Abstract
An odorant-binding protein cDNA (Acer-ASP2) was cloned and characterized from antennae of adult workers of an Asian honey bee, Apis cerana cerana F. (Hymenoptera: Apidae). The full-length open reading frame of Acer-ASP2 cDNA was 429 bp, encoding 142 amino acids. Protein signature analyses revealed that it contained six conserved cysteines with an N-terminal signal sequence of 19 amino acids. The deduced protein sequence shares high homology with Amel-ASP2 from the honey bee, Apis mellifera L., and low similarity with odorant-binding proteins from other species of insects. Immunocytochemical localization showed that Acer-ASP2 was concentrated in the lymph of olfactory sensilla, such as sensilla placodea and sensilla trichodea A. Real-time polymerase chain reaction of Acer-ASP2 transcripts showed that Acer-ASP2 was expressed on antennae but not in other general anatomical regions of the body. Temporally, Acer-ASP2 was expressed at a relatively high level in adults during two periods (9 and 27 vs. 1, 15, and 30 days). This timing is correlated with the production of beeswax and searching behavior for nectar/pollen, respectively. Thus, Acer-ASP2 is a species-specific gene that we propose to be involved in the acquisition of odorant molecules from nectar, pollen, and other general odorant sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects discriminate odorant molecules with sensory organs called olfactory sensilla. Before reaching olfactory receptor neurons in olfactory sensilla, odorant molecules cross an aqueous interface, the sensillar lymph. In the so-called perireceptor events, odorant-binding proteins (OBPs) are the main proteins involved in the interaction between odorants and the elements of the sensillar lymph (Pelosi and Maida 1995; Pelosi 1996). OBPs include pheromone-binding proteins (PBPs) and general odorant-binding proteins (GOBPs), both of which have molecular masses around 16 kDa and contain six conserved cysteine residues paired in three disulfide bridges (Leal et al. 1999; Scaloni et al. 1999). PBPs are present mainly in male insects and are thought to be involved in sex pheromone detection. GOBPs, which are subdivided into the subclasses GOBP1 and GOBP2 based on amino acid sequence homology (Vogt et al. 1991a), seem to play a more general role in olfaction by carrying odorant molecules (Vogt and Riddiford 1981; Vogt and Lerner 1989). GOBPs are highly conserved across species and more abundant in female antennae (Steinbrecht et al. 1995), which suggests that they are not associated with intraspecific pheromone reception, but rather with the perception of nonpheromonal odorants.
Proteins with characteristics of OBPs also have been identified in many orders of insects, such as in Lepidoptera (Vogt et al. 1991b; Maleszka and Stange 1997; Scaloni et al. 1999; Wang et al. 2003; Maida et al. 2005; Xiu and Dong 2007), Diptera (Ozaki et al. 1995; Hekmat-Scafe et al. 1997; Biessmann et al. 2002; Ishida et al. 2002a, 2004; Xu et al. 2003), Heteroptera (Dickens et al. 1995), Coleoptera (Graham et al. 2003; Nagnan-Le Meillour et al. 2004), Orthoptera (Ban et al. 2003), Isoptera (Ishida et al. 2002b), and Hymenoptera (Lu et al. 2007). Twenty-one OBP-like genes have been characterized from the whole genome sequences of the honey bee, Apis mellifera L. (Forêt and Maleszka 2006). Proteins corresponding to specific OBP family member genes from A. mellifera include ASP1 (Danty et al. 1999), ASP2 (Briand et al. 2001), ASP4: AAL60417 (GenBank accession number), ASP5: AAL60422, and ASP6: AAL60421. These have been characterized from the antennae and legs of workers and drones according to their precise molar weights determined by mass spectrometry and N-terminal sequencing (Danty et al. 1998). The antennal specific water-soluble protein, ASP2 (Mr 13,695.2 ± 1.6), was purified and characterized as the first Hymenoptera putative GOBP, based on its specific expression in olfactory areas and higher expression in the worker than in the drone (Danty et al. 1997). Briand et al. (2001) reported that it interacted with a wide range of volatile odorant molecules, but not with the major components of the queen pheromone.
The Asian honey bee, Apis cerana cerana Fabricius (Hymenoptera: Apidae), is an economically important indigenous species with about two million colonies being bred in China. A. cerana cerana has many unique characters, such as the resistance to ectoparasitic mites by a specific grooming behavior and tolerance for low environmental temperatures. The latter quality makes A. cerana cerana an important pollinator of flowering plants at high altitudes (Peng et al. 1987; Chen 2001). Biochemically, OBPs are thought to act as solubilizers and carriers of the lipophilic odorants in the sensillar lymph, as peripheral filters in odor discrimination by selectively binding certain kinds of odorants, as stimulus molecules to the receptor to facilitate signal transduction, as rapid deactivators of odorants after stimulation, and as cleaners to remove unwanted or toxic compounds from the perireceptor space (Vogt and Riddiford 1981; Steinbrecht 1998; Pelosi et al. 2006). Thus, we hypothesize that OBPs may play an important role in the olfactory system of A. cerana cerana related to the search for nectar and pollen and in detection of odorants from ectoparasitic mites.
In this study, we cloned a new OBP-encoding gene (Acer-ASP2) from A. cerana cerana and then expressed the corresponding protein in Escherichia coli. With polyclonal anti-(Acer-ASP2)-serum from purified recombinant Acer-ASP2 antigen, we investigated immunolocalization in the antennae of A. cerana cerana workers, and with real-time polymerase chain reaction (PCR), we characterized the spatiotemporal expression pattern of Acer-ASP2.
Methods and Materials
Insects
Larval, pupal, and adult workers from A. cerana cerana colonies were bred at the Experimental Apiary at Zhejiang University, Hangzhou, China. Bees of known ages were obtained by marking the newly emerged bees from a frame of capped broods kept in an incubator; bees were returned to the hive and collected when required. The developmental stages of workers were classified according to the criteria of Rachinsky et al. (1990) and Michelette and Soares (1993).
Amplification of cDNA Encoding Acer-ASP2
From 100 antennae of adult worker bees of A. cerana cerana, total RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized by using SuperScript™ II Reverse Transcriptase system (Invitrogen). The DNA encoding Acer-ASP2 was amplified by PCR from cDNA with primer pairs (Table 1) designed from ASP2 sequence from A. mellifera (GenBank no. AF393493). The amplification conditions were 4 min at 94°C, followed by 35 cycles of 45 s at 94°C, 45 s at 50°C, 1 min at 72°C, and final extension for 10 min at 72°C. Amplified DNA purified with a gel extraction kit (Qiagen, Valencia, CA, USA) was inserted into pGEM-T vector (Promega, Madison, WI, USA) and was transformed into E. coli TG1 competent cells. Positive colonies, screened for the presence of the insert by restriction analysis by using BamH I and Hind III, were sequenced (Center of Analysis & Measurement, Zhejiang University) and shown to encode the mature protein Acer-ASP2.
Production and Purification of Recombinant Acer-ASP2
The 450-bp fragment excised with BamH I and Hind III from the pGEM-Acer-ASP2 plasmid, was purified with the gel extraction kit (Qiagen) and cloned into pET-30a (+) vector (Novagen, Darmstadt, Germany) digested with the same restriction enzyme, and the recombinant expressed plasmid pET-Acer-ASP2 was transformed into E. coli BL21(DE3) competent cells. Single colonies were grown overnight in 10 ml Luria–Bertani broth (including 30 μg/ml kanamycin). The culture was diluted 1:100 with fresh medium and grown at 37°C until absorbance at OD600 reached 0.4, at which point isopropyl-β-d-thiogalactopyranoside (Merck, Darmstadt, Germany) was added to the culture to a final concentration of 1.5 mM to induce expression of the target products. After 5 h at 28°C, the bacterial cells, harvested by centrifugation and resuspended in lysis buffer [50 mM Tris/HCl (pH 8.0), 100 mM NaCl, TritonX-100 (0.5%), 2 mM ethylenediaminetetraacetic acid], were lysed by sonication and centrifuged again. The inclusion body of recombinant protein Acer-ASP2 was severely precipitated in 1.5 M urea in ddH2O and finally freeze-dried and resuspended (1 mg/ml) in phosphate buffered saline (PBS; pH 7.4) for injection into rabbit for producing antisera.
Preparation of Antisera
Antisera were obtained by injecting an adult male rabbit subcutaneously and intramuscularly with purified inclusion body of recombinant protein of Acer-ASP2 (1 mg/time). The interval between the first and second injection was 21 days, whereas two additional injections were performed at 14-day intervals. The protein was emulsified with an equal volume of Freund's complete adjuvant for the first injection and incomplete adjuvant for further injections. For 10 days after the final injection, the rabbit was bled and the serum was collected for further application.
Western Blot Analysis
After electrophoretic separation under denaturing conditions (12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)), purified Acer-ASP2 was electroblotted on a nitrocellulose membrane by using a Bio-Rad Transblot (Bio-Rad Laboratories, Hercules, CA, USA). After treatment in TBS (containing 3% BSA and 0.05% Tween 20) at 4°C overnight, the membrane was incubated with the crude antiserum against Acer-ASP2 (dilution 1:500) and then with goat antirabbit IgG horse radish peroxidase conjugate (dilution 1:1,000). Immunoreacting bands were treated with the color development solution (2 mg diaminobenzidine in 10 ml TBS, 10 μl 30% H2O2).
Scanning Electron Microscopy
For scanning electron microscopy (SEM), the antennae of worker bees were immersed in osmic acid (1%) for 30 min and in a concentration series of ethanol (50%, 70%, 80%, 90%, 95%, and 100%) for 15 min each. After air drying, samples were mounted on holders and examined by using a SEM of XL30-ESEM (Philips Research, Eindhoven, The Netherlands) after gold coating with K500X sputter coater (Emitech Ltd., Ashford, Kent, UK).
Immunocytochemical Localization
Antennae of worker bees were chemically fixed in a mixture of formaldehyde (2%) and glutaraldehyde (1%) in 0.1 M PBS (pH 7.4) at 4°C for 2–3 h, then dehydrated in an ethanol series and polymerized embedded in K4 M (Sigma, St. Louis, MO, USA) and irradiated with UV at −20°C for 72 h. Ultrathin sections (50–100 nm) were cut with a glass knife on an Ultracut E ultramicrotome (Reichert-Jung, Austria). For immunocytochemistry, the nickel grids adhering to ultrathin sections were floated on droplets of the following solutions on parafilm with sequential steps: ddH2O, BL (50 mM PBS containing 1% bovine serum albumin and 0.02% PEG2000, 10 mM NaCl, and 1% NaN3), primary antiserum diluted with BL (dilution 1:50), twice each on ddH2O, BL, secondary antibody that goat anti-rabbit IgG coupled to 10-nm colloidal gold (AuroProbe EM, GAR G10, Amersham Biosciences, Piscataway, NJ, USA) diluted with BL (dilution 1:20), and three–five times on ddH2O. The results of immunolocalization of Acer-ASP2 were observed and photographed through the transmission electron microscope of JEM-1230 (JEOL Ltd., Tokyo, Japan).
Expression Profiling of Acer-ASP2 with Real-Time PCR
For spatial expression profiling, antennae (A), heads (H), thoraces (Th), abdomens (Ab), wings (W), and legs (L) were dissected from 50 adult worker bees. For expression profiling across ages of worker bees, 7-day-old larvae and pupae, and the antennae of 1, 4, 6, 9, 12, 15, 18, 21, 24, 27, and 30-day-old adult worker bees were prepared. Total RNA of all above material from individual honeybee colonies was extracted separately and cDNA was synthesized as described above. Specific primer pairs were designed based on the primers used for cloning Acer-ASP2 (Table 1) and A. cerana β-actin (GenBank accession no. AB072495). For real-time quantitative RT-PCR, primers for A. cerana β-actin were designed such that the paired primers (Table 1) were expected to amplify a 301-bp fragment. An iCycler iQ™ real-time PCR detection system (Bio-Rad) was used to detect the spatiotemporal transcriptional profiling of Acer-ASP2 mRNA of A. cerana cerana. The total 20-μl reaction system consisted of 1 μl cDNA (about 20 ng), 10 μl iQ™ SYBR® Green Supermix (Bio-Rad), and 10 μM of each primer. The thermal cycling conditions for real-time PCR were 5 min at 94°C, followed by 40 cycles of 30 s at 94°C, 30 s at 50°C, and 30 s at 72°C. PCR reactions were performed in triplicate, and data were processed by using the relative quantification 2−ΔΔCt method (Livak and Schmittgen 2001).
Results
cDNA Sequence of Acer-ASP2
To characterize the Acer-ASP2 cDNA, we designed specific primers and obtained a 393-bp open reading frame (ORF; Fig. 1). After cloning and sequencing, we obtained the cDNA sequence and submitted it to GenBank (accession number DQ449667). The ORF encodes 142 amino acids and contains a hydrophobic signal peptide with 19 amino acids at the N terminus (predicted by the software SignalP 3.0; Bendtsen et al. 2004). By using the proteomics server ExPASy (Gasteiger et al. 2003), the theoretical isoelectric point and molar weight of mature Acer-ASP2 were computed as 4.36 and 15,656.16, respectively, in agreement with other OBPs. The protein has the typical six-cysteine signature of OBPs and has low similarity (14.9–24.4% of identical residues) with OBPs from other insect species (Fig. 2). However, it does have high similarity (98.6%) with Amel-ASP2 (Fig. 2). These results were emphasized in the evolutionary tree from the neighbor-joining analysis (Fig. 3).
Full cDNA sequence of the open reading frame and deduced amino acid sequence of Acer-ASP2 from an Asian honey bee, A. cerana cerana. The asterisk marks the stop codon, six conserved Cys residues are circled, and the 19 amino acid residues in italicized font represent the N-terminal signal peptide sequence
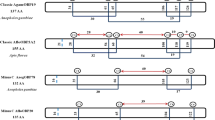
Alignment of deduced amino acid sequence of Acer-ASP2 with homologous proteins from other insect species (GenBank BLASTP). Sequences used in alignment are indicated by the following species abbreviations: Acer Apis cerana cerana, Aaeg Aedes aegypti, Amel Apis mellifera, Asch Anomala schonfeldti, Aruf Anomala rufocuprea, Pdiv Phyllopertha diversa, Nvit Nasonia vitripennis, and Mmed Microplitis mediator. Residues common to most sequences are shaded. Six conserved Cys residues are labeled by asterisks. Signal peptide sequences are highlighted by enclosing them in shaded rectangles
Neighbor-joining (NJ) tree (condensed tree, cutoff value is 50%) of sequences similar to Acer-ASP2. All OBPs from the alignment analysis (Fig. 2) were included in the NJ tree. Bootstrap support values (%) based on 1,000 replicates are indicated
Production and Purification of Acer-ASP2 and Western Blot Analysis with Antisera
To obtain the amounts of recombinant protein Acer-ASP2 suitable for preparation of antisera, we digested the pGEM-Acer-ASP2 plasmid with BamH I and Hind III and the resultant purified DNA was inserted into the expression vector pET-30a (+) and transformed into E. coli BL21(DE3) competent cells. Electrophoretic analysis of the crude cell extracts (pellet and supernatant) under denaturing conditions (12.5% SDS-PAGE) revealed that the recombinant protein Acer-ASP2 migrated as an intense band of 23 kDa (Fig. 4). The protein was recovered almost entirely in the form of an insoluble inclusion body and in high yields (generally, 1 l of culture yielded 50 mg of protein at final purification). Recombinant Acer-ASP2 was purified by precipitation in urea, freeze-dried, and injected into a rabbit as described above. The polyclonal anti-(Acer-ASP2)-serum obtained was applied to detect Acer-ASP2 by Western blot analysis (Fig. 4, WB).
Electrophoretic analysis (12.5% SDS-PAGE) of expression products from E. coli BL21(DE3) cells. The inclusion body protein of Acer-ASP2 was purified by urea with differing concentrations, showing an intense band of 23 kDa, present almost entirely in the pellet. Western blot analysis showed the specificity of anti-(Acer-ASP2)-serum with antigen Acer-ASP2. The low molecular weight protein markers are shown in the lane furthest to the left
Immunocytochemical Localization
Imaging of A. cerana cerana worker antennae by scanning electron microscopy revealed at least six different types chemosensilla: sensilla placodea (sp), sensilla basiconica (sb), sensilla campaniform (scf), and sensilla trichoidea A,B,CD (Fig. 5, which shows only sp and stA). Sensilla placodea, the most numerous type on worker bee antennae, are oval discs. As observed in other honey bees (Ågren 1977, 1978), the plate is raised and resembles a hemispherical dome separated from the surrounding surface by a crevice. These clefts may either result from air drying during sample processing (i.e., an artifact) or may correspond to a lower point at which the plate rim is fastened to the cuticle and the outer rim of the sensillum is raised (Fig. 5, sp). Sensilla trichodea A, which are more numerous than the remaining types listed above, appear slender, glabrous, and bent distally with the tip pointed slightly up. They are characterized by a thick cuticular wall with unbranched outer dendritic segments in the lumen (Fig. 5, stA).
The polyclonal antiserum against Acer-ASP2 was used for immunocytochemical analysis of the antennae. In sections of tarsal segments of antennae, significant gold particles only labeled the sp and the stA. With the sp, gold particles accumulated on the dendrites in the sensillar lymph, but not in the narrow pore areas on the top of the wide brim of the oval discs of the sp (Fig. 6, A1,A2). With the stA, more gold particles accumulated on the dendrites in the trichogen cell; fewer particles accumulated in the outer and inner sensillar lymph in the cell (Fig. 6, B1,B2).
Immunocytochemical localization of OBP in the antennal sensilla of A. cerana cerana. Acer-ASP2 was specifically expressed on the s. placodea (A1 and A2, A2 is an enlargement of the square section in A1) and the s. trichodea A (B1 and B2, B2 is an enlargement of the square section in B1). In this image of the s. placodea, the gold particles were labeled on the dendrites (D) and the sensillar lymph (L), but not in the narrow pore areas (P) on the top of the olfactory sensilla (A2). In this image of the s. trichodea A, more gold particles were labeled on the dendrites in trichogen cell (TC) and fewer particles were labeled in the outer (oL) and inner (iL) sensillar lymph, the cuticular hair wall (C), or the dendritic sheath (DS). Scale bar is 1 μm
Expression Profiling of Acer-ASP2
Based on the normalized relative quantification 2−ΔΔCt method (Livak and Schmittgen 2001) for real-time PCR with SYBR I, spatial expression profiling of Acer-ASP2 revealed that the transcript was specifically expressed on antennal tissue, but never in other anatomical regions tested (based on the final melt curve of real-time PCR; Fig. 7a). On the other hand, the developmental and temporal expression analysis indicated no expression in larvae or pupae, and then two ages in the adult worker bee antenna during which expression was elevated: (1) highest expression between 9 and 15 days (although it declined at the 12 days measurement) and (2) relatively high expression between 27 and 30 days (Fig. 7b). Transcript abundance was calculated based on the difference in threshold cycle (C t) values between Acer-ASP2 and β-actin transcripts.
Relative spatial and developmental quantification of Acer-ASP2 transcripts from various honey bee organs by real-time PCR. In spatial expression profiles (a), the melting curve within real-time PCR showed that Acer-ASP2 was specifically expressed on antennae (A; the high peak at 85–90°C labeled with one asterisk) but was never detected (except as primer dimers) in other organs: heads (H), thoraces (Th), abdomens (Ab), wings (W), and legs (L; the lowest peak at 75–80°C labeled with two asterisks). Another peak representing amplified products of β-actin present in all organs was labeled with three asterisks. In temporal expression profiles (b), Acer-ASP2 was not expressed in larvae and pupae (data not shown in the figure). However, in some adults, the highest levels of relative expression in the antennae occurred at 9 and 27 days (ratio > 2.0), the next highest levels occurred at 1, 15, and 30 days (2.0 > ratio > 1.0), and the lowest levels occurred at 4, 6, 12, 18, 21, and 24 days (ratio < 1.0)
Discussion
In this study, we cloned and identified a novel gene encoding Acer-ASP2, an OBP from the Asian honey bee, A. cerana cerana. The protein has typical hexapod OBP characteristics, e.g., conserved residues of six cysteines, and a similar isoelectric point and molecular weight. When compared with Amel-ASP2 of A. mellifera, the amino acid sequence of Acer-ASP2 is highly similar (98.6%), but less similar (14.9–24.4%) to 13 other OBPs or PBPs (Fig. 2) from more distantly related species of insects.
Immunocytochemistry experiments have shown that OBPs are always localized in the diverse olfactory sensilla in many insects. For example, in Lepidoptera, the GOBPs of Antheraea polyphemus and Bombyx mori were localized only in s. trichodea and s. basiconica and principally in the sensillar lymph around the sensory dendrites (Steinbrecht et al. 1995). Similar anatomical patterns were also described for OBPs from two species of Orthoptera, Schistocerca gregaria and Locusta migratoria (Jin et al. 2005). In male Helicoverpa armigera (Lepidoptera), GOBP2Harm was mainly expressed in the s. basiconica, whereas in females, it was expressed abundantly in the s. trichodea (Wang et al. 2003). In the noctuid moths, Agrotis segetum, Autographa gamma, H. armigera, Heliothis virescens, and Spodoptera littoralis, PBPs were localized predominantly in s. trichodea and GOBP2 in s. basiconica (Zhang et al. 2001). In that study, interspecific immunolocalization utilized antisera raised against the PBP and GOBP2 of A. polyphemus, but the result reflected a good correlation with the stimulus specificity of the receptor cells in these types of sensilla.
Summarizing the immunochemical studies, we can conclude that PBPs of moths always have been localized predominantly in the s. trichodea, which respond to pheromones, whereas the GOBPs have been localized predominantly in the s. basiconica, which are generally involved in detecting plant odors. However, Acer-ASP2 was not found in the s. basiconica of A. cerana cerana (considered to be involved in taste and mechanoreception in A. mellfera; Esslen and Kaissling 1976), but in the s. placodea and s. trichodea A. This suggests that the latter sensillar types may be involved in detecting plant odors in bees. Although the allocation of function to the various types of sensilla in moths and bees is not completely identical, the anatomical placement of OBPs is consistent with their role in detecting general odors in both higher taxonomic groups. Perhaps this is a result of differences in classification of the antennal morphology of moths and bees or in the function of the sensilla in the two species. OBPs are synthesized in the trichogen cells along typical pathways for protein biosynthesis (Steinbrecht et al. 1992). In our study, the heavy labeling of Acer-ASP2 in the trichogen cells of the s. trichodea A supports this concept.
In most insect species, the prevailing model is that OBPs are expressed specifically in the antennae. Our real-time PCR data support this because the Acer-ASP2 mRNA was present in abundance in antennae, but never in other anatomical tissues (e.g., heads, thoraces, abdomens, wings, or legs; Fig. 7a). The anatomical patterns of expression of Acer-ASP2 were similar to those of Amel-ASP2, which has been detected exclusively in the antennae of A. mellifera (Danty et al. 1997). Other OBPs of A. mellifera (e.g., Amel-ASP1, 4, 5, 6) have been reported as antennal specific in workers and drones, but with some localization in legs of drones (ASP1; Danty et al. 1998), equally expressed in the antennae, wings, and legs of various castes and age groups (ASP4 and ASP5) or detected in antennae and legs of both workers and drones (ASP6; Calvello et al. 2005). Furthermore, OBPs have been expressed equally in antennae, wings, and legs of all castes and ages of the paper wasp, Polistes dominulus (Calvello et al. 2003), and in legs and wings of another wasp, Vespa crabro.
From the developmental and temporal expression profiles of Acer-ASP2, we learned that it is not expressed in larvae and pupae, and in adults, there are two periods of elevated expression. The first period occurs at 9 and 27 days (mean expression ratio was 2.7 ± 0.4) and the second occurred at 1, 15, and 30 days (mean ratio was 1.3 ± 0.3). Throughout the remaining times that we assayed for expression, the mean ratio was 0.2 ± 0.03 (Fig. 7b). Because the expression ratios during the first two periods are tenfold greater than that of the lowest period, Acer-ASP2 may play an important functional role in adults during the two periods. At the 9-day timepoint (the highest expression of Acer-ASP2), worker bees are making the transition in maturation and are starting to secrete plentiful beeswax to construct their comb (Yang 1998). After 15 days, maturing bees begin to fly out and forage for nectar and pollen. Their highest level of foraging activity occurs around the 27-day timepoint, which coincides with the second highest level of Acer-ASP2 expression that we recorded. We do not understand why transcript amounts were lower in the intervening periods of 18, 21, and 24 days. However, taken together, our results suggest that Acer-ASP2 may play an important role in the processes of producing beeswax and acquiring the odorant molecules related to foraging for nectar and pollen, or other general odorants.
As a native Chinese bee species, the habitat of A. cerana cerana is threatened by the invasion of exotic bees as well as deteriorating environmental conditions (Tan 2006). Future research necessary to protect this species might involve the molecular mechanism of the unique characteristics of the olfactory system. Recently, the genomic sequencing project of A. mellifera was completed (The Honeybee Genome Sequencing Consortium 2006), and examination of genes related to signal transduction suggested that A. mellifera has fewer genes for gustatory reception and more genes for odorant reception, which is consistent with its ecology and social organization. A. cerana cerana appears to have greater olfactory sensitivity when compared with A. mellifera, so elucidating the biochemical mechanism of the olfactory process in A. cerana cerana should be a high priority in the future.
References
Ågren, L. 1977. Flagellar sensilla of some Colletidae (Hymenoptera: Apoidae). Int. J. Insect Morphol. Embryol. 6:137–146.
Ågren, L. 1978. Flagellar sensilla of two species of Andrena (Hymenoptera: Andrenidae). Int. J. Insect Morphol. Embryol. 7:73–79.
Ban, L., Scaloni, A., D'ambrosio, C., Zhang, L., Yahn, Y., and Pelosi, P. 2003. Biochemical characterization and bacterial expression of an odorant-binding protein from Locusta migratoria. Cell. Mol. Life Sci. 60:390–400.
Bendtsen, J. D., Nielsen, H., Von Heijne, G., and Brunak, S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795.
Biessmann, H., Walter, M. F., Dimitratos, S., and Woods, D. 2002. Isolation of cDNA clones encoding putative odourant binding proteins from the antennae of the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol. Biol. 11:123–132.
Briand, L., Nespoulous, C., Huet, J. C., Takahashi, M., and Pernollet, J. C. 2001. Ligand binding and physico-chemical properties of ASP2, a recombinant odorant-binding protein from honeybee (Apis mellifera L.). Eur. J. Biochem. 268:752–760.
Calvello, M., Guerra, N., Brandazza, A., D'ambrosio, C., Scaloni, A., Dani, F. R., Turillazzi, S., and Pelosi, P. 2003. Soluble proteins of chemical communication in the social wasp Polistes dominulus. Cell. Mol. Life Sci. 60:1933–1943.
Calvello, M., Brandazza, A., Navarrini, A., Dani, F. R., Turillazzi, S., Felicioli, A., and Pelosi, P. 2005. Expression of odorant-binding proteins and chemosensory proteins in some Hymenoptera. Insect Biochem. Mol. Biol. 35:297–307.
Chen, S. L. 2001. The Apiculture Science in China. pp. 123–131. Agricultural Publishing House of China, Beijing, China(in Chinese).
Danty, E., Michard-Vanhee, C., Huet, J. C., Genecque, E., Pernollet, J. C., and Masson, C. 1997. Biochemical characterization, molecular cloning and localization of a putative odorant-binding protein in the honey bee Apis mellifera L. (Hymenoptera: Apidea). FEBS Lett. 414:595–598.
Danty, E., Arnold, G., Huet, J. C., Huet, D., Masson, C., and Pernollet, J. C. 1998. Separation, characterization and sexual heterogeneity of multiple putative odorant-binding proteins in the honeybee Apis mellifera L. (Hymenoptera: Apidae). Chem. Senses 23:83–91.
Danty, E., Briand, L., Michard-Vanhee, C., Perez, V., Arnold, G., Gaudemer, O., Huet, D., Huet, J. C., Ouali, C., and Masson, C. 1999. 7468–7475. Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J. Neurosci. 19:7468–7475.
Dickens, D. J., Callahan, W. P., Wergin, W. P., and Erbe, E. F. 1995. Olfaction in a hemimetabolous insect: antennal-specific proteins in adult Lygus lineolaris (Heteroptera: Miridae). J. Insect Physiol. 41:857–867.
Esslen, J., and Kaissling, K. -E. 1976. Number and distribution of the sensilla on the antennal flagullum of the honeybee (Apis mellifera L.). Zoomorphologie 83:227–251.
Forêt, S., and Maleszka, R. 2006. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 16:1404–1413.
Gasteiger, E., Gattiker, A., Hoogland, C., Ivanyi, I., Appel, R. D., and Bairoch, A. 2003. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784–3788.
Graham, L. A., Brewer, D., Lajoie, G., and Davies, P. L. 2003. Characterization of a subfamily of beetle odorant-binding proteins found in hemolymph. Mol. Cell. Proteomics 2:541–549.
Hekmat-Scafe, D. S., Steinbrecht, R. A., and Carlson, J. R. 1997. Coexpression of two odorant-binding protein homologs in Drosophila: implications for olfactory coding. J. Neurosci. 17:1616–1624.
Ishida, Y., Cornel, A. J., and Leal, W. S. 2002a. Identification and cloning of a female antenna-specific odorant-binding protein in the mosquito Culex quinquefasciatus. J. Chem. Ecol. 28:867–871.
Ishida, Y., Chiang, V. P., Haverty, M. I., and Leal, W. S. 2002b. Odorant-binding proteins from a primitive termite. J. Chem. Ecol. 28:1887–1893.
Ishida, Y., Chen, A. M., Tsuruda, J. M., Cornel, A. J., Debboun, M., and Leal, W. S. 2004. Intriguing olfactory proteins from the yellow fever mosquito, Aedes aegypti. Naturwissenschaften 91:426–431.
Jin, X., Brandazza, A., Navarrini, A., Ban, L., Zhang, S., Steinbrecht, R. A., Zhang, L., and Pelosi, P. 2005. Expression and immunolocalisation of odorant-binding and chemosensory proteins in locusts. Cell. Mol. Life Sci. 62:1156–1166.
Leal, W. S., Nikonova, L., and Peng, G. 1999. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 464:85–90.
Livak, K. J., and Schmittgen, T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408.
Lu, D., Li, X., Liu, X., and Zhang, Q. 2007. Identification and molecular cloning of putative odorant-binding proteins and chemosensory protein from the bethylid wasp, Scleroderma guani Xiao et Wu. J. Chem. Ecol. 33:1359–1375.
Maida, R., Mameli, M., Muller, B., Krieger, J., and Steinbrecht, R. A. 2005. The expression pattern of four odorant-binding proteins in male and female silk moths, Bombyx mori. J. Neurocytol. 34:149–163.
Maleszka, R., and Stange, G. 1997. Molecular cloning, by a novel approach, of a cDNA encoding a putative olfactory protein in the labial palps of the moth Cactoblastis cactorum. Gene 202:39–43.
Michelette, E. R. F., and Soares, A. E. E. 1993. Characterization of preimaginal developmental stages in Africanized honey bee workers (Apis mellifera L.). Apidologie 24:431–440.
Nagnan-Le Meillour, P., Francois, M. C., and Jacquin-Joly, E. 2004. Identification and molecular cloning of putative odorant-binding proteins from the American palm weevil, Rhynchophorus palmarum L. J. Chem. Ecol. 30:1213–1223.
Ozaki, M., Morisaki, K., Idei, W., Ozaki, K., and Tokunaga, F. 1995. A putative lipophilic stimulant carrier protein commonly found in the taste and olfactory systems. A unique member of the pheromone-binding protein superfamily. Eur. J. Biochem. 230:298–308.
Pelosi, P. 1996. Perireceptor events in olfaction. J. Neurobiol. 30:3–19.
Pelosi, P., and Maida, R. 1995. Odorant-binding proteins in insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 111:503–514.
Pelosi, P., Zhou, J. J., Ban, L. P., and Calvello, M. 2006. Soluble proteins in insect chemical communication. Cell Mol. Life Sci. 63:1658–1676.
Peng, Y. S., Fang, Y. Z., Xu, S. Y., and Ge, L. S. 1987. The resistance mechanism of the Asian honey bee Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Inverteb. Pathol. 49:54–60.
Rachinsky, A., Strambi, C., Strambi, A., and Hartfelder, K. 1990. Caste and metamorphosis: hemolymph titers of juvenile hormone and ecdysteroids in last instar honeybee larvae. Gen. Comp. Endocrinol. 79:31–38.
Scaloni, A., Monti, M., Angeli, S., and Pelosi, P. 1999. Structural analysis and disulfide-bridge pairing of two odorant-binding proteins from Bombyx mori. Biochem. Biophys. Res. Commun. 266:386–391.
Steinbrecht, R. A. 1998. Odorant-binding proteins: expression and function. Ann. N. Y. Acad. Sci. 855:323–332.
Steinbrecht, R. A., Ozaki, M., and Ziegelberger, G. 1992. Immunocytochemical localization of pheromone-binding protein in moth antennae. Cell Tissue Res. 270:287–302.
Steinbrecht, R. A., Laue, M., and Ziegelberger, G. 1995. Immunolocalization of pheromone-binding protein and general odorant-binding protein in olfactory sensilla of the silk moths Antheraea and Bombyx. Cell Tissue Res. 282:203–217.
Tan, X. M. 2006. Wild resources of Apis cerana cerana are reducing rapidly. J. Bee (in Chinese) 4:16.
The Honeybee Genome Sequencing Consortium 2006. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443:931–949.
Vogt, R. G., and Riddiford, L. M. 1981. Pheromone binding and inactivation by moth antennae. Nature 293:161–163.
Vogt, R. G., and Lerner, M. R. 1989. Two groups of odorant binding proteins in insects suggest specific and general olfactory pathways. Neurosci. Abstr. 15:1290–1296.
Vogt, R. G., Prestwich, G. D., and Lerner, M. R. 1991a. Odorant-binding-protein subfamilies associate with distinct classes of olfactory receptor neurons in insects. J. Neurobiol. 22:74–84.
Vogt, R. G., Rybczynski, R., and Lerner, M. R. 1991b. Molecular cloning and sequencing of general odorant-binding proteins GOBP1 and GOBP2 from the tobacco hawk moth Manduca sexta: comparisons with other insect OBPs and their signal peptides. J. Neurosci. 11:2972–2984.
Wang, G. R., Wu, K. M., and Guo, Y. Y. 2003. Cloning, expression and immunocytochemical localization of a general odorant-binding protein gene from Helicoverpa armigera (Hubner). Insect Biochem. Mol. Biol. 33:115–124.
Xiu, W. M., and Dong, S. L. 2007. Molecular characterization of two pheromone binding proteins and quantitative analysis of their expression in the beet armyworm, Spodoptera exigua Hübner. J. Chem. Ecol. 33:947–961.
Xu, P. X., Zwiebel, L. J., and Smith, D. P. 2003. Identification of a distinct family of genes encoding atypical odorant-binding proteins in the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 12:549–560.
Yang, C. H. 1998. The development and lives of male and worker bees of Apis cerana cerana. J. Bee (in Chinese) 8:16.
Zhang, S., Maida, R., and Steinbrecht, R. A. 2001. Immunolocalization of odorant-binding proteins in noctuid moths (Insecta, Lepidoptera). Chem. Senses 26:885–896.
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (30270896) to Gao Qi-Kang and (30471127) to Lou Bing-Gan and by a grant from the Natural Science Foundation of Zhejiang (Y307597) to Li Hong-Liang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, HL., Zhang, YL., Gao, QK. et al. Molecular Identification of cDNA, Immunolocalization, and Expression of a Putative Odorant-Binding Protein from an Asian Honey Bee, Apis cerana cerana . J Chem Ecol 34, 1593–1601 (2008). https://doi.org/10.1007/s10886-008-9559-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9559-3