Abstract
We evaluated the disposable non-invasive SpotOn™ thermometer relying on the zero-heat-flux technology. We tested the hypothesis that this technology may accurately estimate the core temperature. The primary objective was to compare cutaneous temperature measurements from this device with blood temperatures measured with the pulmonary artery catheter. Secondary objective was to compare measurements from the zero-heat-flux thermometer indirectly with other routinely used thermometers (nasopharyngeal, bladder, rectal). We included 40 patients electively scheduled for either off-pump coronary artery bypass surgery or pulmonary thromboendarterectomy. Temperatures were measured using zero-heat-flux (SpotOn™), pulmonary artery catheter, nasopharyngeal, rectal, and bladder thermometers. Agreement was assessed using the Bland and Altman random effects method for repeated measures data, and Lin’s concordance correlation coefficient. Accuracy was estimated (defined as <0.5° difference with the gold standard), with a 95% confidence interval considering the multiple pairs of measurements per patient. 17 850 sets of temperature measurements were analyzed from 40 patients. The mean overall difference between zero-heat-flux and pulmonary artery catheter thermometer was -0.06 °C (95% limits of agreement of ± 0.89 °C). In addition, 14 968 sets of temperature measurements were analyzed from 34 patients with all thermometers in situ. Results from the zero-heat-flux thermometer showed better agreement with the pulmonary artery catheter than the other secondary core thermometers assessed. In conclusion, the SpotOn™ thermometer reliably assessed core temperature during cardiac surgery. It could be considered an alternative for other secondary thermometers in the assessment of core temperature during general anesthesia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Unintended perioperative hypothermia, defined as a patient core temperature of less than 36.0 °C, remains a common complication during both general and neuraxial anesthesia and affects patients’ outcome [1]. Perioperative hypothermia is a result of core-to-peripheral redistribution of heat, reduced metabolic heat production during anesthesia, and an increased heat loss to the environment. Potential side effects of hypothermia include coagulopathy and increased blood loss [2], increased incidence of myocardial injury [3] and prolonged action of various drugs [4], as well as postoperative discomfort and shivering [1]. Hypothermia may also lead to an increased risk of infection [5, 6].
The management of unintended perioperative hypothermia is multifactorial. It is well accepted that maintaining perioperative normothermia is crucial, rather than rewarming hypothermic patients [1]. Options are either passive insulation or active warming, with forced air warming being the most efficient method.
Apart from these measures in the prevention and treatment of hypothermia, accurate monitoring of patient core temperature is of pivotal importance. A European survey [7] demonstrated in 2011 that barely 25% of patients under general and 6% of patients under regional anesthesia were monitored intraoperatively. This is conflicting with current NICE guidelines [8] stating that all patients under anesthesia for more than 30 min should have their temperature monitored.
Temperature can be monitored at either core (head, trunk), peripheral (arm, leg) or cutaneous sites of the patient. Peripheral tissue and cutaneous temperatures are typically below core temperature and subject to environment, as well as peripheral vasoconstriction or vasodilatation [9]. Core temperature is tightly regulated and contributes highly to thermoregulatory control, and is deemed most relevant to the patient’s thermal status [1].
The golden standard for measuring core temperature remains the pulmonary artery catheter (except during cardiopulmonary bypass) [10, 11] which has, however, limited indication in routine clinical practice beyond cardiac and transplant surgery [12]. Nasopharyngeal and distal esophageal thermometers are deemed adequate estimators of the core temperature [1]. However, these devices each are subject to several limitations. They cannot be used in awake patients, the insertion depth is likely to influence the accuracy of the temperature measurements and placement of the transesophageal echocardiography probe may interfere with nasopharyngeal or esophageal temperature monitoring [13]. Other options include bladder, rectal, sublingual, axilla and tympanic membrane thermometers. However, the validity of urinary bladder temperature depends critically on urinary flow rates. Moreover, bladder temperature is known to be a poor indicator of core temperature during cardiac surgery with cardiopulmonary bypass [14]. Rectal, sublingual, axilla and tympanic membrane thermometers remain critically operator-dependent and susceptible to artefacts [1]. Hence, it appears obvious that there is a need for a non-invasive way to accurately measure core temperature, especially during cardiac surgery.
An innovative way to non-invasively measure core temperature is using the transcutaneous zero-heat-flux (ZHF) technology. This technique is based on creating a zone of zero heat-flow over the skin surface, thus allowing a simple electronic thermometer to measure the core temperature (or at least the temperature 1 cm to 2 cm below the skin surface) [15, 16]. ZHF was developed in 1971 by Fox et al [15] but has been limited in use due to practical considerations [17]. Only a few years ago a lightweight disposable probe was developed that can easily be used in the clinical setting, now commercially available as SpotOn™. This device offers a non-invasive method of core temperature measurement in clinical circumstances in which invasive methods cannot be applied.
The evidence concerning the accuracy of this ZHF device in measuring the patient core temperature is growing, and study results are promising [18]. The primary goal of this study was to test in a cardiac surgical population the accuracy of the SpotOn™ technology in relation to the gold standard. The secondary goal of this study was to determine how well it performs in comparison with other routinely used core temperature measurements (nasopharyngeal, bladder and rectal thermometers) and to assess its clinical relevance (Fig. 1).
Cartoon of the isothermal zone as a result of zero-heat-flux technology [19]. Reproduced with permission © 3M 2013
2 Methods
2.1 Ethics
Study approved by the local ethics committee on March 25, 2019 and written informed consent was obtained from participating patients. All patients were enrolled at a tertiary care academic hospital between May 3, 2019 and September 24, 2019.
2.2 Study design
Prospective observational study in a cardiac surgical population comparing the SpotOn™ thermometer to measurements from pulmonary artery catheter, nasopharyngeal, bladder and rectal thermometers.
2.3 Patients
We included 40 patients electively scheduled for either off-pump coronary artery bypass surgery (OPCAB) or pulmonary thromboendarterectomy (PTEA). In these patients, standard pulmonary artery catheter monitoring is used as part our institutional standards. Patients were asked to participate if they were older than 18 years, able to understand and sign an informed consent form in Dutch and able to receive temperature assessments through a pulmonary artery catheter and ZHF, bladder, rectal and nasopharyngeal thermometers. They were free to discontinue participation in the investigation at any time, and without prejudice to further treatment.
Exclusion criteria included diabetes mellitus (considering the possibility of impaired thermoregulation, including reduced skin blood flow [20]), the presence of skin or soft tissue disorders on the forehead area covered directly by the ZHF device (e.g. pressure ulcers, clinically significant psoriasis, dermatitis or any other interruption of skin integrity that would be affected by direct heat), or lack of space on the patient’s forehead due to presence of other devices (e.g. near infrared spectrometry, bispectral index monitor).
Preoperative assessments included room temperature and subject weight, height, age and medical history.
In accordance with our cardiac surgery protocol, general anesthesia was induced with sufentanil, propofol and rocuronium, and maintained with remifentanil, dexmedetomidine and sevoflurane. Norepinephrine was started in all patients in continuous infusion after placement of the central-venous catheter, in a dose of 0.01 μg/kg/min and adjusted to clinical need.
Intravenous fluids were administered through a warming system (Ranger Blood and Fluid Warming System, 3M, Germany). An underbody resistive heating mattress (Alpha Plus Patient Warming System, Inditherm Medical, UK) was used when the patient was not on cardiopulmonary bypass, set at 40 °C. Room temperature was set at 22 °C before induction and 18 °C after surgical draping.
2.4 Measurements
Temperatures were measured using a zero-heat-flux thermometer (SpotOn, 3M, Germany), pulmonary artery catheter (Swan-Ganz CCOmbo V Continuous Cardiac Output Catheter, Edwards Lifesciences, USA), nasopharyngeal thermometer (GRI Medical & Electronic Technology, China), rectal thermometer (GRI Medical & Electronic Technology, China), bladder thermometer (GRI Medical & Electronic Technology, China). The ZHF thermometer was placed immediately after being transferred to the operating table, according to the manufacturer’s written guideline: first the sensor was connected to the SpotOn control unit (already connected to the Philips Intellivue MX800 patient monitor). After prepping the site with an alcohol wipe, the sensor was then placed above the right orbital ridge. A ten-minute interval was allowed for thermal equilibration. After induction of anesthesia and intubation, rectal and bladder thermometers were placed. The pulmonary artery catheter was subsequently inserted by the anesthesia resident using dynamic pressure readings from the catheter tip. Finally, the nasopharyngeal thermometer was placed. After all thermometers were in place, another ten-minute interval was allowed for thermal equilibration. Subsequent temperature readings were taken in 30-s intervals thereafter using an automatic registration of the patient monitor (Philips Intellivue MX800, Philips Medizin Systeme, Germany) into the patient data management system (Klinisch Werkstation, University Hospitals Leuven, Belgium). The period on cardiopulmonary bypass was excluded for obvious reasons (lack of pulmonary artery blood flow). The last thermometry data were recorded upon closing of the sternum.
2.5 Statistical methods
Statistical advice was obtained before study commencement and after study conclusion from the Leuven Biostatistics and Statistical Bioinformatics Centre (L-BioStat), KU Leuven, Belgium.
After automatic data registration into the patient data management system (Klinisch Werkstation, University Hospital Leuven) and completion of the study, data was automatically extracted into Excel (Microsoft Office Excel, Microsoft, USA). Analyses were performed using SAS software (version 9.4 of the SAS System for Windows).
The percentage of correct measurements were estimated (defined as <0.5° difference with the gold standard), with a 95% confidence interval considering the multiple pairs of measurements per patient. Estimation was performed using a ratio estimator for the variance of clustered binary data [21].
Agreement was assessed using the Bland and Altman random effects method for repeated measures data, for the estimation of the mean bias, standard deviation of the bias, and 95% limits of agreement (LoA) [22].
Lin’s concordance correlation coefficient (CCC) was calculated [23].
Precision was also assessed using percentage error as defined by Montenij et al [24].
3 Results
3.1 Patient characteristics
Sixty eight patients scheduled for off-pump coronary artery bypass (OPCAB) or pulmonary thrombo-endarterectomy (PTEA) requiring a pulmonary artery catheter were screened. 28 patients were excluded because of diabetes mellitus.
Thirty seven men and 3 women were included. All patients were ASA class 4. Of the 36 patients scheduled for OPCAB, 5 were required to go on full cardiopulmonary bypass because of hemodynamic instability intraoperatively.
Due to technical issues, 6 rectal thermometer readings were discarded (5 during OPCAB and 1 during on-pump CABG). To compare ZHF to the other thermometers a subset analysis was performed on 34 patients with all thermometers in situ. In total, 17 850 sets of temperature measurements were analyzed from 40 patients.
The ZHF sensors were well tolerated in all patients, without occurrence of any adverse events. No sensors failed intraoperatively (Table 1).
3.2 Comparison of zero-heat-flux with pulmonary artery thermometer in all patients
Temperature measurements from the PAC ranged between 34.6 °C and 37.8 °C.
The mean overall difference with ZHF was −0.06 °C (95% LoA ± 0.89 °C). In 89% of measurements (95% CI 82;96%), ZHF temperatures were within 0.5 °C of PAC temperatures. Lin’s concordance correlation coefficient for all patients was 0.73 (95% CI 0.72;0.74). Percentage error was 2.46.
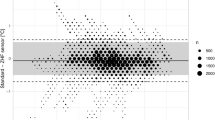
Results are presented in Fig. 2.
3.3 Comparison of zero-heat-flux, nasopharyngeal, bladder and rectal with pulmonary artery thermometer in all and subset patients
17 850 sets of temperature measurements were analyzed from 40 patients for comparison of zero-heat-flux, nasopharyngeal and bladder thermometers with pulmonary artery thermometer. Results are presented in Table 2 and Figs. 2, 3 and 4.
Subsequently, 14,968 sets of temperature measurements were analyzed from 34 patients with all thermometers in situ: 26 patients undergoing OPCAB, 4 on-pump CABG and 4 PTEA. The results are presented in Table 2 and Fig. 5.
4 Discussion
In this prospective observational study performed on 40 patients scheduled for either OPCAB or PTEA, the ZHF thermometer reliably assessed core temperature. Mean overall bias between ZHF and PAC temperatures in all included patients was −0.06 °C (95% LoA ± 0.89 °C). In 89% of measurements (95% CI 82;96%), ZHF temperatures were within 0.5 °C of PAC temperatures.
The findings from our study are consistent with the results from 3 clinical studies comparing measurements from the ZHF with the PAC in a similar setting [18]: Eshragi et al. [16] compared the ZHF thermometer to measurements from the PAC in 105 patients undergoing elective cardiac surgery (mean bias −0.08 °C, 95% LoA ± 0.88 °C); Mäkinen et al. [25] compared the ZHF thermometer to measurements from the PAC in 15 patients undergoing elective coronary artery bypass grafting, aortic valve replacement or reconstruction of the ascending aorta (mean bias −0.05 °C, 95% LoA ± 0.51 °C); Gómez-Romero et al. [26] compared the ZHF thermometer to measurements from the PAC in 41 patients undergoing elective cardiac valve surgery (mean bias + 0.21 °C, 95% LoA ± 2.50 °C).
When comparing the results from the ZHF to the results from the nasopharyngeal, bladder and rectal thermometers, differences were small (especially considering a clinical context). In this mixed cardiac surgical population, the ZHF thermometer showed the best agreement with the PAC measurements (mean bias −0.06 °C, 95% LoA ± 0.89 °C), followed by the nasopharyngeal thermometer (mean bias −0.16 °C, 95% LoA ± 2.50 °C), bladder (mean bias + 0.18 °C, 95% LoA ± 0.68 °C) and rectal thermometers (mean bias + 0.32 °C, 95% LoA ± 0.85 °C). In addition to its accuracy, the big advantage of the ZHF thermometer is being completely non-invasive compared to the (minimal) invasiveness of the other thermometers. Complications with currently used thermometers are rare but do occur, especially from nasopharyngeal probes and in children [27,28,29]. No complications occurred when using the ZHF thermometer in our study. Considering the non-invasive character of this thermometer, it also shows great promise in the field of locoregional anesthesia but studies have yet to be performed in this specific setting [18].
As mentioned earlier, unintended perioperative hypothermia should be avoided as it may cause complications [1]. While after cardiac arrest [30], traumatic brain injury [31] or during cardiopulmonary bypass [32], therapeutic hypothermia has long been considered beneficial, recent evidence questions the value of hypothermia in these situations [33]. During our OPCAB procedures, while using an underbody resistive heating mattress and covering the patient whenever possible, we still recorded a temperature of less than 36.0 °C in 30.5% of measurements.
On the other hand, deep hypothermic cardiac arrest (DHCA) is still pursued during aortic arch surgery and PTEA to protect heart and brain [34]. Debate remains which grade and duration of hypothermia is considered safe [35], and which thermometer best reflects core temperature during these rapid thermal perturbations and low temperatures. The SpotOn™ device shows promise in assessing core temperature in the normothermic to mild hypothermic range, and might even be useful in assessing core (or at least cerebral cortex [36]) temperature during DHCA. Measurements of questionable accuracy were however previously documented during hypothermia below 32 °C [25] and further research is warranted in these procedures.
Our study highlights some drawbacks of the clinical use of the currently available (secondary) core thermometers.
The use of the PAC is limited mainly to specific cardiac and transplant procedures [12], and is deemed inaccurate during cardiopulmonary bypass considering the lack of blood flow in the pulmonary artery and intermittent administration of cold cardioplegia solutions [11].
Placing a nasopharyngeal thermometer can also cause complications, particularly nose bleeding (exacerbated when giving heparin intraoperatively) [27]. When placed too deep, it is easily dislocated when using transesophageal echography which may explain the increased bias in our study.
The bladder thermometer seems most accurate in our study, but bladder cannulation is not routinely indicated during all types of anesthesia, has inherent risks and accuracy is dependent of urinary flow rate [14].
Our study has some limitations. First, we decided for a clinical study with the placement of the thermometers performed by the standard surgical team, without checking the exact depth of the nasopharyngeal thermometer (as this is not routine practice). Second, our study population consists mainly of men. Finally, when looking at the Bland–Altman plots, we suspect a lag in rewarming of the different compartments after cardiopulmonary bypass, thus increasing bias. The ZHF thermometer seems less susceptible to this phenomenon compared to the other thermometers.
The major strength of this study is the clinical setting, with the placement of the thermometers performed by the normal surgical team to limit study bias.
5 Conclusion
In conclusion, the non-invasive SpotOn™ thermometer reliably assessed core temperature during cardiac surgery excluding cardiopulmonary bypass. It could be considered an alternative for other currently available secondary thermometers in the assessment of core temperature during general anesthesia.
Its reliability in deep hypothermia and impact on clinical outcome remains to be studied.
References
Sessler DI. Thermoregulation and heat balance. Lancet. 2016;387(10038):2655–64. https://doi.org/10.1016/S0140-6736(15)00981-2.
Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108(1):71–7. https://doi.org/10.1097/01.anes.0000296719.73450.52.
Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627–34. https://doi.org/10.1503/cmaj.050011.
Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80(5):1007–14. https://doi.org/10.1213/00000539-199505000-00027.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334(19):1209–15. https://doi.org/10.1056/NEJM199605093341901.
Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876–80. https://doi.org/10.1016/S0140-6736(01)06071-8.
Torossian A. Survey on intraoperative temperature management in Europe. Eur J Anaesthesiol. 2007;24(8):668–75. https://doi.org/10.1017/S0265021507000191.
National Institute for Health and Care Excellence (NICE). Hypothermia: prevention and management in adults having surgery, NICE Clinical guideline. NICE Guidel CG65. 2016;(April 2008).
Niven DJ, Gaudet JE, Laupland KB, Mrklas KJ, Roberts DJ, Stelfox HT. Accuracy of peripheral thermometers for estimating temperature: a systematic review and meta-analysis. Ann Intern Med. 2015;163(10):768–77. https://doi.org/10.7326/M15-1150.
Launey Y, Larmet R, Nesseler N, Malledant Y, Palpacuer C, Seguin P. The accuracy of temperature measurements provided by the edwards lifesciences pulmonary artery catheter. Anesth Analg. 2016;122(5):1480–3. https://doi.org/10.1213/ANE.0000000000001242.
Engelman R, Baker RA, Likosky DS, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical Practice Guidelines for Cardiopulmonary Bypass - Temperature Management During Cardiopulmonary Bypass. Ann Thorac Surg. 2015;100(2):748–57. https://doi.org/10.1016/j.athoracsur.2015.03.126.
Cowie BS. Does the pulmonary artery catheter still have a role in the perioperative period? Anaesth Intensive Care. 2011;39(3):345–55. https://doi.org/10.1177/0310057x1103900305.
Wang M, Singh A, Qureshi H, Leone A, Mascha EJ, Sessler DI. Optimal depth for nasopharyngeal temperature probe positioning. Anesth Analg. 2016;122(5):1434–8. https://doi.org/10.1213/ANE.0000000000001213.
Sato H, Yamakage M, Okuyama K, et al. Urinary bladder and oesophageal temperatures correlate better in patients with high rather than low urinary flow rates during non-cardiac surgery. Eur J Anaesthesiol. 2008;25(10):805–9. https://doi.org/10.1017/S0265021508004602.
Fox RH, Solman AJ, Isaacs R, Fry AJ, MacDonald IC. A new method for monitoring deep body temperature from the skin surface. Clin Sci. 1973;44(1):81–6.
Eshraghi Y, Nasr V, Parra-Sanchez I, et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg. 2014;119(3):543–9. https://doi.org/10.1213/ANE.0000000000000319.
Yamakage M, Namiki A. Deep temperature monitoring using a zero-heat-flow method. J Anesth. 2003;17(2):108–15. https://doi.org/10.1007/s005400300026.
Conway A, Bittner M, Phan D, et al. Accuracy and precision of zero-heat-flux temperature measurements with the 3MTM Bair HuggerTM Temperature Monitoring System: a systematic review and meta-analysis. J Clin Monit Comput. 2020. https://doi.org/10.1007/s10877-020-00543-6.
SpotOn Temperature Monitoring System Model 370 Operator’s Manual. https://multimedia.3m.com/mws/media/879803O/operators-manual-english.pdf. Accessed 1 Mar 2021
Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature. 2016;3(1):119–45. https://doi.org/10.1080/23328940.2015.1131506.
Mccarthy WF, Medical M. The estimation of sensitivity and specificity of clustered binary data. Stat Data Anal. 2002;(SUGI31):1–10.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82. https://doi.org/10.1080/10543400701329422.
Lin LI. A concordance correlation coefficient to evaluate reproducibility Author (s ): Lawrence I-Kuei Lin Published by : International Biometric Society Stable URL : http://www.jstor.org/stable/2532051 REFERENCES Linked references are available on JSTOR for thi. Biomatrics. 1989;45(1):255–268.
Montenij LJ, Buhre WF, Jansen JR, Kruitwagen CL, De Waal EE. Methodology of method comparison studies evaluating the validity of cardiac output monitors: a stepwise approach and checklist. Br J Anaesth. 2016;116(6):750–8. https://doi.org/10.1093/bja/aew094.
Mäkinen MT, Pesonen A, Jousela I, et al. Novel zero-heat-flux deep body temperature measurement in lower extremity vascular and cardiac surgery. J Cardiothorac Vasc Anesth. 2016;30(4):973–8. https://doi.org/10.1053/j.jvca.2016.03.141.
Gómez-Romero FJ, Fernández-Prada M, Fernández-Suárez FE, et al. Intra-operative temperature monitoring with two non-invasive devices (3M Spoton® and Dräger Tcore®) in comparison with the Swan-Ganz catheter. Cir Cardiovasc. 2019;26(4):191–6. https://doi.org/10.1016/j.circv.2019.06.002.
Van Zundert A, Wyssusek K, Vivian V. Verification of Nasopharyngeal temperature probes - they are not always where you think they are! Anesth Analg. 2016;123(5):1338–9. https://doi.org/10.1213/ANE.0000000000001542.
Kuo CP, Wong CS, Borel CO, et al. Cerebrospinal fluid rhinorhea after thermometer insertion through the nose. Anesth Analg. 2004;99(2):617–9. https://doi.org/10.1213/01.ane.0000124680.59440.0f.
Wass CT, Long TR, Deschamps C. Entrapment of a nasopharyngeal temperature probe: an unusual complication during an apparently uneventful elective revision laparoscopic nissen fundoplication. Dis Esophagus. 2010;23(1):33–5. https://doi.org/10.1111/j.1442-2050.2009.00968.x.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206. https://doi.org/10.1056/nejmoa1310519.
Maekawa T, Yamashita S, Nagao S, et al. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015;32(7):422–9. https://doi.org/10.1089/neu.2013.3197.
Investigators TWH. Randomised trial of normothermic versus hypothermic coronary bypass surgery. Lancet. 1994;343(8897):559–63. https://doi.org/10.1016/S0140-6736(94)91519-9.
Urits I, Jones MR, Orhurhu V, et al. A comprehensive update of current anesthesia perspectives on therapeutic hypothermia. Adv Ther. 2019;36(9):2223–32. https://doi.org/10.1007/s12325-019-01019-z.
Damberg A, Carino D, Charilaou P, et al. Favorable late survival after aortic surgery under straight deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2017;154(6):1831-1839.e1. https://doi.org/10.1016/j.jtcvs.2017.08.015.
Gupta P, Harky A, Jahangeer S, Adams B, Bashir M. Varying evidence on deep hypothermic circulatory arrest in thoracic aortic aneurysm surgery. Texas Hear Inst J. 2018;45(2):70–5. https://doi.org/10.14503/THIJ-17-6364.
Pesonen E, Silvasti-Lundell M, Niemi TT, Kivisaari R, Hernesniemi J, Mäkinen MT. The focus of temperature monitoring with zero-heat-flux technology (3M Bair-Hugger): a clinical study with patients undergoing craniotomy. J Clin Monit Comput. 2019;33(5):917–23. https://doi.org/10.1007/s10877-018-0227-z.
Funding
This work was supported by 3M (Machelen, Belgium) by supplying 40 single-use sensors and 1 control unit with sensor cable, monitor cable and power unit during the study period. 3 M was not involved in the study design, collection analysis and interpretation of data, writing of the report, nor decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CV, AL and EVG. The first draft of the manuscript was written by CV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of UZ/KU Leuven, Herestraat 49, B 3000 Leuven (internal reference S61212, Belg. Reg-nr: B322201838291) on March 25, 2019.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent to publish
Written informed consent was obtained from all individual participants included in the study.
Data availability
Anonymous temperature readings are available (MS Excel).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verheyden, C., Neyrinck, A., Laenen, A. et al. Clinical evaluation of a cutaneous zero-heat-flux thermometer during cardiac surgery. J Clin Monit Comput 36, 1279–1287 (2022). https://doi.org/10.1007/s10877-021-00758-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00758-1