Abstract
Monitoring of transcranial electrical motor evoked potentials (tcMEP) during carotid endarterectomy (CEA) has been shown to effectively detect intraoperative cerebral ischemia. The unique purpose of this study was to evaluate changes of MEP amplitude (AMP), area under the curve (AUC) and signal morphology (MOR) as additional MEP warning criteria for clamping-associated ischemia during CEA. Therefore, the primary outcome was the number of MEP alerts (AMP, AUC and MOR) in the patients without postoperative motor deficit (false positives). We retrospectively reviewed data from 571 patients who received CEA under general anesthesia. Monitoring of somatosensory evoked potentials (SSEP) and tcMEP was performed in all cases (all-or-none MEP warning criteria). The percentages of false positives (primary parameter) of AMP, AUC and MOR were evaluated according to the postoperative motor outcome. In the cohort of 562 patients, we found significant SSEP/MEP changes in 56 patients (9.96%). In 44 cases (7.83%) a shunt was inserted. Nine patients (1.57%) were excluded due to MEP recording failure. False positives were registered for AMP, AUC and MOR changes in 121 (24.01%), 148 (29.36%) and 165 (32.74%) patients, respectively. In combination of AMP/AUC and AMP/AUC/MOR false positives were found in 9.52% and 9.33% of the patients. This study is the first to evaluate the correctness of the MEP warning criteria AMP, AUC and MOR with regard to false positive monitoring results in the context of CEA. All additional MEP warning criteria investigated produced an unacceptably high number of false positives and therefore may not be useful in carotid surgery for adequate detection of clamping-associated ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently published studies have indicated transcranial electrical motor evoked potentials (tcMEP) to be a useful tool to detect focal ischemia within the corticospinal tract during carotid endarterectomy [1,2,3,4]. Therefore, tcMEP monitoring has been described to compliment the more traditional and established monitoring of somatosensory evoked potentials (SSEP). This combination seems to give important information about both global hemispheric ischemia and focal ischemia of the CST. The evaluation of the MEP monitoring suggests the modality to be highly feasible in the intraoperative setting during CEA. The rate of technical failure of tcMEP monitoring was found to be less than 2% and is therefore comparable to that of SSEP recording [1, 4].
However, the criteria for therapeutic intervention in cases of cerebral ischemia during carotid artery cross-clamping differ between the EP modalities in the literature. A reduction of the SSEP amplitude below 50% is a generally recognized marker for therapy requiring cerebral hypoperfusion. The complete loss of MEP signals has been described exclusively as warning criteria in all publications concerning MEP monitoring during CEA [1,2,3,4,5].
Additional MEP warning criteria have been investigated in the literature describing intracranial or spinal procedures, including reduction of amplitude (AMP) < 50% [6], morphologic changes (MOR) [7], increments of stimulation threshold level or drop of area under the curve (AUC) [8]. Segura et al. [8] investigated a multiparametric alarm criterion combining criteria targeting an earlier detection of potential neuronal damage in spinal deformity surgery. Unfortunately, none of the investigations could produce clear evidence that the additional MEP warning criteria alone or in combination are superior to the criterion of tcMEP loss. According to a recently published overview on criteria for MEP monitoring [9], only a decrement of MEP amplitude (< 50%) suggests a tendency to be more beneficial in preventing false negatives and postoperative motor deficits during cerebral vascular or tumor surgery.
However, in all of the analyses in the literature, therapeutic interventions during ICA cross-clamping were exclusively related to a loss of MEP recordings. Thus, this study is unique in investigating the correctness of the additional MEP warning criteria AMP, AUC and MOR and the combination thereof, with false positives as the primary outcome during carotid endarterectomy. Second, additional MEP warning criteria were evaluated in order to assess their potential to reduce false negative monitoring results of the established warning criteria.
2 Methods
The analysis of patients was approved by the Institutional Review Board by the ethical committee of Saxony, Germany (EK-BR-8/12-1).
2.1 Patients and procedural protocols
We retrospectively reviewed the monocentric medical records of 571 patients who underwent elective CEA at the Klinikum St. Georg gGmbH, Leipzig, Germany between 2011 and 2016. CEA was performed by a limited number of experienced anesthesiologists and vascular surgeons. Either synthetic patch graft and bypass or eversion CEA techniques were used. The neurologic status and evidence of cerebral infarction on computerized tomography or magnetic resonance imaging were preoperatively reviewed in patients with a history of stroke or transient ischemic attack. The diagnostic imaging was utilized within the admission of these patients or during earlier hospital stay. Patients with ICA stenosis were ranked as symptomatic or asymptomatic according to commonly used criteria [10, 11]. Patients with acute preoperative stroke requiring emergency intervention were not included in the study since patients entering our hospital with acute stroke requiring emergency surgery do not follow the standardized anesthesia and neurophysiological protocol. They also do not commonly receive neurophysiological monitoring.
An additional neuroanesthesiologist who was trained and certificated in the assessment of intraoperative neurophysiological monitoring performed the neurophysiological testing including the MEP recording. The clinical outcome of motor function was evaluated by the same anesthesiologist for all patients postoperatively. The motor function was first reviewed in the operation room after tracheal extubation in the presence of the vascular surgeons and additionally after transfer to the postoperative unit and before removal to the ward. In the case of a motor deficit, the patients were also evaluated every following day until the recovery of the deficit or until the patient’s discharge from the hospital. Furthermore, all patients showing intraoperative EP changes (established criteria) or postoperative motor deficits were formally examined by a neurologist. A transient motor deficit was defined in the case of recovery of the postoperative motor deficit before hospital discharge.
2.2 Anesthesia regime
According to the institutional protocol, we used the same anesthetic technique in all patients. Total intravenous anesthesia (TIVA) was achieved by administering a continuous infusion of propofol (4–7 mg/kg/h) and remifentanil (0.2–0.5 μg/kg/min). Etomidate (0.2 mg/kg) or propofol (2 mg/kg) and remifentanil (0.2–0.5 μg/kg/min) were used for induction of anesthesia, as was rocuronium (0.6 mg/kg). During the period of ICA cross-clamping of the target, arterial systolic blood pressure was set at 10% above preoperative values. If necessary, arterial hypotension was treated, and vasopressors (norepinephrine or Akrinor®: 1 amp. a 2 ml, Cafedrin-1HCl 200 mg and Theoadrenalin-HCl 10 mg) were used. Bradycardia was treated with atropine. In all cases throughout the procedure normothermia and normocapnia were maintained.
2.3 Intraoperative neurophysiologic monitoring
According to the standard practice during CEA in patients under general anesthesia, bilateral mSSEPs, tSSEPs, and tcMEPs of the upper extremities were recorded in all cases. The complex ISIS IOM system with Neuroexplorer software was used (inomed Medizintechnik GmbH, Im Hausgrün 29, 79312 Emmendingen, Germany). In addition, computerized Electroencephalogram (EEG) was elicited using the bispectral index (BIS; BIS-Modul for Philips M1034A with BISx, frontal montage, Aspect Medical Systems Inc., Framingham, MA, USA) for evaluating depth of anesthesia (target value: 35–45). EEG was not analyzed for cerebral ischemia.
Cortical SSEPs were recorded bilateral from the scalp using corkscrew electrodes (SDN 530751, inomed Medizintechnik GmbH, Im Hausgrün 29, 79312 Emmendingen, Germany) at C3′/C4′-Fz and Cz′-Fz according to the 10/20 system after electrical stimulation of the median and tibial nerve (needle electrodes/SDN 530630, inomed Medizintechnik GmbH, Im Hausgrün 29, 79312 Emmendingen, Germany).
Electrical stimulation of the precentral motor cortex for MEP measurement was transcranially delivered between C3 − C4 + 1 cm and C4 − C3 + 1 cm (corkscrew electrodes/SDN 530751, inomed Medizintechnik GmbH, Im Hausgrün 29, 79312 Emmendingen, Germany; intensity range 40–85 mA/constant voltage: 300 mV/impulse duration: 0.5 ms/train: 5/inter stimulus interval: 2 ms/stimulation repetition rate: 2 Hz/monophasic). Suprathreshold stimulation was performed in all patients. Motor evoked potential responses were recorded as standard from the abductor digiti minimi muscle (needle electrodes/SDN 530620, inomed Medizintechnik GmbH, Im Hausgrün 29, 79312 Emmendingen, Germany; bipolar/intramuscular/band pass: 5–2500 Hz).
The last measurements of the mSSEP and tcMEP amplitudes recorded before ICA cross clamping were set as the baseline. Any initial technical failure of MEP recording was registered, and such patients were excluded from further analyses.
2.4 Warning criteria and shunt application
The institutional protocol for neurophysiological warning criteria for therapeutic intervention (elevation of mean arterial blood pressure (MAP) and/or arterio-arterial shunt placement) dur-ing the period of ICA cross-clamping were: (1) decrement of mSSEP, (2) tSSEP amplitudes of more than 50% or (3) the complete loss of tcMEP signals (all-or-none interpretation). Persistence or progression of these defined changes was addressed by increasing the arterial blood pressure up to 20% to 30% of preoperative values achieved within 2 min. In the case of failure of responses recovery shunt insertion was suggested.
2.5 Data analysis
tcMEP amplitude, area under the curve, and number of phases (morphology) data were recorded from three measurements before and during ICA cross-clamping. tcMEP signals (AMP, AUC and MOR) were analyzed postoperatively using MATLAB (Version 2009b, The MathWorks, Inc., Natick, United States). For each measurement, the maximal peak-to-peak amplitude was determined. The area under the curve was calculated by rectification and numerical integration. The morphology of the signal was determined by calculating the number of zero-crossings. The numerical results were saved for further statistical analysis.
In the case of the additional MEP warning criteria [AMP or AUC reduction < 50% and MOR changes from polyphasic to biphasic (polyphasic > 3 zero-crossings)], all patients without therapeutic intervention were investigated for the percentage of false positives for the immediate postoperative motor outcome (primary parameter). Thus, a false positive result was defined as follows: no postoperative motor deficit without any significant intraoperative changes of the established modalities of SEP or MEP (signal loss) but occurrence of changes of the additional warning criteria (AMP or AUC reduction < 50% or MOR changes from polyphasic to biphasic). False positives were registered for each additional criteria alone and in combination (AMP/AUC and AMP/AUC/MOR). In addition, periods of time between alteration of one of the additional MEP warning criteria until the final loss of MEP were evaluated. Secondarily, the proportion of false negatives was calculated. Therefore, a false negative result was defined as postoperative motor deficit without significant intraoperative changes in one of the additional MEP criteria. False negatives of the established combined SEP/MEP, monitoring were analyzed separately. Additionally, in case of one of the additional MEP warning criteria showed significant changes prior a MEP loss, the period of time between both events was analyzed.
3 Results
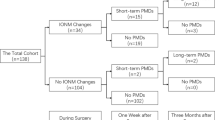
A cohort of 571 patients was reviewed (mean age: 70.46 y/o; female: 185 (32.40%)/male: 386 (67.60%), ASA 2.85). Four hundred and one patients (70.22%) were ranked determined to have a symptomatic ICA stenosis, and 302 (52.88%) patients had a history of ipsilateral stroke. Neurophysiological results were registered in all patients according to a standardized institutional protocol.
Initial MEP recording failed technically in 9 patients (1.57%). Four patients had a history of stroke, whereas MEP recording failed for technical or anesthesia reasons in 5 patients. According to the study protocol, these patients were excluded from further calculations. Intraoperative ischemia was detected by loss of MEP or 50% reduction of the cortical SSEP in 56 (9.96%) of the remaining 562 patients. All patients who showed significant neurophysiological changes received intervention. A shunt was inserted in 44 cases (7.83%), whereas in the remaining 12 cases (2.13%), elevation of the MAP was the only necessary intervention. However, an immediate postoperative deficit was recognized in the same cohort in 15 patients (2.66%). Thirteen patients showed significant intraoperative EP changes, and 2 patients did not (0.35% false negatives). Therefore, for the 504 patients without significant EP changes, no intervention and no postoperative motor deficit were registered as the referential cohort to investigate the primary parameter: false positives according to changes of additional MEP criteria (AMP, AUC and MOR). The results of the analysis of false positives and false negatives based on the additional MEP criteria are shown in Table 1.
The spectrum of postoperative motor deficits is illustrated in Table 2.
In the patients receiving intervention because of an intraoperative MEP loss, transient ischemia occurred in four cases. In one of these patients additional MEP warning criteria were clearly affected before the final MEP loss (AMP: 5:10 min; AUC: 12:45 min).
Otherwise, in five patients without postoperative motor deficit significant changes in at least one of the additional MEP criteria occurred 1–7 min before the MEP loss.
Other than this, in 12 patients receiving intervention following SSEP alerts without MEP loss we found significant changes of at least one of the additional MEP criteria.
4 Discussion
The question regarding the most efficient MEP warning criterion to interpret MEP changes correctly during spinal or cerebral surgery has been a subject of debate for more than a decade [9]. In contrast to D-wave activity, muscle MEP signals are unstable and may show large trial-to-trial variability, especially when a suprathreshold stimulation technique is used [12, 13]. One of the major reasons for the instability of muscle MEPs is the high sensitivity to anesthetic agents [14, 15]. Thus, the disappearance of muscle MEP responses has been used as a strong marker for postoperative motor deficit regardless of the neurosurgical procedure [13]. Studies of established research groups suggest the use of MEP-loss-criterion in spinal surgery to be the most reliable predictor for postoperative motor outcome [12, 15]. In contrast, some authors have demonstrated that changes in tcMEP morphology (from poly- to biphasic waveform) may correlate with postoperative motor dysfunction after intramedullary surgery [7]. The historical debate over the superior MEP warning criterion in spinal monitoring shows some similar aspects to the monitoring of cerebral ischemia. Unfortunately, comparison of both spinal and cerebral MEP monitoring is limited because of profound pathophysiological and methodical differences.
However, following the discussion in the literature, additional criteria of MEP signal interpretation, such as an amplitude reduction > 50%, morphologic changes or increase of stimulation intensity threshold may help to reduce the rate of false negative results. Especially in supratentorial neurosurgery, a potentially earlier recognition of a reduction of MEP amplitude lower than 50% of baseline values tends to be superior to the more robust criterion of MEP loss [16,17,18,19].
However, during carotid endarterectomy, a loss of MEP responses following cerebral ischemia has been described in the literature as the only criterion to initiate shunt application in the period of ICA cross-clamping [1, 2, 5]. According to MEP monitoring during carotid surgery, additional warning criteria have not been previously investigated. Thus, our study was designed to evaluate the validity of the warning criteria of a reduction of amplitude and area under the curve as well as changes in signal morphology during CEA in order to predict a correct postoperative motor outcome. We have therefore chosen a retrospective analysis of false positives as a primary parameter. Since an intraoperative therapeutic intervention was related to a defined SEP changes or MEP loss, the evaluation of false positive results of the additional warning criteria appeared to be a clear statistical parameter with regard to a valid evaluation of outcome prediction. To avoid distorting interventions, the patient cohort without any significant SEP changes or complete MEP loss was investigated. Since we found false positive results when considering AMP, AUC or MOR alone in 24% to 32.7%, the analyzed population of 504 patients seemed large enough, even considering the retrospective character of the study. Interestingly, this large proportion of false positives can obviously be reduced by combining AMP and AUC to 9.52%, but does not reach acceptable low values. The additional inclusion of MOR was also not beneficial to produce a further reduction of the false positive fraction (9.33%). In addition, this study could show evidence that the additional MEP warning criteria provide earlier alert in case of a MEP loss in order to potentially prevent postoperative motor deficit. Our data suggest that these alerts are rare and may be unproportional to the potency to produce false positive MEP results.
When using the criterion of 50% (even to 80%) amplitude decrement in spinal scenarios, similarly to our findings, MEP monitoring was shown to produce a large proportion of false positives and unacceptable positive predictive values [13, 14, 20]. The wide variation of MEP amplitudes as well as of latencies was observed even in neurologically intact patients [21]. As mentioned above, the 50% amplitude reduction criterion was demonstrated to reduce false negative results, especially in supratentorial neurosurgery [18, 19]. Therefore, there is a tendency to use the amplitude decrement as a major criterion in brain tumor surgery and cerebral aneurysm surgery. Differentiated analysis of developments of the area under the curve seems to be similar to changes in peak-to-peak MEP amplitudes, but it is obviously uncommon in routine practice and has only been described for spinal monitoring procedures [8].
However, with regard to carotid surgery, there is no evidence to date showing that more than a 50% reduction in MEP amplitude or area under the curve is superior to the assessment of a signal loss or could potentially decrease the number of false negatives. Thus, in our cohort, there were no patients identified who showed postoperative motor deficit or an intraoperative reduction in amplitude or area under the curve greater than 50% without complete MEP loss or significant SSEP changes. We investigated AUC in addition to the more common parameter AMP to pay attention to the complexity of the MEP wave form [22].
The two patients with false negatives in our population developed postoperative motor deficits without any previous MEP or SEP changes. Unfortunately, the analysis of false negative results of the additional MEP criteria may be incorrect. However, the results of our investigation do not suggest that additional MEP warning criteria have the ability to reduce false negative MEP monitoring. Since the number of false negatives was low, likely because the cohort was too small, the interpretation of this parameter is limited within our study. Although these results are informative in nature, they show a close relation to similar findings in the literature [3]. Regardless of our results, the use of the C3 − C4 + 1 cm montage for MEP stimulation may potentially increase the risk of false negatives.
Due to therapeutic intervention, we are unable to investigate whether or not earlier recognition of critical cerebral perfusion by looking at changes in amplitudes or AUC prior to the final MEP loss may have avoided postoperative transient motor deficit. In addition, the size of the patient cohort may have been too small to review these kinds of results retrospectively. Therefore, we chose false negatives as a secondary parameter and did not study sensitivity or specificity.
The evaluation of significant changes of MEP amplitude, area under the curve or signal morphology as potential warning criteria for the detection of clamping ischemia during CEA was shown to produce an unacceptably high number of false positives in a cohort of 504 patients. Certainly, a combination of AMP, AUC and MOR halved the proportion of false positives. Unfortunately, the reduction did not reach values below 9%. The use of the investigated additional MEP warning criteria may therefore not be beneficial during CEA even considering the retrospective nature of the study.
However, the results also confirm the low rate of false negatives of the established SEP and MEP warning criteria for the evaluation of cerebral perfusion during CEA.
References
Malcharek MJ, Ulkatan S, Marino V, Geyer M, Llado-Carbo E, Perez-Fajardo G, Arranz-Arranz B, Climent J, Aloj F, Franco E, Chiacchiari L, Kulpok A, Sablotzki A, Hennig G, Deletis V. Intraoperative monitoring of carotid endarterectomy by transcranial motor evoked potential: a multicenter study of 600 patients. Clin Neurophysiol. 2013;124(5):1025–30. https://doi.org/10.1016/j.clinph.2012.10.014.
Alcantara SD, Wuamett JC, Lantis JC, Ulkatan S, Bamberger P, Mendes D, Benvenisty A, Todd G. Outcomes of combined somatosensory evoked potential, motor evoked potential, and electroencephalography monitoring during carotid endarterectomy. Ann Vasc Surg. 2014;28(3):665–72. https://doi.org/10.1016/j.avsg.2013.09.005.
Malcharek MJ, Kulpok A, Deletis V, Ulkatan S, Sablotzki A, Hennig G, Gille J, Pilge S, Schneider G. Intraoperative multimodal evoked potential monitoring during carotid endarterectomy: a retrospective study of 264 patients. Anesth Analg. 2015;120(6):1352–60. https://doi.org/10.1213/ane.0000000000000337.
Malcharek MJ, Herbst V, Bartz GJ, Manceur AM, Gille J, Hennig G, Sablotzki A, Schneider G. Multimodal evoked potential monitoring in asleep patients versus neurological evaluation in awake patients during carotid endarterectomy: a single-centre retrospective trial of 651 patients. Minerva Anestesiol. 2015;81(10):1070–8.
Marino V, Aloj F, Vargas M, Spinelli G, Pompeo F, Chiacchiari L, Servillo G, Franco E. Intraoperative neurological monitoring with evoked potentials during carotid endarterectomy versus cooperative patients under general anesthesia technique: a retrospective study. J Neurosurg Anesthesiol. 2018;30(3):258–64. https://doi.org/10.1097/ANA.0000000000000430.
Journee HL, Berends HI, Kruyt MC. The percentage of amplitude decrease warning criteria for transcranial MEP monitoring. J Clin Neurophysiol. 2017;34(1):22–31. https://doi.org/10.1097/WNP.0000000000000338.
Quinones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, McDermott MW, Weinstein PR. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56(5):982–93. https://doi.org/10.1227/01.NEU.0000158203.29369.37.
Segura MJ, Talarico ME, Noel MA. A multiparametric alarm criterion for motor evoked potential monitoring during spine deformity surgery. J Clin Neurophysiol. 2017;34(1):38–48. https://doi.org/10.1097/WNP.0000000000000323.
MacDonald DB. Overview on criteria for MEP monitoring. J Clin Neurophysiol. 2017;34(1):4–11. https://doi.org/10.1097/WNP.0000000000000302.
Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Harbaugh RE, Dempsey RJ, Caplan LR, Kresowik TF, Matchar DB, Toole JF, Easton JD, Adams HP Jr, Brass LM, Hobson RW 2nd, Brott TG, Sternau L. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998;97(5):501–9. https://doi.org/10.1161/01.CIR.97.5.501.
De Fabritiis A, Conti E, Coccheri S. Management of patients with carotid stenosis. Pathophysiol Haemost Thromb. 2002;32(5–6):381–5. https://doi.org/10.1159/000073605.
Deletis V. Intraoperative neurophysiology and methodology for monitoring the motor system. In: Deletis V, Shils J, editors. Neurophysiology in neurosurgery: a modern intraoperative approach. San Diego: Academic Press; 2002. p. 25–51. https://doi.org/10.1016/B978-012209036-3/50004-4.
MacDonald DB, Skinner S, Shils J, Yingling C, American Society of Neurophysiological M. Intraoperative motor evoked potential monitoring—a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013;124(12):2291–316. https://doi.org/10.1016/j.clinph.2013.07.025.
Legatt AD, Emerson RG, Epstein CM, MacDonald DB, Deletis V, Bravo RJ, Lopez JR. ACNS guideline: transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2016;33(1):42–50. https://doi.org/10.1097/WNP.0000000000000253.
Kothbauer K. Motor evoked potential monitoring for intramedullary spinal cord tumor surgery. In: Deletis V, Shils J, editors. Neurophysiology in neurosurgery: a modern intraoperative approach, vol. 1. San Diego: Academic Press; 2002. p. 73–92. https://doi.org/10.1016/B978-012209036-3/50006-8.
Szelényi A, Hattingen E, Weidauer S, Seifert V, Ziemann U. Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging. Neurosurgery. 2010;67(2):302–13. https://doi.org/10.1227/01.NEU.0000371973.46234.46.
Szelényi A, BuenodeCamargo A, Flamm E, Deletis V. Neurophysiological criteria for intraoperative prediction of pure motor hemiplegia during aneurysm surgery. Case report. J Neurosurg. 2003;99(3):575–8. https://doi.org/10.3171/jns.2003.99.3.0575.
Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: intraoperative changes and postoperative outcome. J Neurosurg. 2006;105(5):675–81. https://doi.org/10.3171/jns.2006.105.5.675.
Neuloh G, Schramm J. Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg. 2004;100(3):389–99. https://doi.org/10.3171/jns.2004.100.3.0389.
Langeloo DD, Journee HL, de Kleuver M, Grotenhuis JA. Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery A review and discussion of the literature. Neurophysiol Clin. 2007;37(6):431–9. https://doi.org/10.1016/j.neucli.2007.07.007.
Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996;100:375–83. https://doi.org/10.1016/0168-5597(96)95728-7.
van Dongen EP, ter Beek HT, Schepens MA, Morshuis WJ, Langemeijer HJ, de Boer A, Boezeman EH. Within-patient variability of myogenic motor-evoked potentials to multipulse transcranial electrical stimulation during two levels of partial neuromuscular blockade in aortic surgery. Anesth Analg. 1999;88(1):22–7. https://doi.org/10.1097/00000539-199901000-00005.
Funding
There were no funding sources supporting this study or the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kathrin Thoma and Celine Wegner are employees of inomed Medizintechnik GmbH, Im Hausgrün 29, 79312 Emmendingen, Germany. There are no other patents, products in development or other marketed products to declare. This does not alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials. No other authors have potential conflicts of interest to be disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malcharek, M.J., Hesse, J., Hesselbarth, K. et al. Warning criteria for MEP monitoring during carotid endarterectomy: a retrospective study of 571 patients. J Clin Monit Comput 34, 589–595 (2020). https://doi.org/10.1007/s10877-019-00345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00345-5