Abstract
Within the genus Papilio, the P. glaucus group contains the most polyphagous Papilio species within the Papilionidae. The majority of Papilio species are associated with hostplants in the families Rutaceae and Apiaceae, and characterizing most are secondary metabolites called furanocoumarins. Recent phylogenetic studies suggest that furanocoumarin metabolism is an ancestral trait, with the glaucus group derived from ancestors associated with furanocoumarin-containing Rutaceae. In this study, we examined this relationship by conducting a gravimetric analysis of growth that used various concentrations of the furanocoumarin xanthotoxin. Papilio multicaudatus, the putative ancestor of the glaucus group, includes at least one furanocoumarin-containing rutaceous species among its hostplants; this species can consume leaf tissue containing up to 0.3% xanthotoxin with no detectable effect on relative growth rate, relative consumption rate, or efficiency of conversion of ingested food. As is the case for other Papilio species, xanthotoxin metabolism is mediated by cytochrome P450 monooxygenases (P450s). Ingestion of xanthotoxin by ultimate instar P. multicaudatus increases activity up to 30-fold in a dose-dependent fashion. Midguts of induced larvae can also effectively metabolize six other furanocoumarins, including both linear (bergapten, isopimpinellin, imperatorin) and angular (angelicin, sphondin) forms. A metabolite of xanthotoxin in the frass from xanthotoxin-treated larvae, identified as 6-(7-hydroxy-8-methoxycoumaryl)-acetic acid by MS–MS and NMR analyses, is identical to one from the frass of P. polyxenes. The occurrence of this metabolite in two swallowtails and the presence of a second metabolite of xanthotoxin, 6-(7-hydroxy-8-methoxycoumaryl)-hydroxyethanol in the frass of both P. polyxenes and Depressaria pastinacella are consistent with the suggestion that lepidopterans share as the first step of xanthotoxin metabolism the P450-mediated epoxidation of the furan ring 2′–3′ double bond.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 40 years ago, Ehrlich and Raven (1964) demonstrated that phylogenetic patterns of hostplant use within the Lepidoptera can provide insights into the evolution of specialization and adaptation to plant chemical defense. In coevolutionary interactions, key innovations in behavior or physiology are thought to underlie host use patterns and facilitate hostplant switches; molecular methods now allow the identification and characterization of genes encoding enzymes that play key roles in this reciprocal adaptive process (Berenbaum et al., 1996). The swallowtail butterflies of the family Papilionidae, a cosmopolitan group of 500+ species, has been the focus of coevolutionary studies for many years (Feeny, 1992). Early estimates of phylogenetic relationships within the family were based on morphological characteristics, larval food plant associations, and geographic distributions. Munroe (1961) split Papilio into five sections, which corresponded to differences in utilization of the principal food plants in the families Annonaceae, Lauraceae, Rutaceae, and Apiaceae (Berenbaum, 1995). Plants in the Apiaceae and Rutaceae contain furanocoumarins as defense chemicals (Berenbaum, 1983). Papilio sections II and IV generally utilize species in the family Apiaceae and Rutaceae as hostplants (Scriber et al., 1991; Scriber, 1995), whereas section III species, which tend to have more polyphagous diets, only occasionally use these furanocoumarin-containing hostplants (Cohen et al., 1992). There are no known associations between species in sections I and V and furanocoumarin-containing plants.
Prior to molecular analysis, sections III and V were believed to be basal within the genus Papilio based largely on male genitalia, morphology, and larval hostplant preferences. Analyses have utilized allozyme variations (Hagen and Scriber, 1991), mitochondrial RNA (Sperling, 1993; Sperling and Harrison, 1994; Caterino and Sperling, 1999; Reed and Sperling, 1999), and DNA sequencing (Aubert et al., 1999; Vane-Wright et al., 1999; Yagi et al., 1999) to elucidate relationships within the genus. Zakharov et al. (2004), conducting a meta-analysis to generate an accurate phylogeny, split the genus into two distinct lineages, one including the subgenera Princeps and Papilio (sensu stricto) (section II) and the other including the subgenera Heraclides, Pterourus, and Chilasa (sections IV, III, and V, and I, respectively). Although Pterourus is paraphyletic, the glaucus group is considered the most advanced of these clades. According to this analysis, P. multicaudatus is the basal species within section III, the glaucus group in the subgenus Pterourus.
In both polyphagous section III species and oligophagous section II species, the ability to utilize furanocoumarin-containing hostplants in Apiaceae and Rutaceae is associated with metabolism by cytochrome P450 monooxygenases (P450s), which are detoxificative enzymes that catalyze the NADPH-associated reductive cleavage of oxygen to produce a functionalized product and water. The genes encoding these enzymes (http://drnelson.utmem.edu/CytochromeP450.html) constitute one of the largest superfamilies known. The furanocoumarin-metabolic activity of CYP6B proteins in Papilio species is associated with the probability of encountering hostplant furanocoumarins. Catalytic activity was compared in two closely related CYP6B4 and CYP6B17 groups in the polyphagous congeners Papilio glaucus and Papilio canadensis (Li et al., 2003). Generally, P450s from P. glaucus, which feeds occasionally on furanocoumarin-containing hostplants, display higher activities against furanocoumarins than those from P. canadensis, which normally does not encounter furanocoumarins. These P450s in turn catalyze a larger range of furanocoumarins with lower activity than CYP6B1, a P450 from Papilio polyxenes, which feeds exclusively on furanocoumarin-containing apiaceous hostplants. Reconstruction of the ancestral CYP6B sequences using maximum likelihood predictions and comparisons of the sequence and geometry of their active sites to those of contemporary CYP6B proteins indicate that hostplant diversity is inversely related to substrate specificity. These predictions suggest that, in the lineage leading to Papilio P450s, the ancestral highly versatile CYP6B protein presumed to exist in a polyphagous ancestor evolved through time into a more efficient and specialized CYP6B1-like protein in Papilio species with continual exposure to furanocoumarins.
P. multicaudatus specializes on only three genera of plants within three families: Rutaceae, Rosaceae, and Oleaceae. One host genus, Ptelea, is known to contain furanocoumarins (Murray et al., 1982). The purpose of our study was to examine furanocoumarin disposition in this species, putatively basal to the polyphagous species in section III, in order to determine whether (1) tolerance is intermediate between that of section II furanocoumarin specialists and section III generalists, (2) inducibility of furanocoumarin metabolism is conserved between sections, and (3) metabolites generated by P450-mediated metabolism, reflective of the detoxificative transformation, are similar across sections. Interpreting detoxificative metabolism of hostplant phytochemicals in a phylogenetic context can allow for reconstruction of host shifts and diversification.
Methods and Materials
Insects
Adult P. multicaudatus were captured at Kitt Peak in Pima County, Arizona, at 5000 ft elevation on 11 August 2004 and subsequently transported to Champaign County, Illinois. The captive butterflies were allowed to mate within a 1 × 1 × 1-m screen mesh cage. Juvenile Ptelea trifoliata and Prunus serotina trees were placed within the cage for oviposition. The eggs were allowed to hatch within the cage. Second instar larvae were removed from the cage and placed in 15l rearing tubs. Each rearing tub was constructed of durable, opaque plastic lined with moist paper towels. Air vents were cut into the lids. Fresh P. serotina leaves were exchanged on a daily basis. No rearing tub contained more than 30 larvae at any time. The caterpillars were maintained at 25°C and 16:8 light/dark photoperiod for the duration of the experiment.
Reagents for Detoxification Studies
Xanthotoxin used in gravimetric analyses of performance was obtained from Sigma (St. Louis, MO, USA). Other furanocoumarins, used as substrates in metabolism assays, including angelicin and isopimpinellin from Indofine Chemical Co. (Belle Mead, NJ, USA), bergapten and psoralen from Sigma (St. Louis, Mo, USA), and imperatorin from Feinbiochemical (Heidelberg, Germany); sphondin was a gift from Ralph Mumma. NADPH was obtained from Sigma, and HPLC solvents from Fisher Scientific L.L.C. (Pittsburgh, PA, USA).
Gravimetric Estimates of Xanthotoxin Tolerance
Using ultimate instar P. multicaudatus larvae, we conducted a gravimetric estimate of performance with varying concentrations of xanthotoxin, a linear furanocoumarin, topically applied to the hostplant. Fifth instar larvae were collected from the rearing tubs within 2 hr of the final larval molt. Each caterpillar was weighed and placed in a 9-cm Petri dish with moistened filter paper. Approximately 1 g of P. serotina leaf material, which lacks furanocoumarins, was weighed and topically treated with either acetone or an acetone solution of xanthotoxin. Xanthotoxin solutions of different concentrations were prepared, so that equal volumes could be applied to obtain diets containing xanthotoxin at 0.1, 0.2, 0.3, and 0.4% fresh weight. These concentrations were based on previous studies of P. polyxenes and its host plant, flat-leaf parsley, Petroselinum sativum (Cohen et al., 1989). One hundred larvae were distributed among the five treatments.
Larvae were allowed to feed for 24 hr and then placed in a −20°C freezer for 30 min. Subsequently, larvae, remaining leaf material, and frass were placed in a 60°C drying oven for 24 hr to obtain dry weight. All fresh and dry weights were recorded, and fresh weight was converted to dry weight by using a conversion factor based upon five caterpillars that were frozen and dried within 2 hr of the final molt and five dried P. serotina leaves.
Standard gravimetric parameters of performance were calculated, including relative growth rate (RGR), the efficiency of conversion of ingested food to body substance (ECI), and relative consumption rate (RCR) or weight gain (Waldbauer, 1968). Data were analyzed by using analysis of variance (ANOVA), Dunnett's test, and analysis of covariance (ANCOVA) (Raubenheimer and Simpson, 1992). Regression analysis was conducted to determine the effect of xanthotoxin on relative growth rate.
Inducibility of Xanthotoxin Metabolism
Newly molted fifth instars were individually placed on 1 g fresh weight foliage of P. serotina, topically treated with varying amounts of xanthotoxin dissolved in 250 μl acetone (0, 1, 2, and 4 mg). Four replicate larvae were used for each concentration. After 48 hr, midguts were dissected on ice, and each midgut was homogenized in 600 μl of 0.1 M ice-cold sodium phosphate buffer (pH 7.8) containing 20% glycerol, 1.1 mM EDTA, 0.5 mM PMSF, and 5 μg/ml (w/v) leupeptin. The homogenates were centrifuged at 5000 rpm for 5 min at 4 °C. Metabolism reactions were set up with 30 μl of the supernatant. Each reaction mixture contained 0 (control) or 50 μl of NADPH (10 mg/ml in 0.1 M phosphate buffer, pH 7.8), 2 μl of xanthotoxin (10 mM in methanol), and 468 μl (in control) or 418 μl of 0.1 M phosphate buffer, pH 7.8. The reactions were incubated for 30 min in a 30°C shaking water bath followed by incubation for 5 min at 70°C to inactivate the P450s. The reaction mixture was extracted with 500 μl of ethyl acetate after addition of 5 μl of 1 mM psoralen as an internal standard and centrifuged at 14,000 rpm for 5 min using a benchtop centrifuge at room temperature. Ten microliters of the ethyl acetate phase were removed and analyzed by normal-phase HPLC (Econosphere 5-μm silica column, 150 × 4.6 mm) with a solvent system containing 55% cyclohexane, 42% isopropyl ether, and 3% amyl alcohol to determine the amount of unmetabolized xanthotoxin remaining.
Metabolism of Furanocoumarins by Homogenates of Xanthotoxin-Induced Larval Midguts
To determine the substrate-specificity of P450-mediated furanocoumarin metabolism in P. multicaudatus, metabolism reactions were carried out as described except that homogenate supernatants were prepared from midguts of larvae induced with 4 mg xanthotoxin per gram wild cherry leaves and six furanocoumarin substrates, including the angular furanocoumarins angelicin and sphondin, and the linear furanocoumarins bergapten, imperatorin, isopimpinellin, and psoralen. They were analyzed, with 5 μl of 100 nM xanthotoxin added to the reaction as an internal standard before ethyl acetate extraction.
Isolation and Structural Elucidation of Xanthotoxin Metabolite from Frass
P. multicaudatus larvae starved for 3 hr were presented with foliage of wild cherry treated with 0 mg (control) or 4 mg xanthotoxin/g fresh weight (fw) foliage (treatment). After 24 hr of feeding, frass was collected. To detect the xanthotoxin metabolite in frass and determine a suitable metabolite extraction method, frass from caterpillars on each diet was extracted with water and methanol, and the extracts were analyzed by reverse-phase HPLC (4-μm C-18 column, Waters Novapak, 4.7 × 150 mm). To purify the metabolite, gradient elution (solvent A, 5% glacial acetic acid in water; solvent B, 100% acetonitrile) was performed at a flow rate of 1 ml/min with gradient conditions ranging from 100% A/0% B to 90% A/10% B over 5 min, to 60% A/40% B over 30 min; after 5 min at 60% A/40% B, a gradient was run to 100% A/0% B. The spectra for water and methanol extracts of treatment frass were compared with those of the corresponding control frass at wavelengths between 210 and 400 nm (996 Diode array detector, Waters, Milford, MA, USA); peaks unique to xanthotoxin-fed larvae were considered to represent metabolites.
To obtain sufficient amounts of metabolites to characterize, frass was again collected as described from fifth instars on control or treated (4 mg xanthotoxin/g fw) foliage. The frass collected was extracted with methanol. After drying the extract with N2, the residue was redissolved in the same original volume of HCl-acidified distilled water (pH 2). The water phase was then extracted with the same volume of ethyl acetate after extraction with the same volume of chloroform. The water phase was collected, frozen in liquid nitrogen, and lyophilized. Finally, the residue was resuspended in 0.5 ml methanol for separation by reverse-phase HPLC (as described earlier, except that UV absorbance was set at 325 nm).
Mass spectrometric analysis of purified metabolite was performed at the Mass Spectrometry Service Facility at the University of Illinois at Urbana-Champaign; the instrument used was a Finnigan LCQ Deca XP MAX ion trap mass spectrometer (Thermo Electron Corporation). For MS–MS analysis, a collision energy of 25 was used for the metabolite of MW 250. The 1H NMR spectrum was obtained with a Bruker (Billerica, MA, USA) Avance 400 NMR spectrometer equipped with a 5-mm inverse broadband Z-gradient probe (1H, 400 MHz). The NMR spectrum was recorded in methanol-d 4, which served as the internal reference (1H NMR, 3.30 ppm). Data were analyzed using the Advanced Chemistry Development, Inc., SpecManager 1D Processor and the HNMR Predictor software suite (Toronto, Ontario, Canada).
Results
Gravimetric Estimates of Xanthotoxin Tolerance
There were no statistically significant differences among treatments in consumption rate or efficiency of conversion (Table 1). The only significant effect of treatment evidenced in calculated gravimetric ratios evaluated by ANOVA was for RGR (F = 3.004, df 4, 97, P = 0.022) (Table 1). Growth rates in diet containing 0.2% xanthotoxin were lower than growth rates of caterpillars placed on control diet (P = 0.031), and RGR for caterpillars exposed to 0.4% xanthotoxin were marginally lower than those on control (P = 0.053).
ANCOVAs were performed to compare consumption and weight gain on the five diets. In both analyses, the assumption of homogeneity of slopes was not violated (P > 0.12), but there were no significant differences in consumption or growth among treatments. A marginally significant negative regression of relative growth rate on xanthotoxin concentration (Figure 1) suggests a mild negative effect of ingesting increasing amounts of xanthotoxin (P = 0.061, r 2 = 0.04).
Inducibility of Xanthotoxin Metabolism
Cytochrome P450-mediated xanthotoxin metabolism in the midgut of P. multicaudatus was induced in a dose-dependent fashion, increasing 13-, 16-, and 23-fold when ultimate instars were fed foliage treated with xanthotoxin at 1, 2, and 4 mg/g fw, respectively; constitutive levels of metabolism, in the absence of furanocoumarins, were very low (Figure 2). Larvae with midgut metabolism induced by ingestion of hostplant treated with xanthotoxin (4 mg/g fw) were capable of metabolizing six furanocoumarins other than xanthotoxin, including not only linear furanocoumarins (bergapten, imperatorin, isopimpinellin, psoralen) but also angular furanocoumarins (angelicin, sphondin). Metabolism rates were 4.38, 12.95, 6.28, 12.62, 4.97, and 10.89 nmol/min per midgut for angelicin, bergapten, imperatorin, isopimpimpinellin, psoralen, and sphondin, respectively (Figure 3).
Isolation and Structural Elucidation of Metabolite
Comparison of UV spectra of aqueous and methanolic extracts of frass from caterpillars on control and xanthotoxin diets revealed only a single metabolite, which absorbed strongly at 325 nm. This was isolated and purified by reverse-phase HPLC after chloroform and ethyl acetate extraction (Figure 4) and found to have a molecular weight of 250 (Figure 5). Collection of the metabolite fraction from reverse-phase HPLC and subsequent drying by speed vacuum method yielded sufficient crystalline material to perform structure-elucidation analyses.
Isolation of metabolite in frass on reverse-phase HPLC column (C-18 column, Waters Novapak, 4.7 × 150 mm) after chloroform and ethyl acetate extraction. (A) Depicts the extract of frass from P. multicaudatus larvae fed leaves treated with acetone (control); (B) depicts the extract of frass from P. multicaudatus larvae fed leaves treated with xanthotoxin dissolved in acetone (treatment).
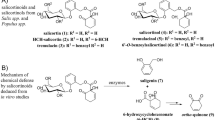
Identification of the isolated metabolite was accomplished by both MS–MS and NMR spectroscopy. The MS–MS spectrum of the metabolite shows that the coumarin ring is intact, but the furan ring is no longer closed (Figure 5). Proton NMR shows a singlet of three protons at δ = 3.98 ppm corresponding to the 8-methoxy group. The protons at C-3 and C-4 give rise to two doublets at δ = 6.22 and 7.85 ppm, respectively, whereas the isolated proton at C-5 shows a singlet at δ = 7.20 ppm. A singlet of two aliphatic protons at δ = 3.67 indicates the adjacent presence of a carboxylic acid resulting from metabolic cleavage of the furan ring (Figure 6). Thus, both MS–MS and NMR spectra suggest that the metabolite in frass is HCA, 6-(7-hydroxy-8-methoxycoumaryl)-acetic acid (Figures 5 and 6).
Discussion
Physiological and biochemical analyses confirm that P. multicaudatus, the putative ancestor to the glaucus group swallowtails, maintains the ancestral capacity to tolerate furanocoumarins, in contrast with the more derived species, P. canadensis and P. glaucus, which have a reduced ability to tolerate these compounds (Li et al., 2002) (Table 1). This conclusion is supported by larval hostplant associations; most section III Papilio species utilize Lauraceae as primary larval hostplants and Magnoliaceae as secondary larval hostplants. P. multicaudatus and P. glaucus are the only two section III species known to use Rutaceae as larval hostplants (Aubert et al., 1999).
As is the case for both Section II (Cohen et al., 1989) and Section III swallowtails (Li et al., 2003), xanthotoxin is a strong inducer of its own P450-mediated metabolism. When larvae were induced with 0.4% fresh weight xanthotoxin, the rate of xanthotoxin metabolism increased to over 5.23 nmol/min per midgut, more than 20 times the rate of its constitutive metabolism (0.23 nmol/min per midgut). Compared with the Section II black swallowtail, P. polyxenes, in which induction increased xanthotoxin metabolism 3- to 8-fold over constitutive levels at dietary concentrations of 0.1 to 0.5%, and the section III tiger swallowtail, P. glaucus, in which induction increased 30-fold over constitutive levels at dietary concentrations of 0.2% (Figure 2, Li et al. 2001), induced metabolism and maximum tolerance increased induction approximately 20-fold in P. multicaudatus at dietary concentrations of 0.2% fresh weight xanthotoxin, levels that are intermediate, as predicted. This intermediate inducibility and tolerance are consistent with the frequency with which furanocoumarins are encountered by these species. P. polyxenes, all of whose hosts contain furanocoumarins, maintains high constitutive levels of furanocoumarin metabolism that are only slightly inducible before maximum capacity is reached; P. multicaudatus, one-third of whose known hosts contain furanocoumarins, maintains intermediate constitutive levels of furanocoumarin metabolism that are substantially inducible; and P. glaucus and P. canadensis, which rarely and never encounter furanocoumarins in their respective diets, maintain vanishingly low levels of constitutive xanthotoxin metabolism that are highly inducible if furanocoumarins are encountered (Li et al., 2003).
Like the other more generalized section III swallowtails, P. multicaudatus larvae can effectively metabolize not only xanthotoxin, but six other furanocoumarins, including linear and angular forms. In the section III swallowtails, this ability to metabolize a diverse array of furanocoumarins is attributable in part to a greater number of furanocoumarin-inducible P450 genes, each of which has broad substrate specificity (Li et al., 2003). CYP6B4, CYP6B17, and CYB6B2, which are all furanocoumarin-inducible in P. glaucus, and CYP6B26, which is furanocoumarin-inducible in P. canadensis (Li et al., 2001), have overlapping substrate specificities, and all can metabolize both linear and angular furanocoumarins (Li et al., 2003). In contrast, in the furanocoumarin specialist P. polyxenes, the enzyme CYP6B1 (Cohen et al., 1992) is furanocoumarin-inducible, but it is highly specialized and can metabolize angular furanocoumarins only to a limited extent and cannot metabolize imperatorin, a linear furanocoumarin. Whether P. multicaudatus owes its tolerance to furanocoumarins to multiple furanocoumarin-inducible P450s with broad substrate specificities, like the other glaucus group swallowtails, remains to be determined.
Some insight into the nature of the metabolic transformations that contribute to P. multicaudatus furanocoumarin tolerance can be gained by an examination of the structure of the metabolites produced. Only a single metabolite was isolated and identified from frass. The metabolite, HCA (Figures 5, 6, and 7), likely arises as a result of cleavage of the furan ring at the 2′–3′ position, because the electron density at that position is higher than at the 3–4 position, and oxidative pathways usually prefer electron-rich π bonds (Schmid et al., 1980). This metabolite has also been found in frass of P. polyxenes as well as in the noctuid generalist Spodoptera frugiperda after xanthotoxin consumption. Both of these species produce a second metabolite, 6-(7-hydroxy-8-methoxycoumaryl)-hydroxyacetic acid (HCHA) (Ivie et al., 1983); HCHA constitutes the sole xanthotoxin metabolite in frass of the oecophorid caterpillar Depressaria pastinacella, which feeds exclusively on furanocoumarin-containing species in the apiaceous genera Pastinaca and Heracleum (Nitao et al., 2003). Collectively, these findings suggest that the furan ring is an important site of metabolic alteration of xanthotoxin in Lepidoptera. In this context, we suggest that P450-mediated epoxidation at the 2′–3′ position on the furan ring is the first step of xanthotoxin metabolism in P. multicaudatus (Figure 7), as is postulated to occur in P. polyxenes, S. frugiperda (Ivie et al., 1983), and D. pastinacella (Ivie et al., 1983; Nitao et al., 2003).
Formation of an epoxide as part of a metabolic transformation presents a toxicological challenge to organisms. In general, epoxides are highly reactive, so the epoxide metabolites of furanocoumarins, if they accumulate in any quantity, may seriously interfere with normal physiological function. In mammals, this problem is resolved by use of rapid conjugation of xanthotoxin with glucuronic acid, glutathione, and sulfate to increase their stability and hydrophilicity (Hayes and Pulford, 1995), for ultimate transport and excretion via ATP-binding cassette (ABC) transporter genes (Dean and Annilo, 2005). To date, no conjugates of xanthotoxin or any other furanocoumarins have been found in insects. The widespread occurrence of metabolite products consistent with epoxidation in Lepidoptera that encounter furanocoumarins suggests that conjugation may play a more important role in detoxification and hostplant specialization than has hitherto been realized.
References
Aubert, J., Legal, L., Descimon, H., and Michel, F. 1999. Molecular phylogeny of swallowtail butterflies of the tribe Papilionini (Lepidoptera: Papilionidae). Mol. Phylogenet. Evol. 12:156–167.
Berenbaum, M. R. 1983. Coumarins and caterpillars: a case for coevolution. Evolution 37:163–179.
Berenbaum, M. R. 1995. Chemistry and oligophagy in the Papilionidae, pp. 27–38, in J. M. Scriber (ed.). Swallowtail Butterflies: Ecology and Evolutionary Biology. Scientific Publishers, Gainesville.
Berenbaum, M. R., Favret, C., and Schuler, M. A. 1996. On defining “key innovations” in an adaptive radiation: cytochrome P450s and Papilionidae. Am. Nat. 148:S139–A155.
Caterino, M. S. and Sperling, F. A. H. 1999. Papilio phylogeny based on mitochondrial cytochrome oxidase I and II genes. Mol. Phylogenet. Evol. 11:122–137.
Cohen, M. B., Berenbaum, M. R., and Schuler, M. A. 1989. Induction of cytchrome P450-mediated detoxification of xanthotoxin in the black swallowtail. J. Chem. Ecol. 15:2347–2354.
Cohen, M. B., Schuler, M. A., and Berenbaum, M. R. 1992. A host-inducible cytochrome P-450 from a host-specific caterpillar: molecular cloning and evolution. Proc. Natl. Acad. Sci. USA 89:10920–10924.
Dean, M. and Annilo, T., 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 6:123–142.
Ehrlich, P. R. and Raven, P. H. 1964. Butterflies and plants: a study in coevolution. Evolution 18:586–608.
Feeny, P. 1992. The evolution of chemical ecology: contributions from the study of herbivorous insects, pp. 1–44, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores Their Interactions with Secondary Plant Metabolites, Vol. 2. Academic Press, New York.
Hagen, R. H. and Scriber, J. M. 1991. Systematics of Papilio glaucus and P. troilus species groups (Lepidoptera: Papilionidae): inferences and allozymes. Ann. Entomol. Soc. Am. 84:380–395.
Hayes, J. D. and Pulford, D. J. 1995. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 30:445–460.
Ivie, G. W., Bull, D. L., Beier, R. C., Pryor, N. W., and Oertli, E. H. 1983. Metabolic detoxification: mechanism of insect resistance to plant psoralens. Science 221:374–376.
Li, W., Schuler, M. A., and Berenbaum, M. R. 2001. Molecular analysis of multiple CYP6B genes from polyphagous Papilio species. Insect Biochem. Mol. Biol. 31:999–1011.
Li, W., Petersen, R. A., Schuler, M. A., and Berenbaum, M. R. 2002. CYP6B cytochrome P450 monooxygenases from Papilio canadensis and Papilio glaucus: potential contributions of sequence divergence to host plant associations. Insect Mol. Biol. 11:543–551.
Li, W., Schuler, M. A., and Berenbaum, M. R. 2003. Diversification of furanocoumarin-metabolizing cytochrome P450 monooxygenases in two papilionids: Specificity and substrate encounter rate. Proc. Natl. Acad. Sci. USA 100:14593–14598.
Munroe, E. 1961. The classification of the Papilionidae (Lepidoptera). Can. Entomol., Suppl. 17:1–51.
Murray, R. D. H., Mendez, J., and Brown, S. A. 1982. The Natural Coumarins: Occurrence, Chemistry, and Biochemistry. John Wiley & Sons, Bristol, 702 pp.
Nitao, J. K., Berhow, M., Duval, S. M., Weisleder, D., Waughn, S. F., Zangerl, A., and Bernenbaum, M. R. 2003. Characterization of furanocoumarin metabolites in parsnip webworm, Depressaria pastinacella. J. Chem. Ecol. 29:671–682.
Raubenheimer, D. and Simpson, S. J. 1992. Analysis of covariance: an alternative to nutritional indices. Entomol. Exp. Appl. 62:221–231.
Reed, R. D. and Sperling, F. A. H. 1999. Interaction of process partitions in phylogenetic analysis: An example from the swallowtail butterfly genus Papilio. Mol. Biol. Evol. 16:286–297.
Schmid, J., Prox, A., Reuter, A., Zipp, H., and Koss, W. H. 1980. The metabolism of 8-methoxypsoralen in man. Eur. J. Drug Metab. Pharmacokinet. 5:81–92.
Scriber, J. M. 1995. Overview of swallowtail butterflies: taxonomic and distributional latitudes, pp. 3–8, in J. M. Scriber, Y. Tsubaki, and R. C. Lederhouse (eds.). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publ., Gainesville.
Scriber, J. M., Lederhouse, R., and Hagen, R. 1991. Foodplant and evolution within P. glaucus and P. troilus species groups (Lepidoptera: Papilionidae), pp. 341–373, in P. W. Price, T. M. Lewinsohn, and W. W. Benson (eds.). Plant–Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons, New York.
Sperling, F. A. H. 1993. Mitochondrial DNA variation and Haldane's rule in the Papilio glaucus and P. troilus species group. Heredity 71:227–233.
Sperling, F. A. H. and Harrison, R. G. 1994. Mitochondrial DNA variation within and between species of the Papilio machaon group of swallowtail butterflies. Evolution 48:408–422.
Vane-Wright, R. I., Raheem, D. C., Cieslak, A., and Vogler, A. P. 1999. Evolution of the mimetic African swallowtail butterfly Papilio dardanus: Molecular data confirm relationships with P. phorcas and P. constantinus. Biol. J. Linn. Soc. Lond. 66:215–229.
Waldbauer, G. P. 1968. The consumption and utilization of food by insects. Adv. Insect Physiol. 5:229–288.
Yagi, T., Sasaki, G., and Takebe, H. 1999. Phylogeny of Japanese papilionid butterflies inferred from nucleotide sequences of mitochondrial ND5 gene. J. Mol. Evol. 48:42–48.
Zakharov, E. V., Caterino, M. S., and Sperling, F. A. H. 2004. Molecular phylogeny, historical biogeography, and divergence time estimates for swallowtail butterflies of the genus Papilio (Lepidoptera: Papilionidae). Syst. Biol. 53:193–215.
Acknowledgments
The authors gratefully acknowledge the help of Dr. Furong Shun in MS–MS analysis of the metabolite. This work was supported by NSF IBN0212242 to MRB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, W., Berhow, M.A., Zangerl, A.R. et al. Cytochrome P450-Mediated Metabolism of Xanthotoxin by Papilio multicaudatus . J Chem Ecol 32, 523–536 (2006). https://doi.org/10.1007/s10886-005-9018-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-9018-3