, H. ligniperda, and A. ferus, and whether non-host volatiles can disrupt the process. Specifically, our objectives were (i) to test the effects of a range of trap colors (that are similar or dissimilar to host material) in the presence or absence of host volatiles, and (ii) to investigate whether non-host volatiles characteristic of broadleaved, non-host trees can disrupt host location processes when emitted either by non-host plants, topically applied green leaf volatiles, or dispensers containing green leaf volatiles. Ultimately, the aims were to understand the host selection behavior of saprophytic bark beetles and longhorn beetles, and to enable the development of management strategies based on manipulation of host attraction and disruption.
Methods and Materials
Effects of Visual and Olfactory Cues on Trap Catch
Ten sites were selected in four Pinus radiata forests in the Nelson region, New Zealand: Golden Downs (NZTM E1592524; N5401801), Kainui (E1595200; N5405404), Moutere (E1602469; N5427872), Lee Valley (E1613407; N5417323). All sites had been harvested within the previous 12 mo. We used 200 flight intercept traps (20 treatments × 10 replicates, see Supplementary Material 1) in different colors (black, green, red, white, yellow, clear), mostly of a cross-vane design, made from MulfluteTM polypropylene sheets (600 × 210 mm; Mulford International, Christchurch, New Zealand), topped with a piece of MulfluteTM (210 × 210 mm), and a funnel and catch-cup attached to the bottom for collecting insects. Clear traps of the same design and dimensions were made from Lexan polycarbonate sheets (SABIC Innovation Plastics, Pittsfield, MA, USA). Black funnel traps were standard eight-funnel traps (PheroTech, Delta, BC, Canada) of the same height and width as the cross-vane traps. To describe the colors of the traps we tested, spectral reflectance measurements were made for the panel and funnel traps as well as Pinus radiata bark (see below and Supplementary Material 2).
Ethanol (Nuplex Specialties NZ, Mt. Wellington, New Zealand) and α-pinene (PINECHEM 500, Lawter (N.Z.), Mt. Maunganui, New Zealand) were provided in separate 150 ml dispensers (450 × 50 mm, 150 μm polyethylene tubing with felt wick strips inside, otherwise as described in Brockerhoff et al. (2006b)). Ethanol and α-pinene were applied at release rates of ca. 0.02 g/day and 0.76 g/day, respectively. The non-host volatiles used were two green leaf volatiles (GLV), (E)-2-hexen-1-ol and (Z)-3-hexen-1-ol (both from Bedoukian, Danbury, USA), applied in separate 20 ml polyethylene tubing dispensers (200 × 50 mm, otherwise as described above). Release rates from GLV dispensers were ca. 0.01 g/day and 0.008 g/day, respectively.
Trap catches were compared with different combinations of trap type, host-based attractants, and non-host repellents (control, attractant, repellent, and attractant+repellent) as shown in Supplementary Material 1. Effects of repellents were tested only for the subset of black, white, and clear panel traps. Traps were placed 20 m apart, suspended from steel Y-posts at about 1.4 m height, and positioned such that no two traps of similar color or treatment were adjacent. Traps were monitored fortnightly from November 2008 until May 2009, and moved one position clockwise at each monitoring to avoid position effects. In addition, traps in Kainui Forest were monitored at 3 hr intervals to record daily activity patterns during part of the peak flight and to assess whether flights occur more during light or dark, as this is relevant to the understanding of the role of vision and responses to colors. This was done over six 24 hr periods from 2 February 2009 (1:30 pm) until 5 February 2009 (10:30 am) and from 18 January 2010 (10:30 am) until 21 January 2010 (1:30 pm).
Effects of Synthetic Non-Host Volatiles on Attack of Pine Logs
Effects of topically applied, non-host green leaf volatiles (GLVs) on attack of P. radiata log billets by H. ater and H. ligniperda were assessed in Chaneys Forest, NE Christchurch (NZTM E1573380; N5192655). This trial was set up on 23 September 2009 during the spring flight of H. ater and H. ligniperda. Trees of the same age, diameter and similar bark thickness were felled and cut into 0.5 m log sections of about 200 mm diam. Fifty logs were placed in a recently harvested part of the forest from which most larger-diameter logging debris had been cleared. Two eight-funnel traps with ethanol and α-pinene (see above) were installed nearby to monitor beetle activity while the logs were in the field. Traps were cleared weekly, and beetles caught were recorded. Five replicates of ten logs were set out in parallel lines (about 50 m between lines) with logs ca. 20 m apart. Each trap log was placed in an east–west direction to standardize log positions with respect to insolation.
GLVs, (E)-2-hexen-1-ol and (Z)-3-hexen-1-ol in silicon oil solution (50 ml, 30 % GLV, 70 % oil), were applied with a paint brush to every second log within each replicate, covering the logs entirely. The remaining logs were left untreated as controls. Bark beetle attacks of logs were assessed weekly by counting the number of beetle gallery entrances and marking them with mapping pins. For the final assessment, all logs were taken to the laboratory on 16 November 2009. At that time, each log was bagged individually and placed in a freezer (-20 ° C) to stop further beetle activity. The bark of each log was removed later with a chisel, and the number of colonizing adult beetles present was counted. No teneral adults from the next generation were present.
Effects of Natural Non-Host Volatiles on Attack of Pine Logs
To test the effects of naturally occurring green leaf volatiles on bark beetle attack of pine logs, five sites in Chaneys Forest were selected that all contained areas with patches of understory vegetation of broadleaved fabaceous shrubs, mainly Cytisus scoparius, Ulex europaeus, and Lupinus arboreus in varying proportions, as well as open areas with no understory vegetation. As related Fabaceae are known to emit (E)-2-hexen-1-ol and (Z)-3-hexen-1-ol (Thöming et al. 2014), these shrubs are likely to emit green leaf volatiles; and as these are not emitted by pines, they can be considered non-host volatiles for pine bark beetles. Fifty 0.5 m-long logs were placed at the five study sites (10 logs per site). At each site, five pairs of logs were distributed such that each pair consisted of one log placed in an area without understory, and another log placed nearby among understory shrubs, with at least 50 m distance between pairs. Assessment of beetle attack of logs was as for the topically applied GLV trial (above), weekly in the field, and a final assessment in the laboratory. Six logs were lost over the trial period.
Measurement of Spectral Reflectance
We measured the spectral reflectance of Pinus radiata bark from a freshly cut tree and of the materials from which panel traps and Lindgren funnel trap were made, using samples measuring 100 × 80 mm. Samples were housed individually within a box lined with black-out cloth, which absorbs scatter light, prevents reflection from the box walls, and ensures that only the light reflected from the material was measured. Natural sun light was focused onto the samples through a N-BK7 lens (25.4 mm diam, focal length f = 100 mm) into an anodized aluminium lens tube (30 mm long, 25.4 mm diam). The spectral quality of reflected light was measured as the average of 20 scans with a 5 ms scanning speed using an Ocean Optics USB2000-NIR-VIS fibre spectrometer (OceanOptics, FL, USA). The relative intensity of reflected light was calculated as a ratio of the sampled wavelength and the peak reflected wavelength for each material tested. The spectral composition of the clear trap material (Lexan polycarbonate sheet) could not be determined as it transmits all wavelengths in the visible region.
Statistical Analysis
Statistical analyses were performed using R version 3.1.1 . We used generalized estimating equations (GEE) with Poisson errors (R-package geepack, Halekoh et al. (2006)) to test for differences between trap catches of the target beetle species caught with different trap colors with and without olfactory attractants (lures) across the 10 sites. The GEE approach was used instead of generalized linear mixed effects models because it could account for overdispersion and within-site correlation using ‘site’ as cluster identification. We applied species-specific GEE models that contained ‘seasonal trap catch’ (the sum of insects caught within a season) as response variable, and ‘color’ (including funnel traps serving as reference) as well as ‘attractant’ (ethanol and α-pinene vs. unbaited control) and their interaction as explanatory variables. The significance of the explanatory variables was assessed via backwards selection using Wald tests and the quasi-likelihood criterion QICu (Pan 2001; Zuur et al. 2009). GEE parameter estimates and variance-covariance matrices were used for multiple comparison tests using Tukey contrasts (Hothorn et al. 2008) (R-package BSagri; R-package multcomp). Resulting P-values were adjusted using Benjamini and Yekutieli’s (2001) method that controls the false discovery rate.
Effects of attractant and/or repellent addition on catch numbers of the same beetle species were tested using generalized linear mixed models (GLMMs) with negative binomial error distribution and logit link function [R package glmmADMB, (Skaug et al. 2013)]. Species-specific models contained ‘trap treatment’ (factor with four levels: ‘control’, ‘repellent’, ‘attractant’, and ‘attractant + repellent’) as explanatory variable and the factor ‘site’ as a random term. The effects of topically applied GLVs or naturally emitted non-host volatiles on bark beetle attack of logs were tested using species-specific GLMMs with negative binomial error distribution, logit link function, and ‘site’ as a random term [R package glmmADMB, Skaug et al. (2013)]. In all GLMMs, the significance of the fixed terms was determined via backwards selection using likelihood-ratio tests and Akaike’s Information Criterion (AIC; Zuur et al. 2009).
Results
Effects of Visual and Olfactory Cues on Trap Catch
Large numbers of beetles of the three target species were caught during the main experiment of this study, including 274,594 H. ligniperda, 7842 H. ater, and 16,301 A. ferus. The main effects, trap color and attractant, and the color × attractant interaction were all highly significant (Fig. 1; Wald tests, all P < 0.001, see Supplementary Material 3). For traps without attractants, black and red panel traps tended to have the highest catches, whereas clear and white traps tended to have the lowest, and these were significantly different from some of the other trap colors (Fig. 1). For example, for H. ligniperda, catches in black panel traps differed significantly from those in green, yellow, and white panel traps, while for A. ferus, catches in black traps differed from those in clear and white traps. Catches in clear traps also differed from those in red, green, and yellow traps, as well as in black funnel traps. For H. ater, red panel traps had significantly more catches than clear traps (Fig. 1). This overall preference for black and red traps is consistent with the spectral reflectance of pine bark, which has a similar peak intensity as our red traps and high levels of intensity overlapping with our black traps (Supplementary Material 2).
Mean (± SE) trap catch of Hylastes ater, Hylurgus ligniperda, and Arhopalus ferus caught in colored panel traps and black funnel traps without and with attractants (α-pinene and ethanol) (N = 10 sites). Different lower case letters indicate statistically significant differences between trap colors within attractant and species, whereas different upper case letters denote statistically significant differences between attractants within trap color and species (multiple comparison tests with Tukey contrasts, α = 0.05). Note: Y-axis for H. ligniperda plotted on two different scales
Traps with α-pinene and ethanol as attractants caught considerably more beetles than traps without attractants, although the extent of this varied by species (approximately twice as many A. ferus, six times as many H. ater, and 200 times as many H. ligniperda). These means, along with the respective χ 2 values for color and attractant (Supplementary Material 3), indicate that host attractants were comparatively more important in H. ligniperda than in H. ater and A. ferus. The relative responses to trap color and type differed between traps with and without attractant, given the significant color × attractant interaction. Attractant-baited black panel traps again tended to have the highest catches, while attractant-baited clear and white traps had the lowest catches for most species. However, black funnel traps caught significantly fewer H. ater and A. ferus than black panel traps when attractants were used.
Trapping in three-hour intervals showed that there were clear peaks in the daily activity of H. ater, H. ligniperda, and A. ferus (Fig. 2). Trap catches of all three species exhibited two peaks of activity between 07:30 am and 10:30 am and between 7:30 pm and 10:30 pm. During the southern hemisphere summer when the data were gathered, these periods represent morning (post-dawn) and evening (including dusk) when there is typically low wind, low to moderate light, and moderate to warm temperatures (Fig. 2).
Green leaf volatiles (GLVs) had a significant repellent effect on trap catch of H. ligniperda (27–55 % reduction in catch), a marginally insignificant effect for H. ater (P = 0.056), and no effect for A. ferus (Fig. 3, Supplementary Material 4). However, there was no significant attractant × repellent interaction in any of the species (Supplementary Material 4). When we compared the relative effect size of attractants (α-pinene and ethanol) and repellents (GLVs), the effect of attractants exceeded the effect of repellents (as indicated by the larger χ 2 values) in all three species (Supplementary Material 4).
Mean (± SE) trap catch of Hylastes ater, Hylurgus ligniperda, and Arhopalus ferus per trap treatment; C, control; R, repellent; A, attractant; A+R, attractant + repellent. Different lower case letters indicate statistically significant differences between trap treatments (multiple comparison tests with Tukey contrasts, α = 0.05; see text and Supplementary Material for details). Note: Y-axis plotted at different scales and with two vertical axes for H. ligniperda
Effects of Non-Host Volatiles on Attack of Pine Logs
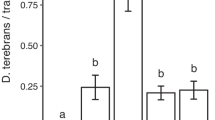
Logs treated with GLVs in silicon oil were attacked by significantly fewer (about half as many) H. ligniperda than control logs (Fig. 4, Supplementary Material 5). For H. ater, GLV treatment had a similar effect (Fig. 4), but the difference was marginally non-significant (P = 0.05, Supplementary Material 5); however, the rate of attack by H. ater generally was low reflecting its lower abundance at the study site. Both species were in flight during the trial period, but catches of H. ligniperda exceeded those of H. ater by about 100:1 (e.g., 46 H. ligniperda vs. 0.43 H. ater per day in mid-October 2009). No attack of pine logs by A. ferus was observed in our trial.
Mean (± SE) number of Hylastes ater and Hylurgus ligniperda found on logs treated with green leaf volatiles (GLV) and untreated logs (control) (N = 5 sites, ** P < 0.01, ns: not significant; see text and Supplementary Material for details)
Pine logs placed among an understory of broadleaved shrubs that naturally emit non-host GLVs were attacked considerably less than logs placed in open areas. The number of H. ligniperda found on logs that had been placed in areas with understory vegetation was significantly reduced, by about 75 %, compared with logs in the open forest without an understory (Fig. 5, Supplementary Material 6). For H. ater no significant treatment effect was found (Fig. 5, Supplementary Material 6); however, attacks by H. ater generally were rarer.
Mean (± SE) number of Hylastes ater and Hylurgus ligniperda found to attack logs placed in ‘open’ areas of pine stand without understory and in pine stands with broadleaved understory vegetation that emits natural non-host volatiles (N = 5 sites, ** P < 0.01, ns: not significant; see text and Supplementary material for details)
Discussion
Host-specific bark beetles and longhorn beetles that feed and reproduce under the bark and in the wood of particular tree species must be able to locate and recognize host material of a suitable species. For saprophytes, the host material also must be of an appropriate condition, as neither live trees nor material that is too decayed can be colonized. For the three pine-infesting beetles we studied, our results indicate that visual and olfactory host and non-host cues together with their interactions are important in the host location process, and that the relative importance of these cues is species-specific. While it is well-known that host volatiles such as α-pinene and ethanol play an important role in host location and recognition by bark beetles and longhorn beetles, including the species studied here (Brockerhoff et al. 2006b; Miller 2006; Miller and Rabaglia 2009; Schroeder and Lindelöw 1989; Suckling et al. 2001), our study contributes new insight into the roles of olfactory and visual cues and their interactions during host location. Although the shape and dark color of funnel traps and panel traps is presumed to visually mimic tree trunks, few prior studies have systematically examined this, especially for such a comprehensive range of trap colors and interactions with olfactory cues as employed in our study (but see Campbell and Borden 2006, 2009; Strom and Goyer 2001).
Trap color was a significant factor in attraction to traps in all three species we studied, although without the concurrent application of olfactory cues, differences in trap catch among the seven color/design treatments were not as striking. However, clear, white and yellow traps, which least resembled host tree trunks and bark, yielded lower catches overall than traps of darker colors. These observations are consistent with the similarities in the spectral reflectance of some of our traps and pine bark (Supplementary Material 2), considering that insect vision typically covers the range from about 350 nm (ultraviolet) to 700 nm (red/infrared), with some variation among species (Briscoe and Chittka 2001). The brightness of different colors probably is also perceived by insects. Although A. ferus is known for its crepuscular flight and activity behavior (Suckling et al. 2001), we observed that all three species in our study fly to some degree during the morning and evening hours of daylight, and they should, therefore, be able to see objects and perceive color to some extent. However, the lack of a strong color response and the more noticeable response difference between darker and lighter-colored traps seem to confirm the conclusions of Strom and Goyer (2001) that color is less important in bark beetle host location than the presence of a dark, rather than light-colored, silhouette.
The importance of olfactory host cues differed among the species we studied. For the two bark beetles, host volatiles were more important during the host location process compared with the longhorn beetle. Hylastes ater catches with host-characteristic visual and olfactory cues (i.e., traps with a black tree trunk-like silhouette emitting α-pinene and ethanol) were about ten-fold higher than for a similar trap without olfactory cues. This effect was even more striking in H. ligniperda where the use of host volatiles increased trap catch by more than 100-fold. By contrast, for A. ferus, host volatiles increased trap catch only slightly (less than double), albeit significantly, compared with unbaited black traps (see also Brockerhoff et al. 2006b). Furthermore, in this species, visual cues had a slightly larger effect than volatiles: black panel traps caught more than twice as many A. ferus as clear panel traps. Collectively, this indicates that for A. ferus, visual cues are more important during host location than olfactory cues, contrary to the situation with the two bark beetles. Our results for the two pine bark beetles are consistent with other studies, which documented strong attraction of conifer-infesting species to α-pinene and ethanol (e.g., Miller and Rabaglia 2009). However, A. ferus showed only a weak response to α-pinene and ethanol, contrasting with the findings of Miller (2006) who found the combination of these compounds to be a strong attractant for a number of longhorn beetles attacking pine, including the congeneric Arhopalus rusticus nubilus. It cannot be ruled out that A. ferus requires some additional attractant compounds. For longhorn beetles in particular, it is not uncommon that host volatiles are less effective as attractants than combinations of host attractants and various pheromone compounds (see Hanks et al. 2012). Nevertheless, host volatiles can be important synergists with other attractants and beetle pheromones (Hanks et al. 2012), and whether this applies to A. ferus requires more research.
The addition of olfactory non-host cues [i.e., green leaf volatiles (GLVs)] resulted in a moderate reduction in trap catch of the bark beetles, but had no apparent effect on A. ferus. Topical application of these GLVs to fresh pine logs resulted in a similar (moderate) reduction in bark beetle attack of pine logs (no attack by A. ferus was observed). These and other GLVs have been shown to repel or reduce attraction for a number of conifer-infesting bark beetles and longhorn beetles (Huber and Borden 2001a; Jactel et al. 2011; Suckling et al. 2001; Zhang and Schlyter 2004). The lack of a repellent effect of GLVs on A. ferus was an unexpected result because a previous study found significant repellent effects of GLVs (Suckling et al. 2001); however, that study used walking and oviposition bioassays that tested close-range responses, whereas our trapping study assessed longer-range responses of flying beetles, which is the probable reason for this difference.
Our comparison of attack of pine logs placed among an understory of broadleaved shrubs and vs. open areas aimed to test whether naturally emitted non-host green leaf volatiles can provide a similar effect as artificially applied GLVs, as predicted by the semiochemical diversity hypothesis (Zhang and Schlyter 2003). While attacks of pine logs in the understory treatment were substantially reduced, especially for H. ligniperda, this effect is unlikely to be due solely to non-host volatiles, as the understory probably also created physical and visual barriers. However, this combination of non-host volatiles and other barriers to insect colonization is clearly involved in the biodiversity effects described from mixed forests that tend to be less prone to insect attack than forests of lower diversity of plant species. Tree-feeding insects have been shown to cause less damage and to be less abundant in forests composed of several tree species than in single-species forests (Jactel and Brockerhoff 2007). Furthermore, mixtures of tree species that are phylogenetically more distant, such as conifers and broadleaved trees, have been found to regulate populations of tree-feeding insects more effectively than mixtures of trees of more closely related species (Castagneyrol et al. 2014; Jactel and Brockerhoff 2007). This effect is known as associational resistance which is defined as “direct and indirect interactions between plants in close proximity, in which the influence of one plant on another … decreases the likelihood of detection by, and/or vulnerability of a focal plant to, herbivores (above and beyond its innate ability to avoid detection or damage owing to herbivory)” (Barbosa et al. 2009; see also Castagneyrol et al. 2014). Closely-related tree species tend to emit relatively similar volatiles and share other traits, so it is not surprising that the host range of pine bark beetles and borers typically includes many species of pine, irrespective of their geographic origin (Branco et al. 2015).
We suggest associational resistance can also occur within single-species forests, such as the pine forests in which our study took place, when an understory of distantly related species is present, broadleaved shrubs in our case. While disruption of host location has been demonstrated by way of experimental application of non-host cues for numerous conifer feeding beetles (Byers et al. 2004; Campbell and Borden 2006, 2009; Goyer et al. 2004; Huber and Borden 2001a, b; Strom and Goyer 2001; Suckling et al. 2001; Zhang and Schlyter 2004), and in the present study, our findings suggest that mixed species planting and the encouragement of a diverse understory vegetation may reduce the impact of pine beetles.
In summary, our study shows that the host-finding behavior of bark beetles and longhorn beetles relies on both olfactory and visual cues from both host and non-host plant species, but to varying extents, with the two bark beetles relying more on olfactory cues, and the longhorn beetle more on visual cues. Based on our results and previous work on numerous other bark beetles and longhorn beetle, it is clear that such host-finding processes can be complex and show a surprising amount of variation among species. Such research is not only relevant for our understanding of the ecology of these insects, which are prominent biological invaders world-wide, but also for the development of ‘greener’ and more sustainable methods for the management of detrimental species, such as stimulo-deterrent diversion and push-pull techniques (e.g., Pickett et al. 2014) by combining attractants and repellents such that insects are diverted away from timber.
References
Baldwin IT (2010) Plant volatiles. Curr Biol 20:R392–R397
Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z (2009) Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu Rev Ecol Evol Syst 40:1–20
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman & Hall, New York
Branco M, Brockerhoff EG, Castagneyrol B, Orazio C, Jactel H (2015) Host range expansion of native insects to exotic trees increases with area of introduction and the presence of congeneric native trees. J Appl Ecol 52:69–77
Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Annu Rev Entomol 46:471–510
Brockerhoff EG, Hosking GP (2001) Arhopalus tristis (F.)(Coleoptera: Cerambycidae) (= Arhopalus ferus (Mulsant)): Burnt pine longhorn beetle. Forest and Timber Insects in New Zealand 27:1–8
Brockerhoff EG, Bain J, Kimberley M, Knížek M (2006a) Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Can J Forest Res 36:289–298
Brockerhoff EG, Jones DC, Kimberley MO, Suckling DM, Donaldson T (2006b) Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For Ecol Manag 228:234–240
Brockerhoff EG, Kimberley M, Liebhold AM, Haack RA, Cavey JF (2014) Predicting how altering propagule pressure changes establishment rates of biological invaders across species pools. Ecology 95:594–601
Bruce TJA, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects - Finding the right mix. Phytochemistry 72:1605–1611
Byers JA, Zhang QH (2012) Chemical ecology of bark beetles in regard to search and selection of host trees. In: Recent advances in entomological research. Springer, p 150–190
Byers JA, Zhang QH, Birgersson G (2004) Avoidance of nonhost plants by a bark beetle, Pityogenes bidentatus, in a forest of odors. Naturwissenschaften 91:215–219
Campbell SA, Borden JH (2006) Integration of visual and olfactory cues of hosts and non-hosts by three bark beetles (Coleoptera: Scolytidae). Ecol Entomol 31:437–449
Campbell SA, Borden JH (2009) Additive and synergistic integration of multimodal cues of both hosts and non-hosts during host selection by woodboring insects. Oikos 118:553–563
Castagneyrol B, Jactel H, Vacher C, Brockerhoff EG, Koricheva J (2014) Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J Appl Ecol 51:134–141
Chase KD, Kelly D, Liebhold AM, Bader MK-F, Brockerhoff EG (2017) Long distance dispersal of non-native pine bark beetles from host resources. Ecological Entomology
De Jong MCM, Sabelis MW (1988) How bark beetles avoid interference with squatters: An ESS for colonization by Ips typographus. Oikos 51:88–96
Doering TF, Chittka L (2007) Visual ecology of aphids-a critical review on the role of colours in host finding. Arthropod Plant Interact 1:3–16
Drever MC, Goheen JR, Martin K (2009) Species-energy theory, pulsed resources, and regulation of avian richness during a mountain pine beetle outbreak. Ecology 90:1095–1105
Edburg SL, Hicke JA, Brooks PD, Pendall EG, Ewers BE, Norton U, Gochis D, Gutmann ED, Meddens AJH (2012) Cascading impacts of bark beetle-caused tree mortality on coupled biogeophysical and biogeochemical processes. Front Ecol Environ 10:416–424
Goyer RA, Lenhard GJ, Strom BL (2004) The influence of silhouette color and orientation on arrival and emergence of Ips pine engravers and their predators in loblolly pine. For Ecol Manag 191:147–155
Halekoh U, Højsgaard S, Yan J (2006) The R package geepack for generalized estimating equations. J Stat Softw 15:1–11
Hanks LM, Millar JG, Mongold-Diers JA, Wong JCH, Meier LR, Reagel PF, Mitchell RF (2012) Using blends of cerambycid beetle pheromones and host plant volatiles to simultaneously attract a diversity of cerambycid species. Can J For Res 42:1050–1059
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Huber DPW, Borden JH (2001a) Angiosperm bark volatiles disrupt response of Douglas-fir beetle, Dendroctonus pseudotsugae, to attractant-baited traps. J Chem Ecol 27:217–233
Huber DPW, Borden JH (2001b) Protection of lodgepole pines from mass attack by mountain pine beetle, Dendroctonus ponderosae, with nonhost angiosperm volatiles and verbenone. Entomol Exp Appl 99:131–141
Jactel H, Brockerhoff EG (2007) Tree diversity reduces herbivory by forest insects. Ecol Lett 10:835–848
Jactel H, Birgersson G, Andersson S, Schlyter F (2011) Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia 166:703–711
Kelsey RG (1994) Ethanol synthesis in Douglas-fir logs felled in November, January, and March and its relationship to ambrosia beetle attack. Can J For Res 24:2096–2104
Knížek M, Beaver R (2004) Taxonomy and systematics of bark and ambrosia beetles. In: Lieutier F, Day KR, Battisti A, Gregoire J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe - a synthesis. Kluwer, Dordrecht, pp 41–54
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408
McCarthy JK, Hood IA, Kimberley MO, Didham RK, Bakys R, Fleet KR, Brownlie RK, Flint HJ, Brockerhoff EG (2012) Effects of season and region on sapstain and wood degrade following simulated storm damage in Pinus radiata plantations. For Ecol Manag 277:81–89
McCarthy JK, Brockerhoff EG, Didham RK (2013) An experimental test of insect-mediated colonisation of damaged Pinus radiata trees by sapstain fungi. PLoS ONE 8:e55692
Miller DR (2006) Ethanol and (−)-α-pinene: Attractant kairomones for some large wood-boring beetles in southeastern USA. J Chem Ecol 32:779–794
Miller DR, Rabaglia R (2009) Ethanol and (−)-α-pinene: attractant kairomones for bark and ambrosia beetles in the southeastern US. J Chem Ecol 35:435–448
Paine TD, Raffa KF, Harrington TC (1997) Interaction among scolytid bark beetles, their asscoiated fungi, and live host conifers. Annu Rev Entomol 42:179–206
Pan W (2001) Akaike’s information criterion in generalized estimating equations. Biometrics 57:120–125
Pichersky E, Noel JP, Dudareva N (2006) Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 311:808–811
Pickett JA, Woodcock CM, Midega CA, Khan ZR (2014) Push–pull farming systems. Curr Opin Biotechnol 26:125–132
Pureswaran DS, Borden JH (2005) Primary attraction and kairomonal host discrimination in three species of Dendroctonus (Coleoptera: Scolytidae). Agric For Entomol 7:219–230
Schroeder L, Lindelöw Å (1989) Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J Chem Ecol 15:807–817
Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B (2013) Generalized linear mixed models using AD Model Builder
Sopow SL, Bader MKF, Brockerhoff EG (2015) Bark beetles attacking conifer seedlings: Picking on the weakest or feasting upon the fittest? J Appl Ecol 52:220–227
Strom BL, Goyer RA (2001) Effect of silhouette color on trap catches of Dendroctonus frontalis (Coleoptera: Scolytidae). Ann Entomol Soc Am 94:949–953
Suckling DM, Gibb AR, Daly JM, Chen D, Brockerhoff EG (2001) Behavioral and electrophysiological responses of Arhopalus tristis to burnt pine and other stimuli. J Chem Ecol 27:1091–1104
Thöming G, Norli HR, Saucke H, Knudsen GK (2014) Pea plant volatiles guide host location behaviour in the pea moth. Arthropod Plant Interact 8:109–122
Tunset K, Nilssen AC, Andersen J (1993) Primary attraction in host recognition of coniferous bark beetles and bark weevils (Col., Scolytidae and Curculionidae). J Appl Entomol 115:155–169
Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12:479–485
Zhang QH, Schlyter F (2003) Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos 101:299–310
Zhang QH, Schlyter F (2004) Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric For Entomol 6:1–20
Zuur AF, Leno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Statistics for biology and health. Springer, New York
Acknowledgments
We thank Dave Brockerhoff, Belinda Gresham, David Henley, Sebastian Horvath, James McCarthy, Stephen Pawson, Michelle Watson, and the late Colleen Carlson for assistance with field or laboratory work, Nelson Forests, Hancock Forest Management, and Selwyn Plantation Board for access to field sites, and Nadir Erbilgin and two anonymous reviewers for comments on the manuscript. Funding for this study was obtained from the New Zealand government [MBIE core funding to Scion (C04X1104) and a Tertiary Education Commission Scholarship (TEC 3101)].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 162 kb)
Rights and permissions
About this article
Cite this article
Kerr, J.L., Kelly, D., Bader, M.KF. et al. Olfactory Cues, Visual Cues, and Semiochemical Diversity Interact During Host Location by Invasive Forest Beetles. J Chem Ecol 43, 17–25 (2017). https://doi.org/10.1007/s10886-016-0792-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0792-x