Abstract
A study was carried out on the allelopathic potential of four forest species, Tectona grandis, Aleurites fordii, Gliricidia sepium, and Maytenus buxifolia. The most active species, T. grandis, was selected to perform a phytochemical study. A new compound, abeograndinoic acid, was isolated, and elucidation of its structure showed that this compound has an unusual carbon skeleton. A further 21 known terpenoids—including 4 sesquiterpenoids, 8 diterpenes and 9 triterpenes—also were isolated. A biosynthetic scheme for the presence of the new compound is proposed. Bioactivity profiles that used etiolated wheat coleoptiles and phytotoxicity bioassays on the isolated compounds were conducted. The compounds that presented the highest phytotoxic activity are the diterpenes 9 (2-oxokovalenic acid) and 12 (19-hydroxyferruginol).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exotic plant species represent the core of agricultural production in many countries. Cuba is no exception, since most of the agricultural species grown in the country are exotic, having been introduced at some point in the past. A number of exotic timber trees were introduced into Cuba from tropical and subtropical regions. The scientific literature supports the ethnobotanical use of such species as sources of bioactive substances of both pharmacological and agrochemical interest. Examples of this phenomenon include neem (Azadirachta indica A. Juss) (Atawodi and Atawodi 2009), chinaberry (Melia azedarach L.) (Charleston et al. 2006), tamarind (Tamarindus indica L.) (Khanzada et al. 2008), gliricidia (Gliricidia sepium Jack) (Ramamoorthy and Paliwal 1993), wild indigo (Indigofera suffruticosa Mill) (Barros and Teixeira 2008), and sugar-apple (Annona squamosa L.) (Naik et al. 2008).
Better utilization of non-wood forest products (NWFPs) requires knowledge of the ecological relationships of introduced alien species and their potential as sources of bioactive compounds, especially agrochemical and allelopathic potential. The families Celastraceae, Fabaceae, Euphorbiaceae, and Verbenaceae have a wide variety of tree species with a number of biological activities. Several species within these families are distributed in Cuba, and these include Tectona grandis (Verbenaceae), Aleurites fordii (Euphorbiaceae) (Fozdar et al. 1989), Gliricidia sepium (Fabaceae) (Herath et al. 1997), and Maytenus buxifolia (Celastraceae) (González et al. 1997). These species are economically important and have been used in agroforestry systems or for various ethnobotanical applications. However, the possible environmental impact that arises from the introduction of these species has not been evaluated, nor has the possible use of these species as a source of natural chemicals.
Herein, we describe a study of the allelopathic potential of these forest species. The species with the highest allelopathic activity, i.e., teak (T. grandis) (Verbenaceae), was selected for a phytochemical study with the aim of isolating and characterizing the metabolites responsible for the biological activity. Few compounds have been isolated from teak, and most of these are quinones and triterpenes (Rimpler and Christiansen 1977). The family Verbenaceae is characterized in chemical terms by the presence of low levels of diterpenes, with tectograndinol as the only example previously isolated from the genus Tectona (Joshi et al. 1977; Marwani et al. 1997).
The characterization of chemical components from Tectona grandis and the assessment of their activity are likely to provide information to explain the success of this tree culture where it has been used in agroforestry (taungya system). This system involves combining crops such as corn, cotton, cassava, ginger, peanut, soybean, upland rice, and beans with young teak plantations, an approach that results in higher crop yields and better control of the weeds (Wiersum 1982). From an ecological point of view, a move towards sustainable agriculture where weeds can be controlled to some extent by forest species such as T. grandis is desirable. The increased forest area is not only as a source of wood, but as a new potential tool for pest control.
Previously, we reported the isolation of seven apocarotenoids from T. grandis and elucidated the structures of two of them, tectoionols A and B (Macias et al. 2008). As a continuation of our study of this species, a bioassay-guided fractionation of extracts from T. grandis was carried out in order to isolate and identify the chemical constituents and to characterize their potential phytotoxic activities. We report here the isolation and structure elucidation of a new compound, abeograndinoic acid, which has an unusual carbon skeleton. A further 21 known terpenoids, including 4 sesquiterpenoids, 8 diterpenes, and 9 triterpenes, also were isolated. The bioactivity profiles of the isolated compounds were studied.
Methods and Materials
General
IR spectra (KBr) were recorded on a Perkin-Elmer FT-IR Spectrum 1000 or a Matton 5020 spectrophotometer. NMR spectra were run on Varian INOVA-400 and Varian INOVA 600 spectrometers. Chemical shifts are given in ppm with respect to residual 1H signals of CHCl3-d 1 and acetone-d 6 (δ 7.25 and 2.04, respectively), and 13C signals are referenced to the solvent signal (δ 77.00 and 29.80, respectively). Optical rotations were determined by using a Perkin-Elmer model 241 polarimeter (on the sodium D line). HRMS were obtained on VG AUTOESPEC mass spectrometer (70 eV). HPLC was carried out on a Merck-Hitachi instrument, with RI detection, using three different Merck LiChrospher columns: RP-18 (10 µm, 250 × 10 mm), SI 60 (5 µm, 250 × 4 mm), and SI 60 (10 µm, 250 × 10 mm).
Plant Material and Aqueous Extracts
Leaves and bark of Tectona grandis, leaves and flowers of Gliricidia sepium, and leaves of Maytenus buxifolia and Aleurites fordii were collected between the months February and March (2003) in Ciudad de La Habana and were identified by MsC. Lutgarda González. Voucher specimens of each species (80613, 80614, 80921, and 80922, respectively) were deposited at the Jardín Botánico de Cuba.
Vegetal material was dried at room temperature in the shade. Dried materials of each species (50 g) were extracted with water (450 ml) for 24 h at room temperature in the dark—except for leaves of G. sepium and A. fordii, which were extracted with 900 ml of water.
Extraction and Isolation
Dried leaves of Tectona grandis (5 kg) were extracted with water (35 l) for 24 h at room temperature in the dark. The aqueous solution was extracted with CH2Cl2 and then with EtOAc at room temperature. Details of the extraction procedure and the bioassays on the extract have been described previously (Macias et al. 2008). DCM/H2O and DCM extracts were the most active.
The DCM/H2O extract (8.8 g) was chromatographed on SiO2 using mixtures of hexane/EtOAc of increasing polarity, acetone and methanol to afford twelve fractions: A1–L1.
Fraction E1 (0.750 g, hexane/EtOAc, 17:3–1:4) was subjected to CC on Sephadex LH-20 using mixtures of n-hexane/chloroform/methanol (3:1:1) in order to remove chlorophylls and other high molecular weight compounds. The residue was separated by CC and HPLC on silica gel using mixtures of chloroform/acetone and hexane/EtOAc to yield compounds 1 (5.0 mg), 2 (0.4 mg), and 3 (4.8 mg). Fraction F1 (0.800 g, hexane/EtOAc, 1:4) was subjected to CC on Sephadex LH-20 using a mixture of hexane/chloroform/methanol (3:1:1). Further purification by C-18 HPLC (water/methanol, 7:13) yielded compound 4 (2.8 mg). Fraction I1 (0.750 g) was subjected to CC on silica gel using mixtures of chloroform/acetone of increasing polarity and methanol. The largest fraction, I16 (0.300 g), was purified by CC on silica gel using mixtures of hexane/acetone to yield compound 21 (1.1 mg).
The DCM (120 g) extract was chromatographed on silica gel (2.0 kg) using hexane/ethyl acetate mixtures of increasing polarity to yield eight fractions: A2–H2.
Fraction C2 (15.00 g) was subjected to CC on silica using 1.5 l of each solvent (hexane, chloroform, ethyl acetate, acetone, and methanol) to afford five fractions: C21–C25. Fraction C22 (0.906 g, CHCl3) was chromatographed by CC on silica gel, using mixtures of hexane/chloroform and chloroform/acetone. Purification by silica gel HPLC yielded 15 (2.3 mg), 5 (2.8 mg), and 13 (60 mg). Fraction C24 (4.900 g, acetone) was subjected to CC on silica gel, using mixtures of hexane/chloroform and chloroform/acetone. Further purification by CC using hexane/ethyl acetate mixtures on silica gel and hexane/chloroform/methanol (3:1:1) on Sephadex LH-20 , that was the stationary phase that best separated these compounds, afforded compounds 12 (12.5 mg), 16 (5.0 mg), 14 (3.2 mg), and 6 (3.3 mg).
Fraction D2 (hexane/EtOAc, 3:2, 7.46 g) was subjected to CC on silica gel, using mixtures of n-hexane/chloroform and chloroform/acetone. Fraction D22 (0.930 g) was chromatographed using mixtures of n-hexane/chloroform and chloroform/acetone to afford, after further purification by HPLC, compounds 17 (3.0 mg), 8 and 10 (3.6 mg). Fraction D23 (1.800 g, n-hexane/chloroform 1:3) was subjected to CC on silica gel using mixtures of n-hexane/chloroform and acetone to afford six fractions D23A–D23F. Fractions D23B (0.485 g, chloroform) and D23C (0.492 g, chloroform) were purified by CC on silica gel using mixtures of n-hexane/acetone and by HPLC to yield compounds 9 (18 mg) and 7 (15.5 mg). Fraction D24 (1.200 g, chloroform/acetone 19:1) was subjected to CC on silica gel, using mixtures of n-hexane/acetone and chloroform/acetone. Compound 4 (3.6 mg) and an isomeric mixture of 18 and 19 (16 mg) were isolated from fraction D24C after silica gel CC chromatography and C-18 HPLC. Fraction D24E (0.059 g) was subjected to CC on silica gel using mixtures of n-hexane/chloroform and chloroform/acetone to yield compound 20 (4.9 mg) after purification by silica gel HPLC. Further chromatography of fraction D24F (0.110 g) by CC and HPLC on silica gel, using mixtures of chloroform/acetone and acetone, afforded 11 (0.7 mg).
Abeograndinoic Acid
(11), colorless oil; [α] 25D −12.9 (c 0.01, CHCl3); IR (KBr) νmax 3410 (OH), 1735 (C=O), 1696 (C=O), 1650 (C=C) cm−1. 1H NMR data (400 MHz) CHCl3 -d 1: δ 9.78 (d, 1.2, H-3), δ 5.67 (br s, H-14), δ 4.13 (dd, 10.2, 4.4, H-2), δ 2.39 (ddd, 10.2, 10.2, 18.0, H-1), δ 2.36 (dd, 10.2, 10.2, H-10), δ 2.16 (d, 3H, 1.2, H-16), δ 2.13 (ddd, 12.9, 12.9, 4.2, H-12), δ 2.05 (ddd, 12.9, 12.9, 4.8, H-12′), δ 1.76 (ddd, 18.0, 10.2, 4.4, H-1′), δ 1.69 (ddd, 12.9, 12.9, 4.8, H-11), δ 1.60 (m, H-8), δ 1.40 (dddd, 13.4, 11.0, 11.0, 4.4, H-7), δ 1.31 (dddd, 13.4, 3.5, 3.5, 3.5, H-7′), δ 1.27 (ddd, 13.4, 4.4, 3.5, H-6′), δ 1.24 (m, H-11), δ 1.19 (m, H-6), δ 1.10 (s, 3H, H-18), δ 1.05 (s, 3H, H-19), δ 0.88 (s, 3H, H-20), δ 0.82 (d, 3H, 6.8, H-17); 13C NMR data (100 MHz) CHCl3 -d 1: δ 208.3 (CH, C-3), δ 164.3.0 (C, C-15), δ 164.1 (C, C-13), δ 114.1 (CH, C-14), δ 78.0 (CH, C-2), δ 65.0 (C, C-4), δ 46.9 (CH, C-10), δ 46.8 (C, C-5), δ 36.7 (C, C-9), δ 36.0 (CH2, C-12), δ 35.9 (CH, C-8), δ 35.2 (CH2, C-1), δ 31.8 (CH2, C-11), δ 30.9 (CH3, C-6), δ 26.2 (CH2, C-7), δ 25.7 (CH3, C-20), δ 20.2 (CH3, C-19), δ 19.4 (CH3, C-16), δ 15.3 (CH3, C-17), δ 12.2 (CH3, C-18); HREIMS m/z 336.2311 (calc. for C20H32O4 m/z 336.2300).
Coleoptile Bioassay
Wheat seeds (Triticum aestivum L. cv. Duro) were sown in 15 cm diam Petri dishes misted with water and grown in the dark at 22±1 ºC for 3 d (Hancock et al. 1964). Roots and caryopses were removed from the shoots. Shoots were placed in a Van der Weij guillotine and the apical 2 mm were cut off and discarded. The next 4 mm of the coleoptiles were removed and used for the bioassay. All manipulations were performed under a green safelight (Nitsch and Nitsch 1956). Compounds were predissolved in DMSO and diluted to the final bioassay concentration with a maximum of 0.1% DMSO. Parallel controls with water and DMSO at the same concentration also were run.
Crude extracts, fractions, or pure compounds to be assayed for biological activity were added to test tubes. Assays were carried out in duplicate. Phosphate-citrate buffer (2 ml) containing 2% sucrose (Nitsch and Nitsch 1956) at pH 5.6 was added to each test tube. Five coleoptiles were placed in each test tube (3 tubes per dilution), and the tubes were rotated at 0.25 rpm in a roller tube apparatus for 24 h at 22ºC in the dark. Coleoptiles were measured following digitalization of their images. Data were statistically analyzed using Welch’s test (Martín Andrés and Luna del Castillo 1990). Data are presented as percent difference from control. Thus, zero represents the control, positive values represent stimulation of the studied parameter, and negative values represent inhibition.
Phytotoxicity Bioassays
The selection of target plants was based on an optimization process developed by us in our search for a standard phytotoxicity bioassay (Macías et al. 2000). Several Standard Target Species (STS) were proposed, including the monocot Allium cepa L. (onion) and dicots Lycopersicon esculentum Will. (tomato), Lepidium sativum L. (cress), and Lactuca sativa L. (lettuce), which were assayed for this study.
Bioassays were conducted using Petri dishes (50 mm diam) with one sheet of Whatman No.1 filter paper as a support. Germination and growth were conducted in aqueous solutions at controlled pH using 10−2 M 2-[N-morpholino]ethanesulfonic acid (MES) and 1 M NaOH (pH 6.0). Compounds to be assayed were dissolved in DMSO (0.2, 0.1, 0.02, 0.01, and 0.002 M), and these solutions were diluted with buffer (5 µl DMSO solution/ml buffer) so that test concentrations for each compound (10−3, 5·10−4, 10−4, 5·10−5, and 10−5 M) were reached. This procedure facilitated the solubility of the assayed compounds. The number of seeds in each Petri dish depended on the seed size. Twenty seeds were used for tomato, lettuce, cress, and onion. Treatment, control or internal reference solution (1 ml) was added to each Petri dish. A similar procedure was used for wheat in 90 mm diam Petri dishes with 10 seeds. Four replicates were used for tomato, cress, onion, and lettuce (80 seeds).
After adding seeds and aqueous solutions, Petri dishes were sealed with Parafilm to ensure closed-system models. Seeds were further incubated at 25ºC in a Memmert ICE 700 controlled environment growth chamber in the dark. Bioassays took 4 d for cress, 5 d for lettuce, tomato, and wheat, and 7 d for onion. After growth, plants were frozen at −10ºC for 24 h to avoid subsequent growth during the measurement process.
The commercial herbicide Logran®, a combination of N-(1,1-dimethylethyl)-N′-ethyl-6-(methylthio)-1,3,5-triazine-2,4-diamine (terbutryn, 59.4%) and 2-(2-chloroethoxy)-N-{[(4-methoxy-6-methyl-1,3,5-triazin-2yl)amino]carbonyl}benzenesulfonamide (triasulfuron, 0.6%), was used as an internal reference in accordance with a comparison study reported previously (Macías et al. 2000). This herbicide was used at the same concentrations (10−3, 5·10−4, 10−4, 5·10−5, and 10−5 M), and under the same conditions as the compounds reported here. Control samples (buffered aqueous solutions with DMSO and without any test compound) were used for all of the plant species assayed.
Evaluated parameters (germination rate, root length, and shoot length) were recorded using a Fitomed© system (Castellano et al. 2001), which allowed automatic data acquisition and statistical analysis by use of the associated software. Data were analyzed statistically using Welch’s test, with significance fixed at 0.01 and 0.05. Results are presented as percent difference from the control. Zero represents control, positive values represent stimulation, and negative values represent inhibition.
IC50 values were obtained after adjusting phytotoxicity data to concentration (logarithmic scale), to a sigmoidal dose-response curve, defined by equation:
Where X indicates the logarithm of concentration, Y indicates the response (phytotoxicity), and Y max and Y min are the maximum and minimum values of the response, respectively. Goodness of fit is described by the determination coefficient (r2). The adjustment and r2 values were obtained using GraphPad Prism® software v. 4.00 (GraphPad Software Inc.).
Results and Discussion
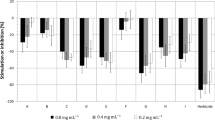
Six aqueous extracts were obtained from leaves and bark of T. grandis (TL, 6 g; TB, 4 g), leaves and flowers of G. sepium (GL, 13 g; GF, 11.5 g), leaves of A. fordii (AL, 8.5 g) and leaves of M. buxifolia (ML, 1.2 g). The extracts were subjected to a bioassay of etiolated wheat coleoptiles. Five dilutions were used in the assay (1,000, 500, 250, 125, and 75 ppm) and these were prepared from the dried aqueous extracts.
The results obtained in the bioassay are shown in Fig. 1, where activity levels correlate with the concentration expressed in ppm. Extracts that showed higher activity levels were TL, ML, TB, and GF, which showed the highest inhibition values at 1,000 ppm, with values of −69, −66, −63, and −60%, respectively. The aqueous extracts of leaves from A. fordii (AL) and G. sepium (GL) at the highest concentrations showed lower inhibitory activity (−41 and −36%, respectively). Among the most active extracts, only the extract from leaves of T. grandis retained activity levels at lower concentrations, and these were close to those observed at the highest concentration. Thus, the activities of these extracts at 500 and 250 ppm were TL (−56 and −53%), ML (−48 and −45%), TB (−45 and −33%), and GF (−38 and −28%). In light of the observed values; its successful use in agroforestry systems; its bioavailability; and the possibility of exploiting non-timber forest products, the leaves of T. grandis were selected for further phytochemical study.
Effect of aqueous extracts on the elongation of etiolated wheat coleoptiles. Values are expressed as percentage from the control and are not significantly different with P > 0.05 for the Mann–Whitney’s test. a Values significantly different with P < 0.01. b Values significantly different with 0.01 < P < 0.05. TL (leaves of Tectona grandis), TB (bark of T. grandis), GL (leaves of Gliricidia sepium), GF (flowers of G. sepium), AL (leaves of Aleurites fordii) and ML (leaves of Maytensus buxifolia)
The study of the allelopathic potential of Tectona grandis was initiated with the study of the DCM extract obtained from the aqueous extract and the DCM extract obtained by direct maceration of the dry leaves. Both extracts were selected on the basis of the bioactivity levels shown in the wheat coleoptile bioassay (Macías et al. 2008).
Spectroscopic data for sesquiterpenes 1 (Gutierrez and Herz 1988), 2 (Zhang et al. 2003), 3 and 4 (Niwa et al. 1978), diterpenes 5 (Zulueta et al. 1995), 6 (Urones et al. 1987), 7 (Marsaioli et al. 1975), 8 (Dekker et al. 1988), 9 (Hasan et al. 1982), and 12 (Cambie et al. 1984), as well as triterpenes 13 (Dellagreca et al. 1990), 14 (Aliotta et al. 1991), 15 (Wenkert et al. 1978), 16 (Brandao et al. 1992), 17 (Seebacher et al. 2003), 18, 19 (Pungitore et al. 2005), 20 (Biessels et al. 1974), and 21 (Takahashi et al. 1974) were identical to those reported previously (see Fig. 2 for structures). This is the first time that sesquiterpenes have been described for the genus Tectona and the first time that compounds 2–6, 8–10, 12, and 21 have been isolated from the family Verbenaceae. Compound 11 is also described for the first time in the literature.
Compound 11 was isolated as a colorless oil from the DCM extract. The mass spectrum of 11 shows a molecular ion at m/z 336.2311, corresponding to a formula C20H32O4. The IR spectrum shows a band at 3,410 cm−1 due to the hydroxyl group, which along with the intense band at 1,696 cm−1 establishes the existence of an acid group in the molecule. Signals at 1,735 and 1,650 cm−1 are assigned to a carbonyl group and a double bond, respectively.
In the 1H-NMR spectrum a characteristic set of signals is observed for the angular methyl groups present in the clerodane diterpene: two signals at δ 0.88 (s, 3H) and 1.05 (s, 3H), corresponding to the methyl groups attached to a quaternary carbon, are assigned to H-20 and H-19, a doublet methyl signal at δ 0.82 (d, 3 H, 6.8) corresponds to H-17 and a signal due to a methyl on a double bond with an E geometry at δ 2.16 (d, 3 H, 1.2, H-16). Moreover, the 1H-NMR spectrum of 11 is similar to that of solidagonal (10) (Bohlmann et al. 1985), with a broad singlet at δ 5.67 (bs, H-14), suggesting the presence of a trisubstituted double bond, and a signal due to an aldehyde group at 9.78 (d, 1.2). These data suggest that this compound could have an abeoclerodane skeleton. The most significant differences observed in the spectrum in comparison to that of 10 are the signal at δ 1.10 (s, 3H), which corresponds to the H-18 methyl located on a quaternary carbon instead of a double bond, and the presence of a signal at δ 4.13 (dd, 10.2, 4.4) due to a proton geminal to a hydroxyl group (g-HSQC C-2 at δ 78.0).
The 1H-NMR-2D-COSY experiment allowed us to establish the following correlations: the signal at δ 4.13 (dd, 10.2, 4.4, H-2) shows coupling with signals at δ 2.39 (ddd, 10.2, 10.2, 18.0, H-1) and δ 1.76 (ddd, 4.4, 10.2, 18.0, H-1′), corresponding to two geminal protons, which in turn are coupled with the signal at δ 2.36 (dd, 10.2, 10.2, H-10). These data imply that the formyl group cannot be attached at C-3, as it is in solidagonal (10).
The presence in the 1H-NMR spectrum of a singlet assigned to H-18 and the correlations observed in the g-HMBC spectrum (Fig. 3) between the signals of H-18 at δ 1.10 and the quaternary carbon at 65.0 (C-4), as well as between C-3 and the aldehydic proton, allow us to place the aldehyde group geminal to C-18. These correlations, together with the previous data, lead us to suggest an unusual carbon skeleton for this compound. The correlations observed in the g-HMBC spectrum (see Fig. 3) allowed us to assign unambiguously all signals in the 1H-NMR and 13C-NMR spectra.
In order to determine the relative stereochemistry of the molecule, a series of 1D NOESY-NOESY experiments was carried out. The observed nOe effects are shown in Fig. 4. The nOe effect between H-14 and H-12 confirms an E stereochemistry for the double bond (Fig. 4).
The spectroscopic data for 11 suggest that this compound has the structure presented in Fig. 2, and we have named this abeograndinoic acid. This diterpene has a rearranged clerodane skeleton. Some furoclerodane compounds with a analogous carbon skeleton and very different functionalization have been described from the family Asteraceae (Jakupovic et al. 1986; Bohlmann and Mungai 1990; Zdero et al. 1990a, b). A biosynthetic route through a pinacolinic rearragement from the corresponding 3,4-dihydroxy derivative was proposed for these compounds. It also has been proposed that the biosynthetic origin of 10 is from a clerodane with a double bond between C-4 and C-5, after subsequent oxidative rupture, cyclization, and dehydration (Bohlmann et al. 1985). In the case of 11, the biosynthesis could start with a clerodane skeleton, which has a double bond between C-2 and C-3. Oxidative rupture of the double bond and subsequent aldolic cyclization would afford compound 11 (Fig. 5).
Coleoptile Bioassay Results
Etiolated wheat coleoptiles bioassay is a fast test (24 h), which is sensitive to a wide range of bioactive substances (Cutler et al. 2000) including plant growth regulators, herbicides (Cutler 1984), antimicrobials, mycotoxins, and assorted pharmaceuticals. (Jacyno and Cutler 1993). The bioactivities on coleoptiles of these compounds were evaluated when sufficient quantities were avaliable (i.e., all cases except 2, 11, and 21). The highest concentration tested for 1, 3, 4, 7–9, 12, 13, and 18–19 was 10−3 M, whereas 5, 6, 10, 14–17, and 20 were tested from 5·10−4 M. It can be seen from Fig. 6 that sesquiterpenes 1 and 4 show inhibitory activity greater than −50% at the maximum concentration.
The results show that compounds 8, 9, and 12 inhibit coleoptile elongation by more than −60% at 10−3 M. In particular, 8 and 12 present values close to −80% at this concentration. It should be pointed out that compound 10 shows an inhibition value of −84% at 5·10−4 M, which is the highest concentration tested. On the other hand, 5 and 6 show activity levels that are not particularly significant.
The most active compounds were the diterpenes 7, 8, 9, 10, and 12. The activity of 12 decreases rapidly on dilution. The profile of the activity with dilution suggests that the presence of this α,β-unsaturated carbonyl group in the bicyclic system is influential in the activity, whereas the double bond and the carboxylic acid group on the lateral chain are less important. Thus, compound 7 shows an inhibition of −37% at 5·10−4 M, whereas 8, 9, and 10 inhibit the elongation by −72, −55, and −84%, respectively, at the same concetration. Furthermore, the inhibition values are higher than −60% in the cases of 8 and 10 at 10−4 M.
Neither triterpenes nor steroids isolated in this work show relevant activity in this bioassay and, as a consequence, they were not selected for evaluation of phytotoxic activity.
In order to compare compound activities, IC50 values were calculated using the sigmoidal dose-response model. This approach allows comparison of the inhibitory activity of active compounds even when the starting concentrations are different. The order of increasing activity of the tested compounds in this bioassay is: 10 (IC50 = 0.078 mM, R 2 = 0.984) > 8 (IC50 = 0.31 mM, R 2 = 0.965) > 12 (IC50 = 0.41 mM, R 2 = 0.983) > 9 (IC50 = 0.65 mM, R 2 = 0.97) > 1 (IC50 = 0.67 mM, R 2 = 0.998) > 4 (IC50 = 0.96 mM, R 2 = 0.976) > 7 (IC50 = 2.1 mM, R 2 = 0.975).
Phytotoxicity Bioassay Results
The most active compounds were selected for phytotoxicity evaluation. Compounds 1, 4, and 10 were not included in the phytotoxicity bioassay due to the small amounts available (Fig. 7). The concentrations tested were identical to those in the previous bioassay. Standard target species (STS) were Lactuca sativa (lettuce), Lycopersicum esculentum (tomato), Lepidium sativum (cress), and Allium cepa (onion).
Effects of compounds 7–9 and 12 on growth of standard target species. Values are expressed as percentage from the control and are not significantly different with P > 0.05 for the Mann–Whitney’s test. a Values significantly different with P < 0.01. b Values significantly different with 0.01 < P < 0.05
Regarding the dicotyledonous species, the tested compounds did not cause significant effects on germination or growth of Lepidium sativum. The behavior of the evaluated compounds in the germination and growth of Lactuca sativa is inhibitory in almost all cases. The highest inhibitory effects on the germination were caused by 9, which shows activity values similar to the herbicide Logran® at the highest concentrations (10−3 M, −89%; 5·10−4 M, −86%; 10−4 M, −68%) and higher values at lower concentrations (5·10−5 M, −48%; 10−5 M, −36%). With regard to the third dicotyledonous species, Lycopersicum esculentum, the effects on germination were of low significance. Root growth of tomato was stimulated by diterpenes 9 and 12, with values higher than 20% for all concentrations, whereas the effects on shoots were not relevant.
In the monocotyledon species Allium cepa, the most affected parameter was root growth. Compounds 9 (10−3 M, −55%; 5·10−4 M, −33%) and 12 (10–3 M, −44%) were inhibitory, with 9 being the most active. The effects on germination and shoot development were not significant, with 12 being the most active compound with values around −40% at the highest concentration.
In summary, the compounds that present the highest phytotoxic activity are the diterpenes 9 (2-oxokovalenic acid) and 12 (19-hydroxyferruginol). In addition, the general activity of the diterpene 10 (solidagonal acid), with an IC50 value of 78 µM, has to be highlighted, although due to the small amount isolated its phytotoxic activity could not be evaluated.
References
Aliotta, G., Monaco, P., Pinto, G., Pollio, A., and Previteria, L. 1991. Potential allelochemical from Pistia stratiotes. J. Chem. Ecol. 17:2223–2234.
Atawodi, S. E., and Atawodi, J. C. 2009. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem. Rev. 8:601–620.
Barros, G. M. C. C., and Teixeira, S. P. 2008. Pharmacobotanical study of two wild indigo species (Indigofera suffruticosa and Indigofera truxillensis, Leguminosae) with pharmacological properties. Rev. Bras. Farmacogn. 18:287–294.
Biessels, H., Van Der Kerk-van Hoof, A., Kettenes-van Den Bosch, J., and Salemink, C. 1974. Triterpenes from Prunus serotina and P. Lusitanica. Phytochemistry 13:203–207.
Bohlmann, F., and Mungai, G. M. 1990. Rearranged clerodanes and other diterpenes from Microglossa pyrrhopappa. Phytochemistry 29:3233–3241.
Bohlmann, F., Singh, P., Singh, R. K., Joshi, K. C., and Jaicupovic, J. 1985. A diterpene with a new carbon skeleton from Solidago altissima. Phytochemistry 24:1114–1115.
Brandao, M. G., Lacaille-dubois, M. A., Teixera, M. A., and Wagner, H. 1992. Triterpene saponins from the roots of Ampelozizyphus amazonikus. Phytochemistry 31:352–354.
Cambie, R., Cox, R., and Sidwell, D. 1984. Phenolic diterpenoids of Podocarpus ferrugineus and other podocarps. Phytochemistry 23:333–336.
Castellano, D., Macías, F. A., Castellano, M., and Cambronero, R. 2001. Spanish Patent Nº P9901565.
Charleston, D. S., Gols, R., Hordijk, K. A., Kfir, R., Vet, L. E. M., and Dicke, M. 2006. Impact of botanical pesticides derived from Melia azedarach and Azadirachta indica plants on the emission of volatiles that attract parasitoids of the diamondback moth to cabbage plants. J. Chem. Ecol. 32:325–349.
Cutler, H. G. 1984. Fresh look at the wheat coleoptile bioassay, pp. 1–9, in Proceedings of the 11th Annual Meeting of the Plant Growth Regulator Society of America, PGRSA, Boston, MS, USA.
Cutler, S. J., Hoagland, R. E., and Cutler, H. G. 2000. Evaluation of selected pharmaceuticals as potential herbicides: Bridging the gap between agrochemicals and pharmaceuticals, pp. 129–137, in S. S. Narwal, R. E. Hoagland, R. H. Dilday, and M. J. Reigosa Roger (eds.). Allelopathy in Ecological Agriculture and Forestry, Springer, Dordrecht, The Netherlands
Dekker, T. G., Fourie, T. G., Matthee, E., Snyckers, F., and Van Der Schyf, S. 1988. Studies of South African medicinal plants. Part 7. Rhinocerotinoic acid: a labdane diterpene with anti-inflammatory properties from Elytropappus rhinocerotis. Afr. J. Chem. 41:33–35.
Dellagreca, M., Monaco, P., and Previtera, L. 1990. Stigmasterols from Typha latifolia. J. Nat. Prod. 53:1430–1435.
Fozdar, B. I., Khan, S. A., Shamsuddin, T., Shamsuddin, K. M., and Kintzinger, J. P. 1989. Aleuritin, a coumarinolignoid, and a coumarin from Aleurites fordii. Phytochemistry 28:2459–2461.
González, A. G., Jiménez, I. A., Ravelo, A. G., Coll, J., González, J. A., and Lloria, J. 1997. Antifeedant Activity of Sesquiterpenes from Celastraceae. Biochem. Syst. Ecol. 25:513–519
Gutierrez, A. B., and Herz, W. 1988. Guaianolides and other constituents of Helianthus microcephalus. Phytochemistry 27:2225–2228.
Hancock, C. R., Barlow, H. W., and Lacey, H. J. J. 1964. The east malling coleoptile straight growth test method. Exp. Bot. 15:166–176.
Hasan, C. M., Healey, T. M., and Waterman, P. G. 1982. Kolavane and kaurane diterpenes from the stem bark of Xilophia aethiopica. Phytochemistry 21:1365–1368.
Herath, H. M. T. B., Dassanayake, R. S., Priyadarshani, A. M. A., De Silva, S., Wannigama, G. P., and Jamie, J. 1997. Isoflavonoids and a pterocarpan from Gliricidia sepium. Phytochemistry 47:117–119.
Jacyno, J. M., and Cutler, H. G. 1993. Detection of herbicidal properties: scope and limitations of the etiolated wheat coleoptile bioassay. PGRSA Quaterly 21:15–24.
Jakupovic, J., Banerjee, F., Bohlmann, F., King, R. M., and Robinson, H. 1986. New diterpenes from Chiliotrichium rosmariniflolium and Nardophyllum lanatum. Tetrahedron 42:1305–1313.
Joshi, K. C., Singh, P., and Pardasani, R. T. 1977. Chemical components of the roots of Tectona grandis and Gmelina arborea. Planta Med. 31:71–75.
Khanzada, S. K., Shaikh, W., Sofia, S., Kazi, T. G., Usmanghani, K., Kabir, A., and Sheerazi, T. H. 2008. Chemical constituents of Tamarindus indica L. medicinal plant in Sindh. Pak. J. Bot. 40:2553–2559.
Macías, F. A., Castellano, D., and Molinillo, J. M. G. 2000. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 48:2512–2521.
Macías, F. A., Lacret, R., Varela, R. M., Nogueiras, C., and Molinillo, J. M. G. 2008. Bioactive apocarotenoids from Tectona grandis. Phytochemistry 69:2708–2715.
Marsaioli, A., De Freitas, H., and De Paiva, J. 1975. Diterpenes in the bark of Hymenea courbail. Phytochemistry 14:1882–1883.
Martín Andrés, A., and Luna Del Castillo, J. D. 1990. Bioestadística para las Ciencias de la Salud, 3rd ed. Madrid: Norma.
Marwani, E., Kobayashi, A., Kajiyama, S., Fukusaki, E., Nitoda, T., Kanzaki, H., and Kawazu, K. 1997. Tectona grandis callus produces antibacterial triterpene acids not detected in the intact plant. Nat. Prod. Sci. 3:75–80.
Naik, S.M., Jayaprakasha, G. K., and Singh, R. P. 2008. Antioxidant activity of custard apple (Annona squamosa) peel and seed extracts. J. Food Sci. Tech. 45:349–352.
Nitsch, J. P., and Nitsch, C. 1956. Studies on the growth of coleoptile and first internode sections. A new sensitive, straight-growth test for auxins. Plant. Physiol. 31:94–111.
Niwa, M., Igushi, M., and Yamamura, S. 1978. Niomimetic reactions of epoxygermacrane-D. Tetrahedron Lett. 19:4043–4046.
Pungitore C., García, M., Gianello, J., Sosa M., and Tonn, C. 2005. Insecticidal antifeedant effects of Junellia aspera (Verbenaceae) triterpenes and derivates on Sitophilius oryzae (Coleoptera: Curculionidae). J. Stored Prod. Res. 41:433–443.
Ramamoorthy, M., and Paliwal, K. 1993. Allelopathic compounds in leaves of Gliricidia sepium (Jacq.) Kunth ex Walp. and its effect on Sorghum vulgare L. J. Chem. Ecol. 19:1691–1701.
Rimpler, H., and Christiansen, I. Z. 1977. Tectograndinol, a new diterpene from Tectona grandis. Naturforsch (C) 32:724–730.
Seebacher, W., Simic, N., Weis, R., Saf, R., and Kunert, O. 2003. Complete assignments of 1H and 13C NMR resonances of oleanic acid, 18α-aleanic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 41:636–638.
Takahashi, K., Kawaguchi, S., Nishimura, K., Kubota, K., Tanabe, Y., and Takani, M. 1974. Studies on constituents of medicinal plants. XIII. Constituents of the pericarps of the capsules of Euscaphis japonica Pax. Chem. Pharm. Bull. 22:650–653.
Urones, J., De Pascual, T., Marcos, I., Fernández, R., Basabe, P., and Sexmero, J. 1987. Acetophenones and terpenoids from Senecio gallicus. Phytochemistry 26:1113–1115.
Wenkert, E., Baddeley, G. V., Burfitt, I. R., and Moreno, L. N. 1978. Carbon-13 Nuclear Magnetic Resonance spectroscopy of naturally-occurring substances LVII. Triterpenes related to lupane and hopane. Org. Magn. Reson. 11:337–342.
Wiersum, K. F. 1982. Tree gardening and taungya on Java: Examples of agroforestry techniques in the humid tropics. Agroforest Syst. 11:53–70.
Zdero, C., Ahmed, A. A., Bohlmann, F., and Mungai, G. M. 1990a. Diterpenes and sesquiterpenes xylosides from east African Conyza species. Phytochemistry 29:3167–3172.
Zdero, C., Jakupovic, J., and Bohlmann, F. 1990b. Diterpenes and other constituents from Pteronia species. Phytochemistry 29:1231–1245.
Zhang, H., Tan, G., Santarsiero, B., Mesecar, A., Hung, N., Manh, N., Doel, D., Pezzuto, J., and Fong, H. 2003. New sesquiterpenes from Lisea verticillata. J. Nat. Prod. 66:609–615.
Zulueta, M. C. A., Tada, M., and Ragasa, C. Y. 1995. A diterpene from Bidens pilosa. Phytochemistry 38:1449–1450.
Acknowledgement
This research was supported by the Ministerio de Ciencia y Tecnología, Spain (Ministerio de Ciencia e Innovación; Project No. AGL2008-04716/AGR).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Supporting Information. NMR spectra of compound 11 and effects of compounds 7–9 and 12 on the growth of standard target species are available at http://www
ESM 1
(DOC 1059 kb)
Rights and permissions
About this article
Cite this article
Macías, F.A., Lacret, R., Varela, R.M. et al. Isolation and Phytotoxicity of Terpenes from Tectona grandis . J Chem Ecol 36, 396–404 (2010). https://doi.org/10.1007/s10886-010-9769-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9769-3