Abstract
Research on host selection by bark and wood boring insects has concentrated on flight orientation behavior. Less is known of the factors that govern the steps successive to host landing. Here, we discuss chemical factors involved in host acceptance by bark beetles and a new microassay. Adult males and females of Ips typographus were offered an artificial diet treated with various concentrations of different plant-derived compounds (host terpenes and nonhost compounds) in a no-choice mode. Beetles were tested individually in a glass tube for 4 hr, and the length of feeding was measured and compared to a control (diet with only solvent). The first effect was diet rejection, especially when nonhost compounds were tested at high concentrations. Most compounds reduced feeding, in proportion to concentration. Females were fed more readily than males after addition of both host and nonhost compounds. Diet removal was significantly affected by all the tested factors (sex, compound, dose) as well as by their interactions. With increased concentrations, males were more responsive than females to antifeedants, as all compounds (except juglone) showed clear sex differences of diet consumption. 3-Octanol, 1-hexanol, and a Green Leaf Volatile (GLV)-blend (three C6 alcohols) showed the strongest antifeedant effects, which started at a low dose (0.1%) and had a low Effective Dose 50 (ED50, 0.3–1%). In contrast, host monoterpenes, limonene and α-pinene, inhibited feeding at high doses (10–30%) only with ED50 > 10%. The highest Antifeedant Indexes (AFIs) were shown by verbenone, carvone, and 1-hexanol (0.90–1.00). Both host and nonhost compounds inhibited feeding at some concentration. No significant stimulation of feeding by any host compound at concentrations reported in the literature as optimal were found, with the possible exception of α-pinene at low concentrations in females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Host selection by bark beetles (Coleoptera: Scolytidae) is mainly individual and is governed by long-range attractive signals such as pheromones (Wood, 1982; Schlyter and Birgersson, 1999). After a searching period, bark beetles land on a host tree and begin feeding activity (Paynter et al., 1990). If the chosen tree is found unsuitable, then flight is resumed and the process is repeated (Byers, 1995; Wallin and Raffa, 2002). For many xylophagous insects, avoidance of nonhost tree species is due either to lack of nutritional compounds or to the detection of potentially toxic secondary metabolites (Agelopoulos et al., 1999). Decisions regarding oviposition are often discerning and include both host species’ recognition and the assessment of the host’s defensive reactions (Safranyik et al., 1975). Thus, the chemical composition of the medium is the last threshold to be overcome before colonization.
During host selection, both attractive and repellent signals may be followed. The former includes pheromones and kairomones, which act in a behavioral sequence (Wood, 1982; Raffa et al., 1993; Borden, 1997; Schlyter and Birgersson, 1999). The latter includes verbenone and angiosperm volatiles—such as nonhost volatiles (NHV)—which inhibit attraction to pheromones and kairomones in conifer-inhabiting Scolytidae (Borden, 1997; Zhang et al., 2000; Zhang and Schlyter, 2004). The use of NHV protects the potential host tree from being attacked, because the beetle does not recognize the substrate as suitable for reproduction.
The secondary plant metabolites important in insect host selection include mainly alkaloids, flavonoids, and terpenes (Frazier and Chyb, 1995), although quinones and phenols may also be important. Terpenes can either be repellent or attractive according to concentration or insect species. They can first increase then inhibit feeding activity once they have reached a toxic concentration (Reddemann and Schopf, 1996). Field bioassays indicate that different concentrations of bark monoterpenes (α-pinene and limonene) have different effects on the colonization rate of Ips typographus (L.) (Reddemann and Schopf, 1996). Uninfested trees contained lower quantities of monoterpenes compared to colonized trees. Trunks colonized by I. typographus were characterized by a concentration of α-pinene, ranging between 0.08 and 0.35 nmol mg−1 of fresh weight (Reddemann and Schopf, 1996). Similar results have also been obtained for American bark beetle species such as I. grandicollis (Eichhoff) for which α-pinene was not only attractive but also enhanced response to its pheromone (Erbilgin and Raffa, 2000). Consistent attraction to α-pinene is shown by the red turpentine beetle, Dendroctonus valens LeConte (Erbilgin and Raffa, 2000), although (−) α-pinene inhibits response to (+) α-pinene. Little is known about feeding deterrents in tree-inhabiting Coleoptera (Byers, 1995). Ascher et al. (1975) found that females of Scolytus rugulosus (Müller) [= mediterraneus (Eggers)] were deterred from feeding on peach twigs that had been dipped in hexa-methylditin. The number of I. pini (Say) entering a phloem-based medium decreases with increased concentrations of many monoterpenes (Wallin and Raffa, 2000). Evaluation of antifeedants (e.g., carvone) against the large pine weevil, Hylobius abietis L., has shown antifeedant effects of nonhost bark compounds in both sexes (Klepzig and Schlyter, 1999). A higher sensitivity to repellents, however, must be expected in the host-selecting sex. When responding to prelanding signals, males of I. typographus are more sensitive to verbenone (Schlyter et al., 1989) and NHV (Zhang and Schlyter, 2004), whereas females of S. rugulosus are more susceptible to hexa-methylditin than males (Ascher et al., 1975). In Europe, the spruce bark beetle I. typographus is a major mortality factor in mature spruce forests (Picea abies Karsten) (Christiansen and Bakke, 1988). Recent research on control strategies for the protection of conifers has focused on the use of antiattractive semiochemicals such as NHV from nonhost trees, mainly angiosperms (Zhang and Schlyter, 2004).
The aim of the present study was to develop a protocol for testing the feeding performances of bark beetles on an artificial diet, as well as the evaluation of antifeedant effectiveness of host and nonhost compounds on I.typographus.
Methods and Materials
Insect Breeding and Handling
Adults of I. typographus were removed daily from breeding cages kept in climatic rooms (25±1°C, RH = 70%). Specimens having emerged from colonized spruce logs (10-cm diameter, 30 cm long) were sexed (Schlyter and Cederholm, 1981) and starved at room temperature for 24 hr before being tested.
Pilot Studies on Artificial Diet and Tunnels
Preliminary assays were performed to find the best experimental protocol. Tests were carried out using narrow strips of fresh bark, cut from spruce logs and inserted into artificial tunnels consisting of transparent glass tubes (3-mm diameter, 30 mm long). Feeding activity could not be easily quantified, and bark characteristics (thickness, moistness, age, and amount of cork) were extremely variable. Later, an artificial agar-based diet-modified from Šimsek and Führer (1993) (composed of 87.6% water, 2% cellulose, 2.6% glucose, 4.3% starch, and 3.5% agar) was tested. Cellulose was obtained from dry spruce sawdust, whereas starch was added as reground maize flour. After a few minutes heating, the diet was poured into Petri dishes to a thickness of 13 mm; the diet was allowed to cool down for few minutes and was transferred by pressing the previously employed glass tubes into the cold diet (Figure 1), where it was left to dry. A direct estimate of the extent of adult host acceptance and feeding could be deduced from the length of diet removed. Because tunneling and diet removal does not necessarily mean feeding, during preliminary analyses several adults were dissected in order to detect ingested diet. The alimentary tract of all these previously starved insects contained diet particles.
Feeding Tests
Compounds used were grouped into two sets: those from nonhost trees, such as juglone, 3-octanol, 1-hexanol, and a blend of GLV, and those host compounds normally present in low (verbenone and carvone) or high (limonene and α-pinene) quantities in healthy host tissues (P. abies), such as limonene and α-pinene (Table 1). Different concentrations of each compound, diluted in ethyl acetate, were tested on adults of I. typographus (Table 1). Each concentration was tested individually on 20 adults (10 males and 10 females). A 10-μl solution of each compound (Table 1) or the solvent (blank) was added separately to the tube. The latter was kept open for 1 hr at 21°C for ventilation, thus allowing the solvent to completely evaporate. Subsequently, one beetle was inserted into each tube, closed with a plastic cap, and was allowed to feed on the diet for 4 hr under illumination. Previous tests have shown that 4 hr are sufficient for insects to tunnel through a diet. Each beetle was used only once. The amount of diet consumed was measured (mm) by using a graduated stereoscope. For each compound, 20 adults (10 males and 10 females) feeding in tubes containing only diet (solvent blank) were used as control.
Statistical Analysis
Data were analyzed by ANOVA to find differences between sexes, compounds, and their concentrations. Homogeneity of variance was tested using Cochran’s test, and when necessary, data were log-transformed [X′ = log (x + 1)] or arcsin-transformed (X′ = arcsin√P x ) to obtain homogeneous variances. Wherever significant differences occurred, Tukey’s honestly significant difference (HSD) multiple comparison test was applied for mean separation (Zar, 1984). Differences at P ≤ 0.05 were considered significant for ANOVA and for the Effective Threshold (ET) concentration, at which the amount of eaten diet becomes statistically lower than the control. For each compound and concentration, an Antifeedant Index (AFI) varying between −1 (attraction) and 1 (repulsion) (Klepzig and Schlyter, 1999) was calculated as follows: AFI = (C − T)/(C + T), where T = amount of diet consumed in the tested treatment and C = amount of diet consumed in the control. In order to compare the effect of active compounds, Effective Dose 50 (ED50) was calculated. The ED50 concentration was obtained from the linear regression of AFI against the concentration.
Results
Most tested chemicals decreased the feeding activity of adults of I. typographus, with an effect that was concentration-dependent. Sex was a factor both in diet acceptance and in feeding.
Diet Acceptance
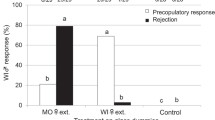
Nonhost compounds had a strong effect on diet acceptance, showing a high level of rejection. At high concentrations, a variable percentage of specimens did not feed at all (Figure 2), whereas in the controls (solvent blanks), all insects were fed. Independently from compound and concentration, diet acceptance was always higher in females than in males (Figure 2). In several cases, males showed a concentration dependence trend similar to that of females, although male rejection started at lower concentrations compared to females. Host compounds, such as α-pinene and limonene, always showed a high feeding frequency (Figure 2), and reduction in feeding started only at very high doses (30 and 10%, respectively).
Diet acceptance frequency in the sexes, estimated as the proportion of insects showing >0 mm removal of diet (feeding) in glass tunnels after 4 hr. Binomial 95% Confidence Intervals. Females: - - -•- - - and males: —□—. For clarity, values for males and females are shifted to the left and right, respectively. Tree symbols, dark or pale, indicate compound origin from angiosperm nonhost or gymnosperm host, respectively.
Diet Consumption
Diet removal was affected by all three of the considered factors: sex, dose, and compound (Table 2). Interactions among these factors were significant, indicating differences in concentration–response slopes due to both sex and compound.
Sex
Sex was the most important factor affecting consumption (Table 2). Males were more susceptible to antifeedants than females, and all compounds showed clear statistical differences between sexes, with the highest feeding responses found in females (Figure 3). Different compounds and doses had different effects on feeding responses of males and females (Table 2 and Figure 3). ED50 was always significantly higher in females, indicating that both host and nonhost compounds had a greater effect on male feeding (Table 3). Feeding performed on control (concentration = 0) showed no consistent difference between sexes (Figures 2 and 3).
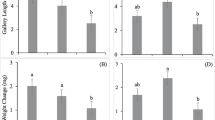
Linear regressions of Ips typographus feeding activity for each tested compound. Females: •, males: □. ED50: Effective Dose halving the feeding performances of the insects (indicated only for Verbenone as an example, see Table 3). For overall means per compound and sex, see Table 2. Data points with the same letter are not different by ANOVA on log(x + 1) followed by the Tukey’s honestly significant difference post hoc test at α = 0.05.
Dose
Concentration was the second most important factor affecting diet removal (Table 2). In general, all compounds showed an antifeedant effect that increased with dose, although this was always stronger in males (Figure 3). In some cases [e.g., (−) carvone, (−) verbenone, and GLV-blend], differences between sexes disappeared at high concentrations, as the effects were strong enough to reduce even female feeding activity (Figures 2 and 3). Compounds and dose showed a significant interaction (Table 2), thus establishing that different compounds are active at different concentrations (Figure 3). Furthermore, I. typographus showed a variable sensitivity to compounds, which became effective (ET) at different concentrations (Table 3). For males, the ET was reached at the lowest dose (0.1%) of many compounds.
Compound
Host compounds always had higher ET and ED50 values than nonhost compounds, indicating lower antifeedant activity. Host monoterpenes, such as α-pinene and limonene, showed low activity as antifeedants; α-pinene at low doses (<1%) acted as a weak stimulus for females (Figure 3), whereas limonene affected diet consumption in females at the highest concentrations only. Oxygenated host monoterpenes (carvone and verbenone) showed similar trends (Figure 3), both having a strong antifeedant effect with a relatively high AFI (Table 3), which depended upon concentration (Figure 4). Nonhost compounds are the more active (Table 3 and Figure 2), with the strongest effect by 3-octanol, which showed both the lowest ED50 value (1.2%) and the highest mean AFI (0.6). Moreover, feeding inhibition caused by nonhost compounds increased rapidly and in a nonlinear way with increasing concentrations, especially for males (Figure 3).
Relative Feeding (AFI)
In general, with the exception of α-pinene at low concentrations, mean AFI was always positive, indicating overall feeding inhibition (Table 3). AFI increased with compound concentration (Figure 4). High concentrations of carvone, verbenone, and 1-hexanol gave the highest AFI values (≈1), meaning total inhibition of feeding. In contrast, α-pinene and limonene showed low antifeedant indexes even at high concentrations. The moderately negative AFI values reported for α-pinene at several low concentrations (Figure 4) indicate a weak stimulating effect on feeding, especially females (Figure 3). At high concentrations (10 and 30%), most compounds showed strong antifeedant effects (AFI ≥ 0.5), whereas at low concentrations (1–3%), strong effects were obtained only for 1-hexanol, GLV-blend, and 3-octanol.
Discussion
Several studies have demonstrated that conifer phloem contains extractable compounds that elicit feeding behavior in bark beetles. Ips paraconfusus Lanier recognizes susceptible ponderosa pines after having entered the phloem (Elkinton et al., 1981). Outer bark extracts of the host pine species stimulate feeding behavior in both the southern pine beetle, Dendroctonus frontalis Zimmermann (Thomas et al., 1981) and the mountain pine beetle, D. ponderosae Hopkins (Raffa and Berryman, 1982). Feeding stimulants have been identified also for bark beetles attacking angiosperms such as Scolytus multistriatus (Marsham) and S. rugulosus (Doskotch et al., 1970; Levy et al., 1974). Literature data suggest that phloem metabolites are mainly responsible for feeding stimulation (Bedard, 1966; Elkinton et al., 1981; Byers, 1995). McNee et al. (2003) reported that several chemicals extracted from ponderosa pine phloem, such as stilbenes, ferulic acid gluciside, and sugars, neither stimulated nor reduced male feeding activity in I. paraconfusus. Nevertheless, few studies have tested the possible antifeedant effect of host extracts on barkbeetles. In the present study, an antifeedant effect, strongly dependent onsex, the concentration, and the origin (host or nonhost) of the compound, wasestablished.
No significant differences between sexes in antennal responses to NHV have been recorded previously (Zhang and Schlyter, 2004), although a higher antennal sensitivity (lower response threshold) would be expected for the host-selecting sex (the males in the case of I. typographus). In this respect, Dickens (1981) reported that the male antennae of I. typographus were 10 times more sensitive to α-pinene than females. Rudinsky et al. (1971) reported that α-pinene and limonene attracted I. typographus adults in a ratio that favored males. High concentrations of verbenone and NHV skew the sex ratio of I. typographus trap samples towards females (Schlyter et al., 1989; Zhang and Schlyter, 2004), being more repellent to males. It was thought that the verbenone released by tunneling males could counteract the effect of the aggregation pheromone and shift the attack to uninfested neighboring trees (Schlyter et al., 1989). Comparing male and female feeding responses, controls did not generally show significant differences between sexes. However, when exposed to nonhost compounds, male feeding was inhibited at relatively low concentrations, ranging between 1 and 3%. Nevertheless, high concentrations of verbenone, carvone, and 1-hexanol decrease female feeding responses as well.
Terpenoids play a fundamental role in host acceptance by conifer-inhabiting bark beetles (Byers, 1995). Limonene, α-pinene, and β-pinene are involved in preexisting and induced defenses against bark beetles (Baier et al., 1999). Compared to many other insects, conifer bark beetles are relatively immune to toxic terpenes, although they can still be lethal at high doses (Everaerts et al., 1988). Some experiments suggest that some monoterpenes are still sufficiently toxic to bark beetles as to significantly influence their ecology. Studies by Sturgeon (1979) of the P. ponderosa/D. brevicomis LeConte association suggest that tree resistance is linked to higher limonene contents. In a study similar to ours, Wallin and Raffa (2000) found that the number of I. pini that entered a phloem-based medium decreased with increased concentrations of most monoterpenes. In particular, the total length of tunnels excavated in the medium decreased with increasing concentrations of α-pinene and limonene (Wallin and Raffa, 2002). Regarding host-tree status, high monoterpene concentrations correspond to trees that have begun to respond to an attack, whereas lower concentrations likely represent constitutive phloem from unattacked trees (Erbilgin and Raffa, 2000). Long exposure to α-pinene- and limonene-saturated vapors can also be lethal for I. typographus adults (Everaerts et al., 1988). Smelyanets and Vasechko (1973), studying the chemotaxis of I. typographus to terpenoids found α-pinene to be repellent at concentrations higher than 3%, whereas limonene was attractive only at concentrations of 0.2–0.6% and repellent above 3–4%. Verbenone, which in many bark beetle species is usually released by tunneling males as a repellent for colonizing adults, shows a concentration-dependent repellence pattern (Schlyter et al., 1989). No previous studies concerning the antifeedant characteristics of verbenone has been conducted. We observed a strong repellent effect at high concentrations, especially to males.
In general, compounds from nonhost trees have strong antifeedant effects. Green leaf volatiles (GLVs) are aliphatic 6-carbon primary alcohols, aldehydes, and acetates found in broad-leaved trees (Visser, 1986). In our experiment, the GLV-blend showed an effective threshold starting at low concentrations. Moreover, I. typographus antennae strongly respond to 1-hexanol (Zhang and Schlyter, 2004). Similar responses have also been found in D. ponderosae, Tomicus piniperda (L.), and T. minor (Hartig), I. duplicatus (Sahlberg), and I. sexdentatus (Boerner) (Zhang and Schlyter, 2004). In our experiment, males of I. typographus were strongly affected by hexanol and GLV, indicating a possible species-specific effect of one or more of these compounds. Among the tested nonhost chemicals, 3-octanol is an 8-carbon alcohol extracted from the bark of European birch species (Betula pendula and B. pubescent) and aspen (Populus tremula) (Zhang et al., 2000). This alcohol showed the strongest effect on the feeding responses of I. typographus, being active at low concentrations (0.1%). Finally, juglone is a quinone derivative (5-hydroxy-1,4-naphthoquinone) already known to inhibit the feeding activity of Periplaneta americana (L.) and S. multistriatus by reacting with aminoacids, especially cysteine (Ferkovich and Dale, 1971). In our study, however, juglone only showed a moderate antifeedant effect. Following starvation, the need for water induces beetles to ingest nonhost or neutral diet, thus masking low antifeedant properties of some compounds. As we found some antifeedant activity in all compounds, the optimal starvation period before test might be longer than that applied in our study. Raffa (1988) hypothesized that phloem colonization does not continue in the presence of repellents, but progresses in the absence of stimulants. This would indicate repellents as the key factor influencing tree colonization. Our results support that hypothesis.
References
N. Agelopoulos M. A. Birkett A. J. Hick A. M. Hooper J. A. Pickett E. M. Pow L. E. Smart D. W. M. Smiley L. J. Wadhams C. M. Woodcock (1999) ArticleTitleExploiting semiochemicals in insect control Pestic. Sci. 55 225–235 Occurrence Handle10.1002/(SICI)1096-9063(199903)55:3<225::AID-PS887>3.0.CO;2-7 Occurrence Handle1:CAS:528:DyaK1MXisVWrtrg%3D
K. R. S. Ascher E. Gurevitz S. Renneh N. E. Nemny (1975) ArticleTitleThe penetration of females of the fruit bark beetle Scolytus mediterraneus Eggers into antifeedant-treated twigs in laboratory tests Z. Pflanzenb. Pflanzenschutz 82 378–383
Baier, P., Bader, R., and Rosner, S., 1999. Monoterpene content and monoterpene emission of Norway spruce (Picea abies (L.) Karst.) bark in relation to primary attraction of bark beetles (Col., Scolytidae), pp. 249–259, in F. Lieutier, W. J. Mattson, and M. R. Wagner (eds.). Physiology and Genetics of Tree Phytophagous Interactions. International Symposium, Gujan, France, 31 August–5 September, 1997.
W. D. Bedard (1966) ArticleTitleA ground phloem media for rearing immature bark beetles (Scolytidae) Ann. Entomol. Soc. Am. 59 931–938
J. H. Borden (1997) Disruption of semiochemical-mediated aggregation in bark beetles R. T. Cardé A. K. Minks (Eds) Pheromone Researche: New Directions Chapman and Hall New York 421–438
J. A. Byers (1995) Host-tree chemistry affecting colonization in bark beetles R. T. Cardé W. J. Bell (Eds) Chemical Ecology of Insects, 2 Chapman and Hall New York 154–213
E. Christiansen A. Bakke (1988) The spruce bark beetle of Eurasia A. A. Berryman (Eds) Dynamics of Forest Insect Populations. Patterns, Causes, Implications Plenum Press New York 479–503
J. C. Dickens (1981) ArticleTitleBehavioral and electrophysiological responses of the bark beetle Ips typographus to potential pheromone components Physiol. Entomol. 6 251–261 Occurrence Handle1:CAS:528:DyaL38XotlSqtA%3D%3D
R. W. Doskotch S. K. Chatterji J. W. Peacock (1970) ArticleTitleElm bark derived feeding stimulants for the smaller European elm bark beetle Science 167 380–382 Occurrence Handle1:CAS:528:DyaE3cXptVOqsg%3D%3D
J. S. Elkinton D. L. Wood L. E. Browne (1981) ArticleTitleFeeding and boring behavior of the bark beetle, Ips paraconfusus, in extracts of ponderosa pine phloem J. Chem. Ecol. 7 209–220 Occurrence Handle10.1007/BF00988649
N. Erbilgin K. F. Raffa (2000) ArticleTitleOpposing effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones J. Chem. Ecol. 26 2527–2548 Occurrence Handle10.1023/A:1005532612117 Occurrence Handle1:CAS:528:DC%2BD3cXptV2mt70%3D
C. Everaerts J.-C. Grégoire J. Merlin (1988) The toxicity of Norway spruce monoterpenes to two bark beetle species and their associated W. J. Mattson J. Levieux C. B. Dagan (Eds) Mechanisms of Woody Plant Defenses Against Insects—Search for Pattern Springer Berlin Heidelberg New York 335–344
S. M. Ferkovich M. N. Dale (1971) ArticleTitleNaphthoquinone inhibitors of Periplaneta americana and Scolytus multistriatus feeding: Ultraviolet difference spectra of reactions of juglone, menadione and 1,4-naphthoquinone with amino acids and the indicated mechanism of feeding inhibition Chem.-Biol. Interact. 4 23–30 Occurrence Handle1:CAS:528:DyaE38XlsVOrsQ%3D%3D Occurrence Handle5156334
J. L. Frazier S. Chyb (1995) Use of feeding inhibitors in insect control R. F. Chapman G. DeBore (Eds) Regulatory Mechanisms in Insect Feeding Plenum Press New York 1–42
K. D. Klepzig F. Schlyter (1999) ArticleTitleLaboratory evaluation of plant derived antifeedants against European pine weevil, Hylobius abietis J. Econ. Entomol. 92 644–650 Occurrence Handle1:CAS:528:DyaK1MXnvFOhsr4%3D
E. C. Levy I. Ishaaya E. Gurevitz R. Cooper D. Lavie (1974) ArticleTitleIsolation and identification of host compounds eliciting attraction and bite stimuli in the fruit tree bark beetle, Scolytus mediterraneus J. Agric. Food Chem. 22 376–379 Occurrence Handle10.1021/jf60193a042 Occurrence Handle1:CAS:528:DyaE2cXkvVKks7Y%3D
W. R. McNee P. Bonello A. J. Storer D. L. Wood T. R. Gordon (2003) ArticleTitleFeeding response of Ips paraconfusus to phloem and phloem metabolites of Heterobasidion annosum-inoculated ponderosa pine, Pinus ponderosa J. Chem. Ecol. 29 1183–1202 Occurrence Handle1:CAS:528:DC%2BD3sXjvFyju7Y%3D Occurrence Handle12857030
Q. E. Paynter O. Anderbrant F. Schlyter (1990) ArticleTitleBehavior of male and female spruce bark beetles, Ips typographus, on the bark of host trees J. Insect Behav. 3 529–543
K. F. Raffa (1988) Host orientation behavior of Dendroctonus ponderosae: Integration of token stimuli and host defenses W. J. Mattson J. Levieux C. Bernard-Degan (Eds) Mechanisms of Woody Plant Defenses Against Insects Sringer Berlin Heidelberg New York 369–390
K. F. Raffa A. A. Berryman (1982) ArticleTitleGustatory cues in the orientation of Dendroctonus ponderosae (Coleoptera: Scolytidae) to host trees Can. Entomol. 114 97–104 Occurrence Handle1:CAS:528:DyaL38XitFCks7Y%3D
K. F. Raffa T. W. Phillips S. M. Salom (1993) Strategies and mechanisms of host colonization by bark beetles T. D. Schowalter G. M. Filip (Eds) Beetle–Pathogen Interactions in Conifer Forests Academic Press New York 103–120
J. Reddemann R. Schopf (1996) ArticleTitleThe importance of monoterpenes in the aggregation of the spruce bark beetle Ips typographus (Coleoptera: Scolytidae: Ipinae) Entomol. Gen. 21 69–80
J. A. Rudinsky V. Novak P. Svihra (1971) ArticleTitleAttraction of the bark beetle Ips typographus L. to terpenes and a male produced pheromone Z. Angew. Entomol. 67 179–188
L. Safranyik D. M. Shrimpton H. S. Whitney (1975) An interpretation of the interaction between lodgepole pine, the mountain pine beetle and its associated blue stain fungi in western Canada D. M. Baumgartner (Eds) Management of Lodgepole Pine Ecosystems Washington St. Univ. Press Pullman 406–428
F. Schlyter G. Birgersson (1999) Forest beetles J. Hardie A. K. Minks (Eds) Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants CAB International Oxford, UK 113–148
F. Schlyter I. Cederholm (1981) ArticleTitleSeparation of the sexes of living spruce bark beetles, Ips typographus (L.) (Coleoptera: Scolytidae) Z. Angew. Entomol. 92 42–47
F. Schlyter G. Birgersson A. Leufvén (1989) ArticleTitleInhibition of attraction to aggregation pheromone by verbenone and ipsenol. Density regulation mechanisms in bark beetle Ips typographus J. Chem. Ecol. 15 2263–2277 Occurrence Handle1:CAS:528:DyaL1MXltlWjtrg%3D
Z. Šimsek ParticleVon E. Führer (1993) ArticleTitleKunstliches Nahr- und Brutmedium fur Ips typographus L. (Col; Scolytidae) J. Appl. Entomol. 116 432–439
V. P. Smelyanets G. I. Vasechko (1973) ArticleTitleThe chemotaxis of Ips typographus (Coleoptera: Ipidae) to terpenoids Zool. Zhurnal 52 1089–1092 Occurrence Handle1:CAS:528:DyaE2cXitlWiug%3D%3D
K. B. Sturgeon (1979) ArticleTitleMonoterpene variation in ponderosa pine xylem resin related to western pine beetle predation Evolution 33 803–814 Occurrence Handle1:CAS:528:DyaL3cXjtlWi
Thomas, H. A., Richmond, J. A., and Bradley, E. L. 1981. Bioassay of pine bark extracts as biting stimulants for the southern pine beetle. USDA Forest Service. Research Note SE-302. 5 pp.
J. H. Visser (1986) ArticleTitleHost odor perception in phytophagous insects Annu. Rev. Entomol. 31 121–144
K. F. Wallin K. F. Raffa (2000) ArticleTitleInfluences of host chemicals and internal physiology on the multiple steps of post-landing host acceptance behaviour of Ips pini (Coleoptera: Scolytidae) Environ. Entomol. 29 442–453 Occurrence Handle1:CAS:528:DC%2BD3cXlvFWgur0%3D
K. F. Wallin K. F. Raffa (2002) ArticleTitlePrior encounters modulate subsequent choices in host acceptance behavior by the bark beetle Ips pini Entomol. Exp. Appl. 103 205–218 Occurrence Handle1:CAS:528:DC%2BD38XovVGhs7k%3D
D. L. Wood (1982) ArticleTitleThe role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles Annu. Rev. Entomol. 27 411–446 Occurrence Handle1:CAS:528:DyaL38Xot12lsQ%3D%3D
J. H. Zar (1984) Biostatistical Analysis EditionNumber2 Prentice Hall Englewood Cliffs, NJ
Q.-H. Zhang F. Schlyter (2004) ArticleTitleOlfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles Agric. For. Entomol. 6 1–20 Occurrence Handle1:CAS:528:DC%2BD2cXnsVeru7s%3D
Q.-H. Zhang F. Schlyter G. Birgersson (2000) ArticleTitleBark volatiles from non-host angiosperm trees of spruce bark beetle, Ips typographus (L.) (Coleoptera: Scolytidae): Chemical and electrophysiological analysis Chemoecology 10 69–80 Occurrence Handle1:CAS:528:DC%2BD3cXlvFygtr4%3D
Acknowledgments
We thank L. Ansebo, M. Tasin, F. Loreto, and S. Larsson for comments on earlier drafts, the Björnstorp Estate for provision of logs, and E. V. Jirle for cutting stems for beetle breeding. The authors are also grateful to the Chemical Ecology Section of the Department of Crop Sciences, SLU (Alnarp, Sweden) for providing facilities and laboratories.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faccoli, M., Blaženec, M. & Schlyter, F. Feeding Response to Host and Nonhost Compounds by Males and Females of the Spruce Bark Beetle Ips typographus in a Tunneling Microassay. J Chem Ecol 31, 745–759 (2005). https://doi.org/10.1007/s10886-005-3542-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-3542-z