Abstract
Six dinuclear lanthanide(III) complexes [Ln2(L)6(phen)2] (Ln = EuIII (1), TbIII (2), DyIII (3), HoIII (4), ErIII (5), SmIII (6), L = 2-(thiophen-2-ylselanyl)acetic acid, phen = 1,10-phenanthroline) have been synthesized and characterized by powder X-ray diffraction and single-crystal X-ray diffraction analysis. Single crystal X-ray diffraction analysis showed that complexes 1–6 are isostructural and crystallize in the triclinic space group P-1. The luminescent properties and lifetimes of complexes 1–3 have been studied, which show the characteristic emissions of EuIII (5D0 → 7F0-4), TbIII (5D4 → 7F6-3) and DyIII (4F9/2 → 6H15/2, 13/2, 11/2, 9/2), and the corresponding luminescent lifetimes are 1.24 ms, 6.25 μs and 4.26 μs, respectively. The magnetic properties of the complexes 3 and 4 were also measured and exhibited weak anti-ferromagnetic interactions between LnIII centers. Moreover, the complex 3 showed the properties of the potential single-molecule magnets with the energy barrier (U/kB) 47.50 K and the τ0 9.73 × 10–8 s. In view of this, the DyIII complex 3 displayed the behavior of the luminescent single-molecule magnets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past few decades, the lanthanide complexes have attracted worldwide interests due to their potential applications in the fields of sorption, catalysis, magnetism and photochemistry [1,2,3]. Since the milestone discovery of a terbium(III) bis(phthalocyaninato) complex displaying the phenomenon of single-molecule magnets (SMMs), which are molecular complexes exhibiting slow relaxation of the magnetization and magnetic hysteresis at low temperature, the designs and syntheses of lanthanide-based SMMs have experienced impressive development, and a large number of lanthanide-based SMMs have been reported, and some of them exhibited slow relaxation of the magnetization with high anisotropy barrier [4,5,6,7]. In the last decade, the rational designs of the multifunctional SMMs with other properties, in which the targeted compounds may exhibit different physical features when subjected to various external stimuli, have attracted the attention of many researchers [8,9,10]. Most lanthanide ions possess strong unquenched orbital angular momentum and large spin orbit coupling of the 4f electrons, they not only may contribute to the acquisition of single-molecule magnets but also contribute to the improvement of luminescence performance, so that lanthanide ions are a great choice for constructing the multifunctional SMMs [11,12,13,14]. The luminescent single-molecule magnets are a type of dual functional materials with magnetic and luminescent properties. The incorporation of two properties into one molecular entity is an effective way to obtain dual functional molecule-based materials [15,16,17,18].
The designs and synthesis of the luminescent SMMs requires consideration of many factors, such as metal ions and ligands. Lanthanide(III) ions, especially the Dy(III) ion, have not only a large spin ground state but also magnetic anisotropy, which can meet the two prerequisites for the appearance of the luminescent SMMs [19,20,21,22,23]. On the other hand choosing a suitable ligand can effectively produce an ‘‘antenna effect’’ to promote the regulation of its luminescent properties [24, 25]. Among various types of ligands, 2-thiophene carboxylate acid substituents containing unsaturated bonds and isolated electrons functional groups can transfer efficiently the energy of the π, π* excited state to lanthanide ions to increase their luminescence properties [26,27,28,29,30]. In addition, it is noteworthy that the 1,10-phenanthroline possessing multiple functional groups as chelating ligand can not only helps to stability of constructed complexes but also improve the fluorescence emissions [31, 32].
Given the aforementioned consideration, we chose 2-(thiophen-2-ylselanyl)acetic acid as the main ligand and 1,10-phenanthroline as the auxiliary ligand to synthesize six isostructural lanthanide complexes [Ln2(L)6(phen)2] (Ln = EuIII (1), TbIII (2), DyIII (3), HoIII (4), ErIII (5), SmIII (6) L = 2-(thiophen-2-ylselanyl)acetic acid, phen = 1,10-phenanthroline). The structures of complexes 1–6 display dinuclear structures. The photoluminescence properties of complex 1–3 and the magnetic properties of complexes 3–4 have been investigated in the solid state. Furthermore, the DyIII complex 3 displayed the phenomenon of the potential multifunctional SMMs with fluorescent properties.
Experimental Section
Materials and Physical Measurements
Lanthanide(III) chloride hexahydrate and 1,10-phenanthroline were commercially available and were all used without further purification. 2-(thiophen-2-ylselanyl)acetic acid was prepared by the method reported in the literatures [33, 34]. The contents of C, H and N were depicted in Vario-EL II elemental analyzer. IR spectra were recorded using the conventional KBr pellets technique by a Nicolet-5700 spectrometer (4000–400 cm−1). The powder X-ray diffraction (PXRD) data of the samples were collected on a Rigaku Dmax 2000 X-ray diffractometer with graphite mono chromatized Cu Kα radiation (λ = 0.1542 nm) and 2θ ranging from 5° to 50°. The fluorescence spectra were collected on Edinburgh FLS 1000 fluorescence spectrometer. Magnetic properties were measured using a Quantum Design SQUID VSM magnetometer on polycrystalline samples. All magnetic data were corrected for the diamagnetism of the sample holder and constituent atoms according to Pascal’s constants.
Syntheses of Complexes 1–6
Six dinuclear lanthanide(III) complexes were prepared with a molar ratio of 3:1:1. The syntheses methods of complexes 1–6 are similar, so complex 1 is taken as an example to describe the synthesis steps (Fig. 1).
A mixture of 2-(thiophen-2-ylselanyl)acetic acid (0.30 mmol), 1,10-phenanthroline (0.10 mmol) were dissolved in methanol, adjusting the pH of the solution to 5.8–6.2 with a KOH solution (0.2 mol/L). Then, the resulting solution was added dropwise into the turbid aqueous solution of EuCl3·6H2O (0.10 mmol) under stirring. After stirring at room temperature for 4 h, the filtered solution was left to stand for 10 days, and finally the block single crystals suitable for X-ray analysis were obtained.
[Eu2[(C4H3S)SeCH2COO]6(phen)2] (1): Yield: 49.37%. Elemental analysis (%), calculated for C60H46Eu2N4O12S6Se6: C, 36.34, H, 2.35, N, 2.79. Found: C, 36.52, H, 2.51, N, 2.92. IR data (KBr, cm−1): 3426(w), 3081(w), 1602(s), 1589(m), 1545(m), 1516(s), 1436(s), 1386(s), 1348(w), 1215(w), 1188(w), 1140(w), 1102(w), 1047(w), 961(w), 864(s), 844(s), 773(w), 729(m), 697(m), 687(s), 670(w), 417(w).
[Tb2[(C4H3S)SeCH2COO]6(phen)2] (2): Yield: 48.95%. Elemental analysis (%), calculated for C60H46Tb2N4O12S6Se6: C, 36.05, H, 2.32, N, 2.80. Found: C, 36.22, H, 2.46, N, 2.99. IR data (KBr, cm−1): 3424(w), 3082(w), 1603(s), 1590(m), 1547(m), 1517(s), 1439(s), 1387(s), 1348(w), 1215(w), 1189(w), 1139(w), 1102(w), 1047(w), 963(w), 864(m), 844(s), 773(w), 729(m), 698(m), 688(s), 418(w).
[Dy2[(C4H3S)SeCH2COO]6(phen)2] (3): Yield: 54.37%. Elemental analysis (%), calculated for C60H46Dy2N4O12S6Se6: C, 35.92, H, 2.31, N, 2.79. Found: C, 35.78, H, 2.19, N, 2.63. IR data (KBr, cm−1): 3422(w), 3083(w), 1603(s), 1590(m), 1548(m), 1517(s), 1440(s), 1387(s), 1348(w), 1218(w), 1189(w), 1139(w), 1102(w), 1047(w), 964(w), 864(s), 843(s), 773(w), 729(m), 698(m), 688(s), 671(w), 418(w).
[Ho2[(C4H3S)SeCH2COO]6(phen)2] (4): Yield: 51.67%. Elemental analysis (%), calculated for C60H46Ho2N4O12S6Se6: C, 35.83, H, 2.31, N, 2.79. Found: C, 36.07, H, 2.44, N, 2.95. IR data (KBr, cm−1): 3416(w), 3084(w), 1604(s), 1590(m), 1550(m), 1517(s), 1441(s), 1388(s), 1348(w), 1215(w), 1189(w), 1139(w), 1103(w), 1047(w), 964(w), 864(s), 843(s), 773(w), 729(m), 698(m), 689(s), 672(w), 418(w).
[Er2[(C4H3S)SeCH2COO]6(phen)2] (5): Yield: 32.46%. Elemental analysis (%), calculated for C60H46Er2N4O12S6Se6: C 35.75, H 2.30, N 2.78. Found: C, 35.55, H, 2.15, N, 2.60. IR data (KBr, cm−1): 3439(w), 3085(w), 1604(s), 1590(m), 1551(m), 1517(s), 1443(s), 1388(s), 1347(w), 1215(w), 1190(w), 1139(w), 1103(w), 1048(w), 965(w), 864(s), 843(s), 773(w), 729(m), 698(m), 689(s), 673(w), 418(w).
[Sm2[(C4H3S)SeCH2COO]6(phen)2] (6): Yield: 48.95%. Elemental analysis (%), calculated for C60H46Sm2N4O12S6: C, 36.36, H, 2.34, N, 2.83. Found: C, 36.21, H, 2.22, N, 2.69. IR data (KBr, cm−1): 3422(w), 3082(w), 1598(s), 1588(m), 1542(m), 1516(s), 1430(s), 1385(s), 1215(w), 1187(w), 1139(w), 1101(w), 960(w), 863(s), 843(s), 773(w), 729(m), 696(m), 685(s), 668(w), 417(w).
X-ray Crystallography
Single-crystal X-ray diffraction data for the complexes 1–6 were collected on a Bruker SMART-1000 CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) radiation at room temperature. The data were integrated and corrected for Lorentz and polarization effects using SAINT [35]. Absorption corrections were applied with SADABS [36]. The structures were solved by direct methods and refined by the full-matrix least-squares method on F2 using the SHELXTL crystallographic software package [37]. During the final cycles, the atoms were refined anisotropically. Hydrogen atoms were placed in calculated positions and refined as riding atoms. Some constraints (SIMU, DELU and ISOR instructions in SHELXL2014) on the anisotropic displacement parameters were applied in the refinement procedures to restrain the thermal ellipsoid [37, 38]. Crystallographic data and structural refinement details of complexes 1–6 are summarized in Table 1. Selected bond lengths and bond angles of complex 1–6 are listed in Tables S2 to S7.
Results and Discussion
Crystal Structure Descriptions
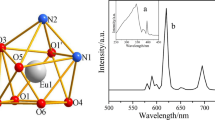
X-ray crystallographic study reveals that complexes 1–6 are isostructural except for the distinction of the lanthanide ion. Each of them is a dinuclear structure and crystallizes in the Triclinic space group P-1 with Z = 1, thus complex 2 is selected as a representative of the six complexes to discuss their structures in detail. As is shown in Fig. 2, complex 2 is composed of two TbIII ions, six deprotonated 2-(thiophen-2-ylselanyl)acetic acid ligand, one 1,10-phenanthroline molecule. Every TbIII centre is surrounded by nine atoms, exhibiting a tricapped triangular prism geometry with seven oxygen atoms and two nitrogen atoms, two oxygen atoms (O1, O2) from one chelating deprotonated 2-(thiophen-2-ylselanyl)acetic acid ligand, three oxygen atoms (O3A, O4, µ2-O5A) from three bridging deprotonated ligands, two oxygen atoms (µ2-O5, O6) from one bridging deprotonated ligand, and two nitrogen atoms (N1, N2) from one chelating 1,10-phenanthroline molecule. Using the program SHAPE 2.1 [39], the continuous shape measures (CShMs) of the TbIII centers relative to the ideal Cs symmetry is calculated to be 5.631. The Tb-O bond distances range from 2.299(5) to 2.481(5) Å, and the Tb-N bond distances range from 2.496(7) to 2.625(6) Å, which are within the bond distances of Tb-O and Tb-N of the previous terbium complexes [40, 41]. The intramolecular TbIII···TbIII distances of 3.828 Å, which are within the of TbIII···TbIII distances reported for lanthanide clusters [42].
The adjacent dinuclear complexes extend along a axis direction to form a one-dimensional (1D) chain through the C–H···O (C5–H5···O4, 2.287 Å) intermolecular hydrogen bond interaction (Fig. 3). Furthermore, such 1D chains assemble into 2D layers supramolecular architecture via the C–H···O (C7–H7···O1, 2.439 Å) hydrogen bonding interactions in the a c plane (Fig. 4).
In complex 2, the deprotonated 2-(thiophen-2-ylselanyl)acetic acid adopt multiple coordination modes with k(O,O′) chelating mode, and μ-1 k(O);2 k(O′) or μ-1 k(O,O′);2 k(O′) bridge modes to connect Tb atoms into dinuclear coordination complex (Fig. 5) [43].
Powder X-ray Diffraction and Thermogravimetric Analyses of Complexes 1–6
In order to evaluate the phase purity of complexes 1–6, powder X-ray diffraction patterns experiments have been performed at the room temperature. The PXRD patterns of complexes 1–6 are shown in Figs. S7 to S12, respectively. Their high purity solid state phases were confirmed by the good matching the measured and simulated PXRD patterns.
The thermogravimetric analyses (TGA) of complexes 1–6 are measured in the temperature range from 25 to 800 °C under nitrogen atmosphere with a heating rate of 20 K min−1 (Fig. S13). The weight loss behaviours of complexes 1–6 are similar and there is only one step of weight loss in the temperature region of 250–310 °C. The TGA results of complexes 1–6 indicate good thermal stability.
Magnetic Properties
Static Magnetic Properties
The variable-temperature magnetic susceptibility measurements for 3 and 4 were carried out over the temperature range of 2 to 300 K in alternating current field of 1 kOe. The plots of χMT and χM−1 versus T are shown in Fig. 6a for 3 and Fig. 6b for 4. The experimental χMT values at 300 K are 29.96 cm3 mol−1 K for 3 and 21.94 cm3 mol−1 K for 4, respectively, which are approached to the expected values of LnIII ions (DyIII: 28.34 cm3 mol−1 K, S = 5/2, L = 5, g = 4/3; HoIII: 28.14 cm3 mol−1 K, S = 2, L = 6, g = 5/4). With decreasing temperature, the χMT values exhibit very slow decrease down to 50 K and then sharply decrease in the lower temperature regions, at 2 K reach a minimum of 9.30 cm3 mol−1 K for 3 and 2.91 cm3 mol−1 K for 4. This behavior should be ascribed to depopulation of the excited Stark sublevels from the crystal-field splitting of LnIII ions and/or antiferromagnetic interactions. This similar features was found in the reported LnIII complexes [Dy2(HMBA)2(MBA)2(DMF)2(H2O)2]∙6H2O and {[Ho2(Hpimda)2(μ4-C2O4)] 2H2O 4H2O}n [44, 45]. By analyzing of χM−1 versus T, the magnetic susceptibility obeys the Curie–Weiss law, χM = C/(T-θ), between 50 and 300 K with Curie constant C are 30.39 cm3 mol−1 K for 3 and 22.93 cm3 mol−1 K for 4, the Weiss constant θ are − 3.83 K for 3 and − 12.80 K for 4, and then the negative Weiss constant values further exhibits weak antiferromagnetic indicated anti-ferromagnetic interactions between LnIII ions [46].
The field dependencies of the magnetization were performed in the magnetic field range 0–70 kOe at 2 K for 3 and 4. As shown in Fig. 7, the magnetization values first increase rapidly and then increase slowly with increased field and reach the values of 6.0 Nβ for 3 and 4.5 Nβ for 4 at 70 kOe. The observed saturation values for 3 and 4 are lower than the calculated values, which may be ascribed to the crystal field effects, low-lying excited states and/or significant magnetic anisotropy. The similar phenomenon was found in the other reported DyIII and HoIII clusters complexes [47, 48].
Dynamic Magnetic Properties
In order to explore the potential slow magnetic relaxation of DyIII complex 3, which may originate from SMMs behavior, alternating current (ac) magnetic susceptibility measurements were performed at various temperatures and frequencies. As shown in Fig. 8, under zero dc fields, both in-phase (χ′) and out-of-phase (χ″) signals clearly were observed in the temperature range of 2.0–10 K, but no peaks maxima were observed even at a low temperature and high frequency. The upturning and tailing of the curve in the low temperature area indicates that there is a significant QTM effect in the system. In order to study the relaxation behavior more clearly, an external magnetic field of 1000 Oe was applied. The QTM in the system was effectively suppressed. As shown in Fig. 9, the out-of-phase (χ″) signals exhibit well-shaped peaks, which shift gradually to lower frequencies with the decrease of temperature. In other words, as shown in Fig. 10, the imaginary part (χ″) can clearly display strong frequency dependence in the frequency range of 2.5–999 Hz, suggesting characteristic magnet relaxation of the SMMs [49, 50]. Furthermore, the relaxation times (τ) are obtained by applying the generalized Debye law in a range from 4.0 to 7.0 K (Fig. 11), giving the parameters α of 3.607 × 10–2–1.795 × 10–1 listed in Table S9. The similar parameter values were observed for other DyIII SMMs [51, 52]. At high temperature range, satisfactory results can be achieved by analyzing ln(τ) versus T−1 using the Arrhenius law τ = τ0exp(U/kBT). As shown in Fig. 12a, the resulting best-fit parameters are an energy barrier U/kB of 47.5 K and τ0 of 9.73 × 10–8 s, and U/kB parameters agrees well with some examples of reported lanthanide clusters (Table S10) [53,54,55,56]. Nevertheless, τ becomes weakly dependent on T with decreasing temperature, which is possibly due to the mixture of the Orbach process and other relaxation processes. Moreover, the suitable fit was obtained by considering contributions from a Raman and an Orbach processes, τ−1 = CTn + τ0−1 exp(− U/kBT), the resulting best-fit parameters are an energy barrier U/kB = 53.28 K, C = 0.29 s−1 K−4.86, n = 4.86, τ0 = 6.48 × 10–8 s. The n value of 4.86 is reasonable value (1 < n < 6), and the τ0 value falls within the normal range (τ0 = 10–6–10–11), which is consistent with the properties of the reported single-molecule magnets [57,58,59]. Based on the above research, the DyIII complex 3 exhibited the behavior of single molecule magnets.
Photoluminescence Properties
The trivalent lanthanide ions are wide researched for their photoluminescence properties in the visible and near-infrared regions, which endows Ln3+ luminescent materials with wide applications in photonic devices. Based on this, the solid-state luminescent properties of the complexes 1–3 have been investigated at room temperature. Their excitation and emission spectra and lifetime decay behaviors are shown in Figs. 13 and 14, corresponding colour coordinates are shown in Fig. S14.
The excitation spectra of complexes 1–3 all show broad band, which are attributed to the π–π* transition of the ligands. Excitation of complex 1 (Fig. 13a), EuIII displays weak characteristic f-f transitions at 396 nm, which can be reasonably assigned to the 7F0 → 5L6 transitions of EuIII. The f–f transitions are weaker than the ligand-bands, which suggest the better sensitization of EuIII ions luminescence through ligand excitation. Such the properties of complex 1 has been also found in other Eu-selenoacetic complex, [Eu2L6(phen)2] (L = 4-fluorophenylselenoacetate) [60]. EuIII complex displays five characteristic sharp emission bands at 580, 592, 615, 652, and 698 nm under the excitation of 348 nm (Fig. 13a), which can be assigned to the 5D0 → 7F0–4 transitions, respectively. The intensity of the 5D0 → 7F2 transition, which is extremely sensitive to site symmetry, is stronger than the 5D0 → 7F1 transition. Therefore, the EuIII ion in complex 1 should occupy sites with a low symmetry and no inversion center should be present for these sites. The similar result is also found in {[Eu(L1)(DMF)(H2O)]·DMF}n (5-(2′-carboxylphenoxy)isophthalic acid) [61]. As shown in Fig. S14a, the CIE chromaticity coordinate values of complex 1 are (0.6629, 0.3367) and ‘‘red’’ emission is emitted. The emission spectra of complex 2 (Fig. 13b), TbIII complex displays four characteristic sharp emission bands at 489, 545, 584, 621 nm under the excitation of 335 nm, which can be assigned to the 5D4 → 7F6–3 transitions, respectively. The most intense peak at 545 nm represents the 5D4 → 7F5 transition, which shows the green light emission for TbIII ions with a CIE chromaticity coordinate of (0.3425, 0.5639) (Fig. S14b). The emission spectra of complex 3 (Fig. 13c), DyIII complex displays four characteristic sharp emission bands at 482, 572, 658, 750 nm under the excitation of 348 nm, which can be assigned to the 4F9/2 → 6HJ (15/2, 13/2, 11/2, 9/2) transitions, respectively. Notably, the DyIII complex emits a yellow light with a CIE coordinate of (0.3873, 0.4317) (Fig. S14c). In addition, it is found that there is no apparent residual ligand-based luminescence emission in complexes 1–3, indicating that EuIII and TbIII and DyIII centres can be efficiently sensitized by the ligands [62, 63].
The luminescence decay curve can be well fitted to the double-exponential function as I = A1 exp(− t/τ1) + A2 exp(− t/τ2) (where τ1 and τ2 are the fast and slow components of the luminescence lifetimes, A1 and A2 are the pre-exponential factors). And then, based on τ = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) and τ is the luminescent lifetime [64, 65], yielding the average lifetimes values of around 1.24 ms for 1, 6.25 μs for 2, 4.26 μs for 3, which are moderate compared to previous reports [66,67,68]. Furthermore, photoluminescence quantum yields of complexes 1 and 3 are also determined by the integration sphere method, which are 23.10% and 1.30%, respectively. The value for complex 2 is too small (< 1%) to be measured accurately. These preliminary results indicate that the energy transfer from the ligands to LnIII ions is efficient [68,69,70].
Conclusions
In summary, new six lanthanide(III) complexes were successfully synthesized and characterized. The single X-ray crystallography study reveal that the lanthanide(III) ions adopt a nine-coordinated tricapped triangular prism geometry and adjacent molecules are connected by hydrogen bonding interactions to form infinite 1D chain structures and 2D layers. The solid-state luminescent spectra demonstrated that complexes 1–3 respectively exhibited strong characteristic emissions. Magnetic studies revealed that the anti-ferromagnetic interactions existed between the adjacent Ln3+ ions complexes 3 and 4. Moreover, the complex 3 showed the phenomenon of the single-molecule magnets with the energy barrier (U/kB) 47.50 K and the τ0 9.73 × 10–8 s. In view of this, the DyIII complex 3 displayed the phenomenon of the luminescent SMMs, which offers valuable information for the designs and syntheses of the lanthanide-based multifunctional single-molecule magnets.
References
A. Topor, D. Liu, C. Maxim, G. Novitchi, C. Train, Z. A. AlOthman, A. A. S. Al-Kahtani, L. Ungur, L. T. A. Ho, L. F. Chibotaru, and M. Andruh (2021). J. Mater. Chem. C 9, 10912–10926.
C. Theppitak, F. Kielar, W. Dungkaew, M. Sukwattanasinitt, L. Kangkaew, S. Sahasithiwat, H. Zenno, S. Hayami, and K. Chainok (2021). RSC Adv. 11, 24709–24721.
S. Dey and G. Rajaraman (2020). Dalton Trans. 49, 14781–14785.
N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara, and Y. Kaizu (2003). J. Am. Chem. Soc. 125, 8694–8695.
F.-S. Guo, M. Day Benjamin, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki, and A. Layfield Richard (2018). Science 362, 1400–1403.
Z. Zhu, C. Zhao, T. Feng, X. Liu, X. Ying, X.-L. Li, Y.-Q. Zhang, and J. Tang (2021). J. Am. Chem. Soc. 143, 10077–10082.
F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Büchner, and A. A. Popov (2017). Nat. Commun. 8, 16098.
J. Long, J. Rouquette, J.-M. Thibaud, R. A. S. Ferreira, L. D. Carlos, B. Donnadieu, V. Vieru, L. F. Chibotaru, L. Konczewicz, J. Haines, Y. Guari, and J. Larionova (2015). Angew. Chem. Int. Ed. 54, 2236–2240.
J.-Y. Wang, Y. Shi, D.-L. Tao, G.-Y. Yin, and Q.-B. Bo (2020). CrystEngComm 22, 4449–4467.
J. Long, R. Vallat, R. A. S. Ferreira, L. D. Carlos, F. A. Almeida Paz, Y. Guari, and J. Larionova (2012). Chem. Commun. 48, 9974–9976.
M. Nie, L. Yang, C. Zhao, H. Meng, L. Feng, P. Jin, C. Wang, and T. Wang (2019). Nanoscale 11, 18612–18618.
G. Cucinotta, M. Perfetti, J. Luzon, M. Etienne, P.-E. Car, A. Caneschi, G. Calvez, K. Bernot, and R. Sessoli (2012). Angew. Chem. Int. Ed. 51, 1606–1610.
D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Büchner, and A. A. Popov (2017). Chem. Commun. 53, 7901–7904.
R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch, and T. Greber (2012). J. Am. Chem. Soc. 134, 9840–9843.
Q.-Q. Su, K. Fan, X.-X. Jin, X.-D. Huang, S.-C. Cheng, L.-J. Luo, Y.-J. Li, J. Xiang, C.-C. Ko, L.-M. Zheng, and T.-C. Lau (2019). Inorg. Chem. Front. 6, 1442–1452.
J.-H. Jia, Q.-W. Li, Y.-C. Chen, J.-L. Liu, and M.-L. Tong (2019). Coord. Chem. Rev. 378, 365–381.
J. Long, Y. Guari, R. A. S. Ferreira, L. D. Carlos, and J. Larionova (2018). Coord. Chem. Rev. 363, 57–70.
H.-F. Wang, J.-X. Tang, H.-H. Zou, and F.-P. Liang (2020). J. Clust. Sci. 31, 1155–1161.
F. Pointillart, B. L. Guennic, S. Golhen, O. Cador, O. Maury, and L. Ouahab (2013). Chem. Commun. 49, 615–617.
R. Sessoli and A. K. Powell (2009). Coord. Chem. Rev. 253, 2328–2341.
S. Chorazy, M. Rams, K. Nakabayashi, B. Sieklucka, and S.-I. Ohkoshi (2016). Chem. Eur. J. 22, 7371–7375.
J. Ruiz, A. J. Mota, A. Rodríguez-Diéguez, S. Titos, J. M. Herrera, E. Ruiz, E. Cremades, J. P. Costes, and E. Colacio (2012). Chem. Commun. 48, 7916–7918.
S. Chorazy, J. Wang, and S.-I. Ohkoshi (2016). Chem. Commun. 52, 10795–10798.
W.-Q. Zhang, R.-F. Zhang, Q.-F. Zhang, S.-L. Zhang, J. Ru, Q.-L. Li, and C.-L. Ma (2019). Solid State Chem. 270, 360–365.
A. S. Kalyakina, V. V. Utochnikova, M. Zimmer, F. Dietrich, A. M. Kaczmarek, R. Van Deun, A. A. Vashchenko, A. S. Goloveshkin, M. Nieger, M. Gerhards, U. Schepers, and S. Bräse (2018). Chem. Commun. 54, 5221–5224.
R. J. Batrice, A. K. Adcock, P. M. Cantos, J. A. Bertke, and K. E. Knope (2017). Cryst. Growth Des. 17, 4603–4612.
K. E. Horner and P. B. Karadakov (2013). J. Org. Chem. 78, 8037–8043.
F. Cagnin, M. R. Davolos, and E. E. Castellano (2014). Polyhedron 67, 65–72.
C.-H. Zhan, F. Wang, Y. Kang, and J. Zhang (2012). Inorg. Chem. 51, 523–530.
Y.-G. Sun, B. Jiang, T.-F. Cui, G. Xiong, P. F. Smet, F. Ding, E.-J. Gao, T.-Y. Lv, K. Van den Eeckhout, D. Poelman, and F. Verpoort (2011). Dalton Trans. 40, 11581–11590.
C.-M. Liu, D.-Q. Zhang, and D.-B. Zhu (2016). Chem. Commun. 52, 4804–4807.
J. Ye, Q. Wang, H. Gao, X. Lu, W. Gong, Y. Lin, and G. Ning (2012). Inorg. Chim. Acta 384, 1–7.
W. H. H. Günther and M. N. Salzman (1972). Ann. N. Y. Acad. Sci. 192, 25–43.
N. Stuhr-Hansen, T. I. Sølling, and L. Henriksen (2011). Tetrahedron 67, 2633–2643.
Bruker Analytical X-ray Systems, Smart & SAINT Software Reference Manuals, Version 6.45 (Bruker Analytical X-ray Systems, Madison, 2003).
G. M. Sheldrick, SADABS an empirical absorption correction program (Bruker Analytical X-ray Systems, Madison, 1996).
G. M. Sheldrick (2015). Acta Crystallogr. Sect. C: Struct. Chem. 71, 3–8.
M. Emami, K. A. Ślepokura, M. Trzebiatowska, N. Noshiranzadeh, and V. Kinzhybalo (2018). CrystEngComm 20, 5209–5219.
D. Casanova, P. Alemany, J. M. Bofill, and S. Alvarez (2003). Chem. Eur. J. 9, 1281–1295.
Y.-Y. Li, N. Ren, S.-M. He, S.-P. Wang, and J.-J. Zhang (2020). Appl. Organometal. Chem. 34, e5418.
C.-W. Jin, Y. Wang, N. Ren, L.-N. Geng, and J.-J. Zhang (2016). J. Chem. Thermodyn. 103, 181–188.
J.-Y. Zhao, N. Ren, and J.-J. Zhang (2021). Thermochim. Acta 699, 178915.
G. B. Deacon, P. C. Junk, W. W. Lee, M. Forsyth, and J. Wang (2015). New J. Chem. 39, 7688–7695.
L. Zhong, W.-B. Chen, X.-H. Li, Z.-J. OuYang, M. Yang, Y.-Q. Zhang, S. Gao, and W. Dong (2020). Inorg. Chem. 59, 4414–4423.
X. Feng, J. Zhao, B. Liu, L. Wang, S. Ng, G. Zhang, J. Wang, X. Shi, and Y. Liu (2010). Cryst. Growth Des. 10, 1399–1408.
L. Hua, F.-W. Zheng, H.-T. Chen, L. Wang, D.-J. Li, L. Yang, F.-J. Han, X.-Y. Duan, T.-T. Liu, and W.-X. Wang (2021). Solid State Chem. 303, 122463.
J.-B. Peng, Y.-P. Ren, X.-J. Kong, L.-S. Long, R.-B. Huang, and L.-S. Zheng (2011). CrystEngComm 13, 2084–2090.
Y. M. Litvinova, Y. M. Gayfulin, A. S. Bogomyakov, D. G. Samsonenko, and Y. V. Mironov (2017). J. Clust. Sci. 28, 3103–3114.
S. Yu, Z. Chen, H. Hu, B. Li, Y. Liang, D. Liu, H. Zou, D. Yao, and F. Liang (2019). Dalton Trans. 48, 16679–16686.
W. Zhang, S.-M. Xu, Z.-X. Zhu, J. Ru, Y.-Q. Zhang, and M.-X. Yao (2020). New J. Chem. 44, 2083–2090.
C. Zhang, X. Ma, P. Cen, X. Jin, J. Yang, Y.-Q. Zhang, J. Ferrando-Soria, E. Pardo, and X. Liu (2020). Dalton Trans. 49, 14123–14132.
S.-L. Zhang, S.-S. Li, S.-Y. Zeng, Y. Shi, D.-Q. Wang, and L. Chen (2020). New J. Chem. 44, 2408–2413.
Y.-P. Hua, C.-L. Xue, W.-M. Zhang, Y. Liu, J.-L. Tian, and W.-M. Wang (2021). J. Mol. Struct. 1227, 129510.
Y. Wang, Z. Yuan, Y. Guo, X. Ma, Z. Meng, J. Sha, and H. Zhang (2020). Z. Anorg. Allg. Chem. 646, 1696–1701.
K. Zhang, D. Liu, V. Vieru, L. Hou, B. Cui, F.-S. Guo, L. F. Chibotaru, and Y.-Y. Wang (2017). Dalton Trans. 46, 638–642.
D.-F. Wu, H.-Y. Shen, X.-Y. Chu, W.-J. Chang, L.-H. Zhao, Y.-Y. Duan, H.-H. Chen, J.-Z. Cui, and H.-L. Gao (2018). New J. Chem. 42, 16836–16845.
C.-M. Liu, R. Sun, B.-W. Wang, F. Wu, X. Hao, and Z. Shen (2021). Inorg. Chem. 60, 12039–12048.
J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen, and M.-L. Tong (2016). J. Am. Chem. Soc. 138, 5441–5450.
C.-M. Liu, D.-Q. Zhang, J.-B. Su, Y.-Q. Zhang, and D.-B. Zhu (2018). Inorg. Chem. 57, 11077–11086.
F. Zhang, R.-F. Zhang, J. Ru, J. Wang, Q.-L. Li, and C.-L. Ma (2021). Z. Anorg. Allg. Chem. 647, 1213–1220.
X. Mi, D. Sheng, Y. E. Yu, Y. Wang, L. Zhao, J. Lu, Y. Li, D. Li, J. Dou, J. Duan, and S. Wang (2019). ACS Appl. Mater. Interfaces 11, 7914–7926.
M. V. Marinho, D. O. Reis, W. X. C. Oliveira, L. F. Marques, H. O. Stumpf, M. Déniz, J. Pasán, C. Ruiz-Pérez, J. Cano, F. Lloret, and M. Julve (2017). Inorg. Chem. 56, 2108–2123.
P. Lin, F. Jiang, M. Wu, and M. Hong (2018). Inorg. Chem. Commun. 93, 29–32.
T. Fujii, K. Kodaira, O. Kawauchi, N. Tanaka, H. Yamashita, and M. Anpo (1997). J. Phys. Chem. 101, 10631–10637.
A. de Bettencourt-Dias, P. S. Barber, S. Viswanathan, D. T. de Lill, A. Rollett, G. Ling, and S. Altun (2010). Inorg. Chem. 49, 8848–8861.
Y.-B. Lu, X.-M. Jiang, S.-D. Zhu, Z.-Y. Du, C.-M. Liu, Y.-R. Xie, and L.-X. Liu (2016). Inorg. Chem. 55, 3738–3749.
A. Arauzo, L. Gasque, S. Fuertes, C. Tenorio, S. Bernès, and E. Bartolomé (2020). Dalton Trans. 49, 13671–13684.
M.-F. Wu, M.-S. Wang, S.-P. Guo, F.-K. Zheng, H.-F. Chen, X.-M. Jiang, G.-N. Liu, G.-C. Guo, and J.-S. Huang (2011). Cryst. Growth Des. 11, 372–381.
W. Xu, C.-J. Zhang, H. Wang, and Y. Wang (2017). J. Clust. Sci. 28, 2005–2015.
P. Kalita, P. Nayak, N. Ahmed, J. M. Herrera, K. Venkatasubbaiah, E. Colacio, and V. Chandrasekhar (2020). Dalton Trans. 49, 15404–15416.
Acknowledgements
We thank the National Natural Science Foundation of China (Grant No. 21371087), Natural Science Foundation of Shandong Province (Grant Nos. ZR2018LB003, ZR2020MB019) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material. Crystallographic data for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary numbers CCDC 2124044 - 2124049 for complexes 1-6. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, F., Zhang, RF. et al. Synthesis, Crystal Structures, Photoluminescence and Magnetic Properties of Lanthanide(III) Complexes Based on 2-(Thiophen-2-ylselanyl)acetic Acid Ligand. J Clust Sci 34, 881–892 (2023). https://doi.org/10.1007/s10876-022-02266-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02266-x