Abstract
Chemical analyses performed on the invasive weed Phytolacca americana (pokeweed) growing in industrially contaminated (Ulsan) and noncontaminated (Suwon) sites in South Korea indicated that the levels of phenolic compounds and various elements that include some heavy metals (Al, As, B, Cd, Co, Cu, Fe, Mn, Ni, Pb, and Zn) were statistically higher in Ulsan soils compared to Suwon soils with Al being the highest (>1,116 mg/l compared to 432 mg/l). Analysis of metals and nutrients (K, Na, Ca, Mg, Cl, NH4, N, P, S) in plant tissues indicated that accumulation occurred dominantly in plant leaves with Al levels being 33.8 times higher in Ulsan plants (PaU) compared to Suwon plants (PaS). The ability of PaU and PaS to tolerate stress was evaluated under controlled conditions by varying atmospheric CO2 and temperature and soil pH. When grown in pH 6.4 soils, the highest growth rate of PaU and PaS plants occurred at elevated (30°C) and non-elevated (25°C) temperatures, respectively. Both PaU and PaS plants showed the highest and lowest growth rates when exposed to atmospheric CO2 levels of 360 and 650 ppm, respectively. The impact of soil pH (2–6.4) on seed germination rates, plant growth, chlorophyll content, and the accumulation of phenolics were measured to assess the effects of industrial pollution and global-warming-related stresses on plants. The highest seed germination rate and chlorophyll content occurred at pH 2.0 for both PaU and PaS plants. Increased pH from 2–5 correlated to increased phenolic compounds and decreased chlorophyll content. However, at pH 6.4, a marked decrease in phenolic compounds, was observed and chlorophyll content increased. These results suggest that although plants from Ulsan and Suwon sites are the same species, they differ in the ability to deal with various stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain, air, and soil pollution have damaged natural ecosystems since the beginning of industrial development and urbanization. One byproduct has been atmospheric CO2 enrichment. There is considerable evidence indicating that increased CO2 levels have detrimental effects on terrestrial plants and ecosystems (Korner 1996; Hall et al. 2005). According to global climate change studies, atmospheric CO2 concentrations will almost double within the twenty-first century, thereby, increasing mean surface temperatures on earth by as much as 8°C (Stainforth et al. 2005). Such an upward shift in the earth’s temperature is predicted to have serious impacts on plant communities, but the extent of the effect is largely unknown (Ro et al. 2001; Pagel Brown et al. 2007).
Acid rain, as a result of atmospheric pollution, can have detrimental effects on terrestrial and aquatic ecosystems (US-NAPAP 1991). For example, in forest ecosystems, soil acidification is thought to be responsible for a decline in plant health (Aber et al. 1982; Xin et al. 2007). Acidification of soil causes damage to the fine roots of plants that results in leakage of nutrients and directly impacts the soil microbial communities required for plant health and maintenance (Malinowski et al. 1998; Illmer et al. 2003). For example, studies have shown that mycorrhizal associations present in healthy forest trees are poorly developed or absent in unhealthy trees exposed to acid rain (Dighton and Skeffington 1987).
Industrial pollution is pervasive and may comprise organic and inorganic chemicals that may be toxic to plants and their supporting microbes (Kasurinen et al. 2007). Acidification of soils, a byproduct of industrial pollution, increases metal bioavailability (Bergholm et al. 2003; Wang et al. 2006) and exacerbates the detrimental effects of metal pollutants on plants and microbes. All plants have the ability to perceive stresses such as high CO2, soil acidification, and heavy metal exposure and can initiate complex physiological responses that mitigate impacts of these stresses (Bohnert et al. 1995; Kasurinen et al. 2007). However, few plants are capable of thriving in high stress habitats. The adaptation to stressful habitats is considered to involve changes in the genome (Robe and Griffiths 2000; Schurr et al. 2006) as well as associations with various microbes that are important for health and maintenance of plant systems (Petrini 1996; Rodriguez et al. 2008). Over the last half century, researchers have become concerned about hyperaccumulation of heavy metals in plants due to the alarming increase in industry induced heavy metal contamination and soil acidification (Wang et al. 2005). These metals are easily taken up by roots and translocated to different plant organs (Baker et al. 1994), and high accumulation generally causes growth inhibition and even plant death (Khan and Khan 1983).
The aim of this study was to compare the stress tolerance ability of one plant species growing in industrially contaminated and noncontaminated soils. In South Korea, the invasive weed Phytolacca americana has become established in both contaminated and noncontaminated areas (Park et al. 1999, Kim et al. 2005a, b). Here, we present the first chemical studies of P. americana plants from two different locales: industrially contaminated soils of Ulsan (acidified soil, metals, and heavy metals) and noncontaminated soils from Suwon, South Korea. Collectively, field and laboratory-generated plants were analyzed and compared for chemical composition and plant response to abiotic stresses associated with global warming (elevated temperature and CO2) and acid rain (soil pH).
Methods and Materials
Plant and Soil Collection
P. americana plants and seeds were collected from contaminated Ulsan (Kyunnam Province) and noncontaminated Suwon (Kyunggi Province) soils, South Korea. The organic plant debris layer surrounding the plants was removed and discarded, and the upper 5 cm of soil (approximately 200 g) was collected in plastic baggies and maintained at 4°C until processed.
Endophyte Profile Analysis of Plants and Soil
Greenhouse studies were conducted by propagation of PaU and PaS plants generated from seeds collected from Ulsan and Suwon sites, respectively. For endophyte profile analysis, seeds with their seed coats and plant tissues were surface sterilized in 2% sodium hypochlorite for 10–30 min and rinsed in ten volumes of sterile distilled water. Seeds (N = 100) and plant tissues (N = 6; cut into roots, stem, and leaf sections) were placed on 0.1× potato dextrose agar (PDA) medium supplemented with antibiotics (100 ug/ml ampicillin and streptomycin, 50 μg/ml tetracycline; Redman et al. 2002a). Soils and plant tissues were processed for endophyte colony forming units (CFU) and percentage colonization of plant tissues, respectively. Soil was homogenized in plastic baggies, passed through a 2-mm soil sieve, 3 g resuspended in 30 ml of sterile water, and 100 μg/ml plated onto 0.1× PDA plates. One gram of surface sterilized plant tissues (Redman et al. 2002a) was homogenized, resuspended in 3 ml of STC (1.2 M Sorbitol, 10 mM TRIS pH 8.0, 10 mM CaCl2) and 500 μg/ml were plated onto 0.1× PDA medium. All plates were maintained at 28°C with a 12-h light regime for 10 days and assessed for fungal colonization. Fungi were identified by using standard taxonomic, microscopic, and molecular techniques (Barnett and Hunter 1998; Redman et al. 2002a). CFU analysis was repeated a minimum of three times.
Soil and Plant Chemical Analysis
Soil from Ulsan and Suwon were air-dried and passed through a 2-mm soil sieve. Root and leaf samples of PaU and PaS field plants were oven-dried at 60°C for 48 h and then ground and passed through a 2-mm sieve. Soil and plant tissues were analyzed by using standard protocols for pH, total nitrogen, Cl (Piper 1966), various compounds and metals (B, Al, Co, Cd, Cu, Fe, Mn, Ni, Pb, Zn, As; Agricultural Improving Institute 1988), available phosphate, (Agricultural Improving Institute 1988), suggested exchangeable ions ((Na+, K+, Mg++, NO3−) Page et al. 1982), and total phenolic compounds (Swain and Hillis 1959) see below.
Total Phenolic Compounds
Two hundred grams of fresh PaU and PaS leaf and root tissues were extracted in 1 L of distilled water at room temperature for 48 h and centrifuged at 15,000 rpm for 30 min (Centrikon T-1045, Kontron Co). For soil sampling, the upper 5 cm of soil was collected under a patch of P. americana after removing the organic layer. Ten g of PaU and PaS soils were resuspended in 50 ml of sterile water. The supernatant was collected and stored at 4°C (Inderjit 1996). Total phenolic compounds in plant materials (leaves and roots) and soils were analyzed by the Folin–Denis reagent method (Swain and Hillis 1959). All assays were repeated a minimum of three times.
Chlorophyll Content
The total chlorophyll content (chlorophyll a and b) of leaves was determined by using standard protocols (MacKinney 1941). Four mature leaves that were 15 cm above the pot soil base for each treatment were measured for chlorophyll content. A minimum of ten measurements per leaf was taken and recorded.
Simulated Acid Rain Stress
Uniformly sized PaU and PaS seeds were sterilized for 3 min in 5% sodium hypochlorite solution, and then rinsed with 7–10-fold volume of distilled water. Using a modified bioassay of Lodhi (1976), 30 seeds were sown in a petri dish (diameter of 90 mm) and treated with simulated acid rain (pH 2 to 8) solutions (Park et al. 1999). Seeds were placed in an incubator at 28°C under 400 μmol/m2/s fluorescent lighting with 16/8 h L/D period for 6 days. Seed germination, growth, and biomass were assessed daily. All assays were replicated a minimum of three times.
Statistical Analysis
Data were normally distributed, and significant differences between treatments and controls were calculated with Duncan’s mean separation test for the measured parameters (SAS INSTITUTE 2000). The data given in tables and figures are the mean ± SE.
Results
Chemical analysis of the soils and plants (roots and leaves) from contaminated Ulsan and noncontaminated Suwon sites was performed to measure various elements and metals, pH, and total phenolics (Table 1). Statistical analysis revealed that all of the elements analyzed and total phenolics were significantly higher in Ulsan soils. In addition, Ulsan soil pH (3.84) was significantly more acidic than Suwon soil pH (5.82). Of the metals analyzed, Al, Fe, and Mn were in highest abundance (approximately 3, 129, and 14 times, respectively) in Ulsan soils compared to Suwon soils. A similar pattern was observed in the plant leaf tissues with elevated levels of Al, Fe, and Mn at 50.41, 18.30, and 69.45 mg/l in Ulsan plants (PaU) compared to 1.49, 2.80, and 3.11 mg/l in Suwon plants (PaS). Overall levels of these compounds in roots was lower than in leaf tissues; however, differences between PaU and PaS root tissues were not significant (Table 1). The pH of leaves and plant tissues were similar in both PaS and PaU plants with the pH ranging from 5.92–5.42 and 7.55–7.47 in the leaf and root tissues, respectively (Table 1). Analysis of total phenolic compunds indicated that they were more than three times higher in Ulsan soils compared to Suwon soils. Similarly, total phenolic compounds were approximately twice as high in both the plant leaf and root tissues of PaU plants compared to PaS plants (Table 1).
Nutrient composition of PaU and PaS plant tissues were also analyzed (Table 2). PaU leaf tissues had elevated levels of Cl, NH4, N, P, and S compared to PaS plants with N, Cl and S being 846, 11.8, and 1.98 times higher in PaU leaves, respectively. In contrast, PaS plant leaves had elevated levels of K, Na, Ca, and Mg compared to PaU leaf tissues. With the exception of Ca, Na, Mg, and K, all elements tested were higher in PaU roots compared to PaS root tissues. Of these elements, Cl, N, P, and S were notably higher in PaU roots compared to PaS roots (Table 2).
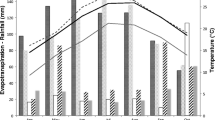
The effects of simulated acid rain (pH 2–6.4) on PaU and PaS plants was measured by determining the percentage seed germination, total phenolic compounds, and chlorophyll levels in plants, as well as plant growth and biomass (Fig. 1). With the exception of chlorophyll content, the overall pattern in each of these assays was similar: PaU plants had higher seed germination (P < 0.001), plant growth, and biomass compared to PaS plants for all simulated acid rain treatments (P < 0.001). The highest seed germination of 100% and 78% and chlorophyll content of 37 and 22 mg/m3 occurred at simulated acid rain pH 2.0 for both PaU and PaS plants, respectively (Fig. 1A and B). The same pattern was observed with plant growth and biomass of shoot at simulated acid rain pH 2.0, with the highest plant growth of 45 and 38 cm, and plant biomass of 5.4 and 4.2 mg occurred in PaU and PaS plants, respectively (Fig. 1C and D). Assessment of plant seed germination, chlorophyll content, plant growth and biomass over a simulated acid rain pH range (2.0–6.4) showed a similar pattern in that the highest levels were achieved at pH 2.0 followed by a marked decrease at pH 3.0–5.0 and a slight increase at pH 6.4 (Fig. 1A–D) for both PaU and PaS plants. In general, an inverse relationship was observed with total phenolic compounds and pH compared to other parameters (% seed germination, plant growth and biomass), with a general increase in total phenolic compounds occurring from pH 2.0 to 5.0 followed by a slight decrease at pH 6.4 for both PaU and PaS plants (Fig. 1B). In contrast, PaS plants exhibited statistically lower total phenolic compound levels than PaU plants but higher chlorophyll content at pH 2–5 (Fig. 1B).
Comparison of A germination rate; B phenolic compounds and chlorophyll levels; C growth and; D dry weight of plants grown from seeds harvested from Ulsan (PaU) contaminated soils and Suwon (PaS) noncontaminated soils field sites. PaS and PaU plants were exposed to simulated acid rain conditions (pH 2, 3, 4, 5) and the control pH of the potting soil was 6.4. TP Total phenolic compound levels; means with the same letter are not significantly different (Duncan’s multiple range test, A, B, C and D = P < 0.001), ±SE of N = 6 measurements each are given
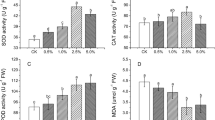
The effects of varying temperature and CO2 levels on PaU and PaS plant health was assessed (Fig. 2). The overall effect of the treatments (I–IV) on growth rates of PaU plants was III > I > IV > II, and PaS plants was I > III > IV > II, with PaU plants being larger (P < 0.001) and maturing faster (onset of flowers and mature leaves; recorded observation; data not shown) than PaS plants. PaU and PaS plants were compared and analyzed for chlorophyll content, plant growth, and total phenolic levels when exposed to treatments I–IV; PaU plants were statistically higher for plant growth and total phenolics compared to PaS plants (Fig. 2A and C). In contrast, PaU plants exhibited statistically lower chlorophyll levels when compared to PaS plants (Fig. 2B). Although PaU and PaS plants were the same age, PaU plants were larger, and tissues were more mature when compared to PaS plants (personal observation, Yong Ok Kim). Leaves were broader and more mature and, as such, exhibited a lighter green color and subsequent decrease in chlorophyll content. The reverse was true for total phenolics with levels in PaU higher when compared to PaS plants.
Comparison of A Total growth; B Chlorophyll content; C Phenolic compound levels of plants grown from seed harvested from Ulsan (contaminated soils) and Suwon (noncontaminated soils) field sites. PaS and PaU plants were exposed to treatments I ambient CO2 (360 µmol mol−1 CO2) and temperature (25°C); II double ambient CO2 (650 µmol mol−1 CO2) and ambient temperature (25°C); III increased temperature (30°C) with ambient CO2; and IV increased temperature (30°C) and double ambient CO2. TP total phenolic compound levels; means with the same letter are not significantly different (Duncan’s multiple range test, A and B = P < 0.001, C = P < 0.01), ±SE of N = 10 measurements each are given
Six PaU and PaS field plants and 100 seeds from both field sites were surface sterilized and plated on microbial growth media to determine the plant and seed endophyte profiles. The surrounding rhizosphere soil was collected and analyzed to determine the composition of the soil microbial community. All six of the PaU plants had a fungal endophyte (Glomeralla acutata) associated with the leaf and root tissue, as did 97.25% of seeds. No such fungal associations were found in PaS plants and seeds. Analysis of the soils surrounding the plants indicated that the same fungal endophyte was present in Ulsan and absent in Suwon soils (Table 3, P < 0.01).
Discussion
In 1979, P. americana was introduced into South Korea and became an important ecological problem by 1993 when it had spread throughout the country, displacing many native species (Park 1995). P. americana is widely distributed in contaminated areas of Korea, and a correlation between plant distribution and pollution absorption from soils has been reported (Park et al. 1999). Little is known about Phytolaccaceae ecology with the exception of a few papers that deal with the invasive threat of P. americana in Korea (Kim et al. 2005a, b). The extent of the effects of pollution on plants and the time frame for adaptation to stressful habitats in unknown but thought to involve a combination of plant genome changes and associations with microbes that can hasten the adaptation (within a growing season) in a non-Darwinian manner (Rodriguez et al. 2008). Our studies are the first to address the effects of pollution and global warming related stresses of P. americana via plant chemical analysis and the surrounding rhizosphere soils from two different habitats. Interestingly, PaU plants growing in the Ulsan contaminated site were larger (overall height, leaf size and number), more robust, and growing more densely than PaS plants growing in noncontaminated Suwon soils (personal observation, Yong Ok Kim).
It is not surprising that PaU plants had higher concentrations of phenolic compounds in the leaves and roots when compared to PaS plants, since plants exposed to metal or acid stress often produce secondary metabolites that are thought to play roles in plant health and survival (Inderjit 1996; Kasurinen et al. 2007). Terpenoids, flavonoids, alkaloids, and phenolics are the most common allelochemicals, with the latter allelochemical being abundant under field conditions (Seneviratne and Jayasinghearachchi 2003). Phenolic compounds are known to affect seed germination, seedling growth, cell division, fungal activity, protein synthesis, and enzyme activity (Callaway et al. 2004; Vivanco et al. 2004). It has been reported that phenolic-induced oxidative damage is mediated by heavy metals (Sakihama and Yamasaki 2002) and that accumulation of these metals occurs by conjugation with phenolics in various plant organs (Santiago et al. 2000; Lavid et al. 2001). Loponen et al. (2001) studied foliar concentrations of phenolics in contaminated and control areas to detect early symptoms of heavy-metal pollution in birch trees. They found that in heavily polluted areas, atmospheric stress factors appear to be correlated with the accumulation of phenolics in birch leaves (Andrea et al. 2006). In addition, exposure of plants to simulated low-pH acid rain (pH 3.0) conditions resulted in the appearance of necrotic spots on the leaf blades and subsequent accumulation of phenolics in necrotic areas (Sant’Anna-Santos et al. 2006).
Our studies showed that P. americana preferentially accumulates metals in leaf tissues compared to root tissues. This was not expected since several studies indicate that plants preferentially accumulate metals in root tissues (Rauser and Meuwly 1995). For example, Nishizono et al. (1987) reported the heavy metals, Cd, Cu, and Zn, accumulated in roots of Athyrium yokoscense compared with other organs. Lubben and Sauerbeck (1991) also suggested that roots are the first site of accumulation of heavy metals and subsequent tolerance to heavy metals (Wu and Bradshaw 1972).
Seed germination of both PaU and PaS plants decreased when exposed to simulated acid rain with the lowest germination rates occurring at pH 5.0 and 4.0, respectively. Although seed germination of PaU and PaS increased at pH 6.4, the highest percentage germination occurred at the lowest pH (2.0), and germination rates for PaU were higher than PaS at all pH levels tested. The growth length, dry weight, and overall health of PaU and PaS were also highest at pH 2.0, with all values greater for PaU. These results are in contrast to those of others (Fan and Wang 2000) that showed simulated acid rain conditions of pH 2.0 caused foliar damage, reduced chlorophyll content, and reduced seedling growth; while seedling growth was simulated at pH levels between pH 3.5 and 5. Regardless, increased germination and growth of PaU plants at pH 2.0 suggests that PaU plants have more tolerance to acid rain than PaS plants.
The phenolic levels of both PaU and PaS plants significantly increased with pH under simulated acid rain conditions, and the phenolic content of PaU plants was higher than that of PaS plants (Fig. 1). Interestingly, in other plants species (Mimosa artemisiana, Gallesia integrifolia, and Norway spruce), enhanced accumulation of phenolics and development of necrotic spots on leaves occurred upon exposure to acidic pH (3.0; Sant’Anna-Santos et al. 2006). Although phenolic accumulation was directly proportional to pH in P. americana, plant growth was inversely proportional to pH, and lesions did not develop at low pH. Therefore, we surmise that the acidic nature of soils and enhanced bioavailability of compounds do not have a negative impact on growth or disease symptom development on PaU plants.
Exposure of plants to different environmental treatments (altered CO2, temperature, and soil pH) resulted in altered growth rates (Fig. 2). PaU plants grew larger and matured faster in all treatments compared to PaS plants. In addition, the treatments affected plant growth differently with PaU plants having highest growth in treatment III (ambient CO2 and increased temperature) while PaS plants had the greatest growth in treatment I (ambient CO2 and temperature). Although the significance of this is not yet known, it demonstrates that PaU and PaS plants are physiologically different.
The chlorophyll content of leaves was greater for PaS plants than PaU plants in every treatment. A contributing factor to the observed overall decrease in plant chlorophyll content in PaU plants growing in contaminated soils is that they grew more quickly (both overall plant height and overall size and number of leaves), and the leaves measured were physiologically more mature than PaS plants. Chlorophyll levels in both plants were highest when the ambient CO2 levels were doubled. However, measurements of the growth, chlorophyll content, and phenolic levels did not show a significant difference between treatment I of PaU plants when compared to PaS plants. Mature flowers and aging leaves in PaU plants appeared earlier than in PaS plants and, as such, is reflected as a decrease in chlorophyll content in PaU plants. Similarly, Loponen et al. (2001) and Peltonen et al. (2005) reported that elevated CO2 increased phenolic compound levels by 25%, with phenolic compound levels 21% higher in samples from heavily contaminated smelter areas than from noncontaminated areas. We observed a similar phenomenon with higher levels of phenolics in PaU plants than PaS plants, especially in the roots of plants exposed to treatment II (25°C, 650 ppm CO2) and IV (30°C, 650 ppm CO2).
Total phenolic levels in the leaves and roots of treatment I were less than that of the other treatments, and the growth rates decreased, while chlorophyll content increased for treatments II and IV. This suggests that there may be a relationship between growth rates, total phenolic levels, and chlorophyll content.
Microbial analysis showed that a single dominant fungal endophyte (>97%) was present in the contaminated (PaU) and absent in the noncontaminated (PaS) site samples. The endophyte was identified as G. acutata, which was isolated from leaves, roots, and seeds, and was also abundant in the surrounding soils (Table 3). Interestingly, the endophyte was in highest abundance (84.53 CFU) in PaU leaves where the highest accumulation of metals was found. The abundance of the endophyte in Ulsan soils, and the variance in abundance levels in the different plant tissue types, suggest that this endophyte may have an ecologically significant role in PaU plants. Recent studies have shown that symbiotic fungi impart habitat-specific stress tolerances and are responsible for the survival of some plants in high-stress habitats (Redman et al. 2002a; Rodriguez et al. 2008). In addition, fungal endophytes are known to promote plant growth (Redman et al. 2002b; Varma et al. 2006). However, the ecological role of endophytes in contaminated habitats has not been well defined. Studies have shown that fungal symbionts have the ability to confer heavy metal tolerance to plants (Monnet et al. 2001). Some fungi produce siderophore and siderophore-like compounds and are able to sequester iron in the surrounding rhizosphere (Wilhite et al. 2001), while other fungi increase translocation of metals to leaf tissues (Al-Karaki et al. 2001).
Although the mechanism is yet unknown, collectively, these studies provide evidence to support the hypothesis that PaU plants have adapted to and are able to thrive in this industrially contaminated high stress habitat. The PaU plants represent acid-rain and heavy-metal-tolerant ecotypes, while PaS plants do not. Although additional work is required, the results presented here are promising and demonstrate the ability of PaU pokeweed plants to absorb and translocate metals to leaf tissues. As such, the results suggest that this system may be developed as a potential useful phyto-remediation tool to clean-up contaminated soils.
References
Aber, J. D., Hendrey, G. R., Botkin, B., Francis, A. J., and Melillo, J. M. 1982. Potential effects of acid precipitation on soil nitrogen and productivity of forest ecosystems. Water, Air and Soil Pollute 18:405–412.
Agricultural Improving Institute. 1988. Methods of Soil Chemical Analysis. p.450.
Al-Karaki, G. N., Hammad, R., and Rusan, M. 2001. Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 11:43–47.
Andrea, M., Steffen, A., Barbara, K., Gerhard, H. B., Uwe, S., and Hans-Peter, M. 2006. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant, Cell and Environ. 29:126–137.
Baker, A. J., Reeves, R. D., and Hajar, A. S. M. 1994. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. and C. Presi (Brassicaceae). New Phytol. 127:61–68.
Barnett, H. L., and Hunter, B. B. 1998. Illustrated genera of imperfect fungi, p. 240. American Phytopathology Society, St. Paul.
Bergholm, J., Berggren, D., and Alai, G. 2003. Soil acidification induced by ammonium sulphate addition in a Norway spruce forest in southwest Sweden. Water, Air, and Soil Pollution 148:87–109.
Bohnert, H. J., Nelson, D. E., and Jensen, R. G. 1995. Adaptations to environmental stresses. The Plant Cell 7:1099–1111.
Callaway, R. M., Thelen, G. G., Rodriguez, A., and Holven, W. E. 2004. Release from inhibitory soil biota in Europe may promote invasive plant invasion in North America. Nature 427:731–733.
Dighton, J., and Skeffington, R. A. 1987. Effects of artificial acid precipitation on the mycorrhizae of Scots seedlings. New Phytol. 107:191–202.
Fan, H. B., and Wang, Y. H. 2000. Effects of simulated acid rain on germination, foliar damage, chlorophyll contents and seedling growth of five hardwood species growing in China. Forest Ecol. & Management 126:321–329.
Hall, M. C., Stiling, P., Hungate, B. A., Drake, B. G., and Hunter, M. D. 2005. Effects of elevated CO2 and herbivore damage on litter quality in a scrub oak ecosystem. J. Chem. Ecol. 31:2243–2356.
Illmer, P., Obertegger, U., and Schinner, F. 2003. Microbiological properties in acidic forest soils with special consideration of KCl extractable Al. Water, Air, and Soil Pollution 148:3–14.
Inderjit, M. A. U. 1996. Plant phenolics in allelopathy. Bot Rev. 62:186–202.
Kasurinen, A., Peltonen, P. A., Julkunen-Tiito, R., Vapaavuori, E., Nuutinen, V., Holopainen, T., and Holopainen, J. K. 2007. Effects of elevated CO2 and O3 on leaf litter phenolics and subsequent performance of litter-feeding soil macrofauna. Plant and Soil 292:25–43.
Khan, S., and Khan, N. N. 1983. Influence of lead and cadmium on the growth and nutrient concentration of tomato (Lycopersicon esculentum) and egg-plant (Solanum melongena). Plant and Soil 74:387–394.
Kim, Y. O., Johnson, J. D., and Lee, E. J. 2005a. Allelochemical effects and chemical analysis of leaf extracts from three Phytolaccacese species. J. Chem. Ecol. 31:1175–1186.
Kim, Y. O., Johnson, J. D., and Lee, E. J. 2005b. Phytotoxicity of Phytolacca americana leaf extracts on the growth, and physiological response of Cassia mimosoides. J. Chem. Ecol. 31:2963–2973.
Korner, C. 1996. The response of complex multispecies systems to elevated CO2, pp. 20–42, in B. H. Walker, and W. L. Steffen (eds.). Global Change and Terrestrial Ecosystems. Cambridge University Press, Cambridge.
Lavid, N., Schwartz, A., Yarden, O., and Tel-Or, E. 2001. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the water lily (Nymphaeaceae). Planta 212:323–331.
Lodhi, M. A. K. 1976. Role of allelopathy as expressed by dominating trees in a lowland forest in controlling the productivity and pattern of herbaceous growth. Amer. J. Bot. 63:1–8.
Loponen, J., Lempa, K., Ossipov, V., Kozlov, M. V., Girs, A., Hangasmaa, K., Haukioja, E., and Pihlaja, K. 2001. Patterns in content of phenolic compounds in leaves of mountain birches along a strong pollution gradient. Chemosphere. 45:291–301.
Lubben, S., and Sauerbeck, D. 1991. The uptake and distribution of heavy metals by spring wheat. Water Air and Soil Pollut. 57–58:239–247.
Mackinney, G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140:315–332.
Malinowski, D., Alloush, G., and Belesky, D. 1998. Evidence for chemical changes on the root surface of tall fescue in response to infection with the fungal endophyte Neotyphodium coenophialum. Plant and Soil 205:1–12.
Monnet, F., Vaillant, N., Hitmi, A., Coudret, A., and Sallanon, H. 2001. Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne. Physiol. Plantarum 113:557–563.
Nishizono, H., Ichikawa, J., Suziki, S., and Ishii, F. 1987. The role of the root cell wall in the heavy metal tolerance of Athyrium yokoscense. Plant and Soil 101:15–20.
Page, A. L., Miller, R. H., and Keeney, D. R. 1982. Methods of Soil Analysis, part 2, Chemical and Microbiological Properties, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison.
Pagel Brown, A. L., Day, F. P., Hungate, B. A., Drake, B. G., and Hinkle, C. R. 2007. Root biomass and nutrient dynamics in a scrub-oak ecosystem under the influence of elevated atmospheric CO2. Plant and Soil. 292:219–232.
Park, S. H. 1995. Unrecorded naturalized species in Korea. Korean J. of Species Taxo. 25:123–130.
Park, Y. M., Park, B. J., and Choi, K. R. 1999. pH changes in the Rhizosphere soil of Phytolacca americana. Korean J. Ecol. 22:7–11.
Peltonen, P. A., Vapaavuori, E., and Julkunen-Tiito, R. 2005. Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Global Change Biology 11:1305–1324.
Petrini, O. 1996. Ecological and physiological aspects of host-specificity in endophytic fungi, pp. 87–100, in S. C. Redlin, and L. M. Carris (eds.). Endophytic Fungi in Grasses and Woody Plants. APS, St. Paul.
Piper, C. S. 1966. Soil and plant analysis, p. 1368. Hans, Bombay.
Rauser, W. E., and Meuwly, P. 1995. Retention of cadmium in roots of maize seedling. Role of complexation by phytochelatins and related thiol peptides. Plant Physiol. 109:195–202.
Redman, R. S., Sheehan, K. B., Stout, R. G., Rodriguez, R. J., and Henson, J. M. 2002a. Thermotolerance conferred to plant host and fungal endophyte during mutualistic symbiosis. Science. 298:581.
Redman, R. S., Roossinck, M. J., Maher, S., Andrews, Q. C., Schneider, W. L., and Rodriguez, R. J. 2002b. Field performance of cucurbit and tomato plants colonized with a nonpathogenic mutant of Colletotrichum magna (teleomorph: Glomerella magna; Jenkins and Winstead). Symbiosis. 32:55–70.
Ro, H. M., Kim, P. G., Lee, I. B., Yiem, M. S., and Woo, S. Y. 2001. Photosynthetic characteristics and growth responses of dwarf apple (Malus domestica Borkh. Cv. Fuji) saplings after 3 years of exposure to elevated atmospheric carbon dioxide concentration and temperature. Trees. 15:195–203. doi:10.1007/s004680100099.
Robe, W. E., and Griffiths, H. 2000. Physiological and photosynthetic plasticity in the amphibious, freshwater plant, Littorella uniflora, during the transition from aquatic to dry terrestrial environments. Plant Cell Environ. 23:1041–1054.
Rodriguez, R., Henson, J., Van Volenburgh, E., Hoy, M., Wright, L., Beckwith, F., Kim, Y. O., and Redman, R. S. 2008. Stress tolerance in plants via habitat-adapted symbiosis. ISME-Nature. 2:406–416. doi:10.1038/ismej.2007.106.
Sakihama, Y., and Yamasaki, H. 2002. Lipid peroxidation induced by phenolics in conjunction with aluminum ions. Bio. Plant. 45:249–254.
Santiago, L. J. M., Louro, R. P., and De Oliveira, D. E. 2000. Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of (Phyllanthus tenellus Roxb.) and their induction by copper sulphate. Ann. Bot. 86:1023–1032.
Sant’anna-Santos, B., Campos, F., Da Silva, L., Azevdgo, A. A., Marcos De Arajo, J., Alves, E. F., Monteiro Da Silva, A., and Aguiar, R. 2006. Effects of simulated acid rain on the foliar micromophology and anatomy of tree tropical species. Environ. Exp. Botany. 58:158–168.
Sas Institute 2000. SAS/STAT Guide for Personal Computers, Version 6.03 ed. SAS, Cary.
Schurr, U., Walter, A., and Rascher, U. 2006. Functional dynamics of plant growth and photosynthesis - from steady-state to dynamics - from homogeneity to heterogeneity. Plant Cell Environ. 29:340–352.
Seneviratne, G., and Jayasinghearachchi, H. S. 2003. Phenolic acids: Possible agents of modifying N2-fixing symbiosis through rhizobial alteration. Plant and Soil. 252:385–395.
Stainforth, D. A., Aina, T., Christensen, C., Collins, M., Faull, N., Frame, D. J., Kettleborough, J. A., Knight, S., Martin, A., Murphy, J. M., Piani, C., Sexton, D., Smith, L. A., Spicer, R. A., Thorpe, A. J., and Allen, M. R. 2005. Uncertainty in predictions of the climate response to rising levels of greenhouse gases. Nature. 433:403–407.
Swain, T., and Hillis, W. E. 1959. The phenolic constituents of Prunus domestica I. The quantitative analysis of the phenolic constitutents. J. Sci. Food Agric. 10:63–68.
US National Acid Precipitation Assessment Program. 1991. Acidic deposition: State of science and technology, Vol. I–IV, ed. P. M. Irving. Government Printing Office, Washington, DC 20402-9352, USA.
Varma, A., Verma, S., Sudha, S. N., Butehorn, B., and Franken, P. 2006. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 6:2741–2744.
Vivanco, J. M., Bais, H. P., Stermitz, F. R., Thelen, G. C., and Callaway, R. M. 2004. Biogeographical variation in community response to root allelochemistry: Novel weapons and exotic invasion. Ecol. Lett. 7:285–292.
Wang, F., Lin, X., and Yin, R. 2005. Heavy metal uptake by arbuscular mycorrhizas of Elsholtzia splendens and the potential for phytoremediation of contaminated soil. Plant and Soil. 269:225–232.
Wang, A. S., Angle, J. S., Chaney, R. L., Delorme, T. A., and Reeves, R. D. 2006. Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant and Soil. 281:325–337.
Wilhite, S. E., Lumsden, R. D., and Straney, D. C. 2001. Peptide synthetase gene in Trichoderman virens. Appl. Environ. Microbiol. 11:5055–5062.
Wu, L., and Bradshaw, A. D. 1972. Aerial pollution and the rapid evolution of copper tolerance. Nature. 238:167–169.
Xin, L. H., Han, S. J., Li, L., Zhou, Y. M., and Zheng, J. Q. 2007. Responses of soil enzymes to long-term CO2 enrichment in forest ecosystems of Changbai Mountains. Northeast Forestry University and Ecological Society of China. 18:119–122.
Acknowledgements
We are grateful to Dr. Ro Hee Myoung for providing CO2 and temperature chamber. Funding was provided in part by NSF (0414463), US/IS BARD (3260-01C) and US Geological Survey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.O., Rodriguez, R.J., Lee, E.J. et al. Phytolacca americana from Contaminated and Noncontaminated Soils of South Korea: Effects of Elevated Temperature, CO2 and Simulated Acid Rain on Plant Growth Response. J Chem Ecol 34, 1501–1509 (2008). https://doi.org/10.1007/s10886-008-9552-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9552-x