Abstract
We determined the quantity and chemical composition of cuticular hydrocarbons of different strains, sexes, and ages of buffalo flies, Haematobia exigua. The quantity of cuticular hydrocarbons increased from less than 1 μg/fly for newly emerged flies to over 11 μg/fly in 13-d-old flies. The hydrocarbon chain length varied from C21 to C29, with unbranched alkanes and monounsaturated alkenes the major components. Newly emerged flies contained almost exclusively C27 hydrocarbons. Increasing age was accompanied by the appearance of hydrocarbons with shorter carbon chains and an increase in the proportion of alkenes. 11-Tricosene and 7-tricosene were the most abundant hydrocarbons in mature H. exigua. Cuticular hydrocarbons of H. exigua are distinctly different from those of horn flies, Haematobia irritans. The most noticeable differences were in the C23 alkenes, with the major isomers 11- and 7-tricosene in H. exigua and (Z)-9- and (Z)-5-tricosene in H. irritans, respectively. Cuticular hydrocarbon analysis provides a reliable method to differentiate the two species, which are morphologically difficult to separate. The differences in cuticular hydrocarbons also support their recognition as separate species, H. exigua and H. irritans, rather than as subspecies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The buffalo fly, Haematobia exigua de Meijere (Diptera: Muscidae) abounds in the Oriental and Australian regions (Seddon, 1967; Williams et al., 1985). H. exigua is an obligate blood feeder spending most of its time on the host (MacQueen and Doube, 1988) and constitutes one of the major animal health problems to dairy and beef producers in northern Australia. The horn fly, Haematobia irritans (L.) a close relative of H. exigua, is found in the Americas, North Africa, and Europe, and is also considered a major arthropod pest of pastured cattle in the US and Canada (Drummond et al., 1986; Kunz, 1986).
Morphological differentiation of the adult fly species is difficult, and Pont (1973) indicated that the various larval stages were probably indistinguishable. While Zumpt (1973) recognized the two as subspecies of H. irritans, Skidmore (1985) subsequently recognized them as two separate species, H. exigua and H. irritans, although acknowledging their close relationship. Species and subspecies status are currently being applied to these flies by various authors. One approach to species identification that could clarify this situation is cuticular hydrocarbon profiling (Lockey, 1991), which has been successfully applied to a number of problem species (Carlson et al., 1993; Page et al., 1997; Brown et al., 1998; Broza et al., 2000; Howard et al., 2003). The cuticular hydrocarbons of H. irritans have been identified (Mackley et al., 1981), with the major components being odd-numbered, straight-chain C21–C29 saturated hydrocarbons and monounsaturated C23, C25, and C27 alkenes. Double bond positions were established through oxidative cleavage followed by gas chromatography-mass spectrometry (GC-MS) of the resulting aldehydes.
In this study, we report the identification of H. exigua cuticular hydrocarbons and variations in their composition and quantity depending on the sex and age of the fly. We employ a more elegant and direct route to the determination of double bond positions through the formation and GC-MS analysis of dimethyl disulfide (DMDS) adducts (Scribe et al., 1988; Carlson et al., 1989). Considering the morphological and behavioral similarities between H. irritans and H. exigua, the resulting information is valuable and useful for taxonomic purposes.
Methods and Materials
Flies
H. exigua were obtained from a colony of a field strain at the DPI Oonoonba Veterinary Laboratory, Townsville (DPI strain), and from the closed laboratory colony at CSIRO Entomology, Indooroopilly (CSIRO strain), Queensland, Australia. Both colonies were cultured on bovine hosts (Stegeman et al., 1996), and the CSIRO strain flies were collected from cattle kept in an insectary. Pupae submitted from Townsville were kept in cardboard cylinders lined with filter paper at 27°C and 70–80% RH, and emerged flies were fed with bovine blood (Anderson, 1995). H. irritans were obtained from the colony of Prof. Butler at the University of Florida, Gainesville, FL, USA.
Hydrocarbon Extraction
One ml of hexane (UltimAR, Mallinckrodt) containing 5 μg n-tetracosane [internal standard (IS)] was added to 20 flies that had been immobilized (−20°C or carbon dioxide). After standing for 5 min, the hexane solution was passed through a silica gel plug (1 g) confined in a Pasteur pipette that was then washed with 5 × 1 ml hexane. The combined hexane solutions were dried with anhydrous sodium sulfate, filtered, evaporated to dryness, and reconstituted with 100 μl hexane. Single fly extractions were carried out analogously using 20-fold lower IS concentration and reconstituted with 5 μl hexane.
Chemicals
(Z)-9-Tricosene was obtained from ISP Fine Chemicals (Columbus, OH, USA).
Methylthiolation of Alkenes
DMDS adducts were prepared according the method of Carlson et al. (1989) with a reaction time of 16 hr at 40°C.
Analysis and Identification
Gas chromatography (GC) was carried out on an HP5890 gas chromatograph equipped with a capillary column (DB-5, J&W; 30 m × 0.25 mm, 0.25 μm film thickness) using nitrogen as the carrier gas (column pressure, 100 kPa) and with a flame ionization detector (FID). The oven temperature was increased (5°C/min) from 200 to 260°C (280°C for DMDS adducts) where it was maintained for 5 min. Data acquisition and processing were performed with an HP3392A integrator with peak area used for the quantitation of components (relative to tetracosane). Coupled GC-MS was performed on an HP5890 GC (same column as described above) with a VG Trio 2000 mass spectrometer. Ionization was by electron impact (EI), and the total ion current was accumulated from m/z 35 to 400 (500 for DMDS adducts) over 1 sec. Selected ion mass spectrometry of high-intensity mass fragments was used for the detection or confirmation of components at low abundance. The stereochemistry of (Z)-9-tricosene was confirmed by comparison of its GC retention time with the standard sample.
Results
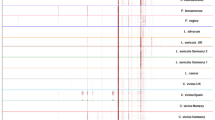
Cuticular hydrocarbon extracts from newly emerged, 2–3, and 13-d-old female H. exigua were analyzed by GC-FID (Figure 1). Relevant hydrocarbon peaks are designated with a reference number (for key, see Table 1). All alkanes were completely resolved from the alkenes. At least partial, but often complete, separation of homologous alkenes with double bonds at 5, 7, and 9 positions was achieved, but the 9 and 11 alkenes coeluted. The hydrocarbon chain length varied from C21 to C29, with a distinct shift in relative quantities to shorter hydrocarbons with increasing age of the flies (Figure 1, Table 1).
Gas chromatography-flame ionization detector traces of cuticular hydrocarbon extracts from female Haematobia exigua; Top: newly emerged; center: 2–3 d old; bottom: 13 d old (IS = internal standard, UK = unknown; for assignments of reference numbers, see Table 1).
From newly emerged flies (DPI strain), only small quantities of hydrocarbons (<1 μg) were extracted, and these were mostly C27 alkenes and alkanes (Table 1). The flies, which were kept in cardboard cylinders and maintained on bovine blood, contained larger amounts of hydrocarbons at older ages, reaching over 11 μg per female fly at 13 d. With increasing age (13 d), the DPI strain had a higher proportion of shorter carbon chains (79% C23) and a higher proportion of alkenes (60%). The CSIRO strain, which had been kept on cattle postemergence, contained at the age of 3 d a similar amount of cuticular hydrocarbons to the DPI strain of the same age, but its hydrocarbon pattern was more similar to that of 13-d-old DPI flies. Eighty and twenty percent of the CSIRO (3 d) and DPI flies (13 d) were gravid, respectively.
Unbranched, odd-numbered C21 to C29 alkanes were recovered from most H. exigua. Heptacosane was the most abundant alkane in young flies, whereas tricosane was found in the highest quantities in mature flies. A series of methyl branched C27 alkanes was detected only in newly emerged flies.
The position of the double bond in C23 to C29 monounsaturated alkenes was established through derivatization with DMDS, GC-MS analysis of the adducts, and correlation of GC retention times of parent compounds and adducts. The presence of abundant fragments (often at or close to 100% abundance) corresponding to cleavage at the original site of unsaturation, now carrying thiomethyl groups at each end, defined the location of the double bond. For instance, four adducts of C23 alkenes showing intense ions at m/z 201/215, 173/243, 145/271, and 117/299 indicated the presence of 11-, 9-, 7-, and 5-tricosene, respectively. The most abundant hydrocarbon in mature (3 and 13 d old) female H. exigua was 11-tricosene, followed by 7-tricosene, which together constituted about 90% of all alkenes. 7-Tricosene was the most abundant hydrocarbon in mature (3 d old) male CSIRO flies. Other C23 alkenes included 5-tricosene and (Z)-9-tricosene, with the latter being detected only in the CSIRO strain in trace quantities. In the early stages of the DPI flies' development, C27 and C25 alkenes dominated the extract, with 7- and 9-heptacosene and 7-pentacosene as the major components.
The cuticular hydrocarbon content of individual H. exigua was consistent in quantity and composition within a sex/age group (Table 2). The standard errors of the means of the major components from replicated hydrocarbon extraction from individual flies were small (typically 5%). Furthermore, the mean values from single fly extractions closely match the normalized values from the batch extractions.
Single fly extracts of female and male H. irritans yielded as major components (Z)-9-tricosene, 5-tricosene, 9-pentacosene, pentacosane, 9-heptacosene, and heptacosane (Table 1).
Discussion
Analyses of H. irritans cuticular hydrocarbons were consistent with those published previously (Mackley et al., 1981; Milstrey, 1983), but were obtained in a more efficient fashion. This was a consequence of the superior resolving power of modern capillary GC columns compared with packed columns, avoiding the necessity for prior column-chromatographic separation of alkenes from alkanes. Structure elucidation of the hydrocarbons was achieved on the basis of GC retention times, methylthiolation, and mass spectrometry of the parent compounds and their adducts. Resolution of all unsaturated positional isomers was obtained with the exception of the 9 and 11 isomers, where the parent compounds as well as the DMDS adducts partially coeluted. However, these isomers could be distinguished by selected ion monitoring of the DMDS adducts, which showed clear differences in the major fragments obtained by EI ionization. The stereochemistry of the double bond could be assigned unambiguously only for (Z)-9-tricosene, the only unsaturated reference hydrocarbon that we were able to obtain. It is likely that all other alkenes share the Z configuration, based on their GC retention times and precedence from the structures of cuticular hydrocarbons from related flies (Mackley et al., 1981).
The cuticular hydrocarbon profile from newly emerged H. exigua was similar to the profile reported from a colony of newly emerged H. irritans (Milstrey, 1983). Normal C25 and C27 and methyl branched-C27 hydrocarbons provided the bulk of the alkanes, and 9-heptacosene and 9-pentacosene largely made up the alkenes. A few additional components not detected previously in H. irritans (Milstrey, 1983) were observed in H. exigua, including 9-nonacosene, 5- and 7-heptacosene, and 7-pentacosene; however, they were present only in small or trace quantities. Branched C27 alkanes were present in newly emerged H. exigua and H. irritans, but were not detected in any other age category in either species. The quantity of alkanes and alkenes extracted was lower in newly emerged H. exigua than in H. irritans (Milstrey, 1983). Similar to H. irritans, there was no marked difference in the composition of cuticular hydrocarbons between newly emerged female and male H. exigua (Table 1), although the quantity of the methyl branched C27 alkanes was lower in male flies.
Significant qualitative and quantitative changes in hydrocarbon composition were observed 2–3 d after the emergence of H. exigua. Shorter carbon chains were more prominent and alkenes constituted about 80% of the total hydrocarbons, compared to less than 35% in newly emerged flies (Table 1). Similar age-related changes in hydrocarbon content have also been observed for H. irritans (Milstrey, 1983). However, qualitative differences between the composition of unsaturated hydrocarbons in H. exigua and H. irritans were obvious. Extraction of 2- to 3-d-old H. exigua kept in a cardboard cylinder since emerging (DPI strain, Table 1) yielded 5- and 7-tricosene, 7- and 9-pentacosene, 7- and 9-heptacosene (major component) and 9-nonacosene. Haematobia irritans (3–4 d old) yielded (Z)-5- and (Z)-9-tricosene (major components), (Z)-9-pentacosene and (Z)-9-heptacosene (Mackley et al., 1981; Milstrey, 1983), and 9-nonacosene (this study, see below). Thus, none of the (Z)-9-tricosene, which was the major alkene component in H. irritans (except newly emerged), was found in the DPI H. exigua. Conversely, 7-tricosene, 7-pentacosene, and 7-heptacosene were detected in H. exigua but not in H. irritans. The extraction of 13-d-old female DPI flies yielded a total of 11 μg cuticular hydrocarbons per fly, with the alkenes comprising 60% of these. Further evidence for the shift to shorter carbon chain length with age is provided by the detection of 11-tricosene and 11-pentacosene in the extracts of 13-d-old females. 11-Tricosene was the major component and, together with 7-tricosene and tricosane, contributed 79% to the total hydrocarbons. An inspection of a subsample of this population indicated that 20% of the female flies were gravid.
Extraction of CSIRO H. exigua, kept on cattle for 3 d postemergence, yielded a similar amount of hydrocarbons to the caged flies of the same age (Table 1). However, the resultant hydrocarbon profile was much more similar to that of 13-d-old DPI flies. All mature H. exigua had predominantly C23 alkenes, with 11- and 7-tricosene as the most abundant isomers. The 3-d-old female CSIRO flies were 80% gravid, and the close resemblance of their hydrocarbon profiles to those of the DPI flies (13 d old, 20% gravid) indicates that the observed change in the hydrocarbon profile is related to developments other than the attainment of sexual maturity. The CSIRO flies also showed quantitative differences between the sexes, with 11-tricosene and 7-tricosene as the major components in female and male H. exigua, respectively. However, results from this study may not fully reflect sexual dimorphism, as the flies were collected from mixed-sex populations, unlike the H. irritans study where pupae were individually contained (Milstrey, 1983). Transfer of cuticular hydrocarbons between female and male flies through mating has been documented (Scott et al., 1988). The CSIRO fly extracts also contained small amounts of (Z)-9-tricosene, the major alkene component of H. irritans. However, (Z)-9-tricosene was only detected in H. exigua with selected ion mass spectrometry, which provided higher sensitivity than total ion current or flame ionization detectors.
Variability in the quantity of major cuticular hydrocarbons extracted from individual H. exigua was low (Table 2). All major components were detected in all samples, and the standard errors of the means were all below 10% and typically around 5%. Thus, single fly extractions provide hydrocarbon profiles that are characteristic of the population with a reasonable degree of certainty.
To complete our study, we extracted one female and one male H. irritans and determined the structure of the unsaturated hydrocarbons by methylthiolation followed by GC-MS. We confirmed (except for the stereochemistry) the previous findings, obtained by ozonolysis of the parent components, that (Z)-9-tricosene, (Z)-5-tricosene, (Z)-9-pentacosene, pentacosane, (Z)-9-heptacosene, and heptacosane are the major cuticular hydrocarbons found in H. irritans (Mackley et al., 1981; Milstrey, 1983). Our H. irritans extracts also contained small quantities of 9-nonacosene, which was not previously reported, probably due to the lower sensitivity resulting from packed rather than capillary GC columns. Although the ages of the H. irritans samples were unknown, the resulting hydrocarbon profile indicated that they were at least a few days old.
Results presented in this study and comparison with previous reports (Mackley et al., 1981; Milstrey, 1983) demonstrate that cuticular hydrocarbon profiles of H. exigua and H. irritans are almost identical in newly emerged flies, but can readily be distinguished in flies older than 2 d (Table 3). This dissimilarity is independent of sex, feeding regimen (in vivo or in vitro), or sexual maturity. For chemotaxonomic differentiation, a simple analysis of C23 alkenes of flies aged 2 d or more provides an unambiguous identification, with each species possessing unique positional isomers (rows in bold italics in Table 3). The major alkenes were the 11- and 7-tricosene in H. exigua (both not detected in H. irritans), and the (Z)-9- and (Z)-5-tricosene in H. irritans. Such a chemotaxonomic differentiation is more reliable and robust than the difficult differentiation based on morphological characters (Zumpt, 1973).
Cuticular hydrocarbons are utilized by insects as species recognition cues (Carlson et al., 1998; Howard et al., 2003). Clear differences in the cuticular hydrocarbon profiles of H. exigua and H. irritans thus provide support for their recognition as separate species. Additional cuticular hydrocarbon profiling and molecular, genetic, morphological, and cross-breeding studies of specimens of both species across their extensive distributions would be desirable to further explore species integrity.
In summary, we determined the qualitative and quantitative composition of cuticular hydrocarbons of different strains, sexes, and ages of H. exigua. We demonstrated that the hydrocarbon profile of mature H. exigua unambiguously differs from that of H. irritans, and that by determining the identity of a few key compounds in cuticular hydrocarbon extracts, fly species can be distinguished.
References
J. M. E. Anderson (1995) ArticleTitleCulturing Haematobia exigua de Meijere (Diptera: Muscidae) in the laboratory J. Aust. Entomol. Soc. 1 17–21
W. V. Brown R. Morton M. J. Lacey J. P. Spradbery R. J. Mahon (1998) ArticleTitleIdentification of the geographical source of adults of the Old World screw-worm fly, Chrysomya bezziana Villeneuve (Diptera : Calliphoridae), by multivariate analysis of cuticular hydrocarbons Comp. Biochem. Physiol., Part B Biochem. Mol. Biol. 119 391–399
M. Broza J. L. Nation K. Milne J. Harrison (2000) ArticleTitleCuticular hydrocarbons as a tool supporting recognition of Gryllotalpa tali and G. marismortui (Orthoptera: Gryllotalpidae) as distinct species in Israel Ann. Entomol. Soc. Am. 93 1022–1030
D. A. Carlson C. Roan R. A. Yost J. Hector (1989) ArticleTitleDimethyl disulfide derivatives of long chain alkenes, alkadienes and alkatrienes for gas chromatography/mass spectrometry Anal. Chem. 61 1564–1571 Occurrence Handle10.1021/ac00189a019
D. A. Carlson S. K. Milstrey S. K. Narang (1993) ArticleTitleClassification of tsetse flies Glossina spp. (Diptera, Glossinidae) by gas chromatographic analysis of cuticular components Bull. Entomol. Res. 83 507–515
D. A. Carlson I. I. Offor S. Elmessoussi K. Matsuyama K. Mori J. M. Jallon (1998) ArticleTitleSex pheromone of Glossina tachinoides—isolation, identification, and synthesis J. Chem. Ecol. 24 1563–1575 Occurrence Handle10.1023/A:1020967918594
R. O. Drummond J. E. George S. E. Kunz (1986) Control of Arthropod Pests of Livestock: A Review of Technology CRC Press Boca Raton, FL
R. W. Howard L. L. Jackson H. Banse M. W. Blows (2003) ArticleTitleCuticular hydrocarbons of Drosophila birchii and D. serrata: Identification and role in mate choice in D. serrata J. Chem. Ecol. 29 961–976 Occurrence Handle10.1023/A:1022992002239 Occurrence Handle12775155
S. E. Kunz (1986) Integrated pest management and Dipteran pests in the new world J. Howell (Eds) Parasitology—Quo Vadit Australian Academy of Science Canberra 659–664
K. Lockey (1991) ArticleTitleInsect hydrocarbon classes: implications for chemotaxonomy Insect Biochem. 21 91–97 Occurrence Handle10.1016/0020-1790(91)90068-P
J. W. Mackley D. A. Carlson J. F. Butler (1981) ArticleTitleIdentification of the cuticular hydrocarbons of the horn fly and assays for attraction J. Chem. Ecol. 7 669–683 Occurrence Handle10.1007/BF00990300
A. Macqueen B. M. Doube (1988) ArticleTitleEmergence, host finding and longevity of adult Haematobia irritans exigua de Meijere (Diptera: Muscidae) J. Aust. Entomol. Soc. 27 167–174
Milstrey, E. G. 1983. Variation in horn fly, Haematobia irritans (L.) hydrocarbons and the effect of altering pheromone levels in the field on wild horn fly populations. M.Sc. Dissertation, University of Florida, Gainesville.
M. Page L. J. Nelson G. J. Blomquist S. J. Seybold (1997) ArticleTitleCuticular hydrocarbons as chemotaxonomic characters of pine engraver beetles Ips spp. in the grandicollis subgeneric group J. Chem. Ecol. 23 1053–1099 Occurrence Handle10.1023/B:JOEC.0000006388.92425.ec
A. C. Pont (1973) ArticleTitleStudies on Australian Muscidae (Diptera). IV. A revision of the subfamilies Muscinae and Stomoxyinae Aust. J. Zool. 21 129–296
D. Scott R. C. Richmond D. A. Carlson (1988) ArticleTitlePheromones exchanged during mating: a mechanism for mate assessment in Drosophila Anim. Behav. 36 1164–1173
P. Scribe J. Guezennec J. Dagaut C. Pepe A. Saliot (1988) ArticleTitleIdentification of the position and the stereochemistry of the double bond in monounsaturated fatty acid methyl esters by gas chromatography/mass spectrometry of dimethyl disulfide derivatives Anal. Chem. 60 928–931 Occurrence Handle10.1021/ac00160a019
H. R. Seddon (1967) Diseases of domestic animals in Australia. Part 2: Arthropod infestations (flies, lice and fleas) Department of Health, Commonwealth of Australia Canberra
P. Skidmore (1985) The Biology of the Muscidae of the world Dr. W. Junk Dordrecht
D. A. Stegeman R. S. Tozer R. W. Sutherst (1996) ArticleTitleProcedures for mass rearing the buffalo fly, Haematobia irritans exigua de Meijere (Diptera: Muscidae) Aust. J. Entomol. 35 77–79
J. D. Williams R. W. Sutherst G. F. Maywald C. T. Petherbridge (1985) ArticleTitleThe southward spread of buffalo fly (Haematobia irritans exigua) in eastern Australia and it survival through a severe winter Aust. Vet. J. 62 367–369 Occurrence Handle3834900
F. Zumpt (1973) The Stomoxyine Biting Flies of the World. Diptera: Muscidae. Taxonomy, biology, economic importance and control measures Gustav Fischer Verlag Stuttgart
Acknowledgments
The authors are grateful to W. M. Doherty (DPI&F), Dr. D. Kemp (CSIRO) and Prof. J. F. Butler (University of Florida) for provision of flies and Meat & Livestock Australia for partial funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urech, R., Brown, G.W., Moore, C.J. et al. Cuticular Hydrocarbons of Buffalo Fly, Haematobia exigua, and Chemotaxonomic Differentiation from Horn Fly, H. irritans. J Chem Ecol 31, 2451–2461 (2005). https://doi.org/10.1007/s10886-005-7112-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-7112-1