Abstract
We describe a simple approach for the synthesis of palladium nanoparticles (Pd NPs) using Melia azedarach leaf extract. The synthesized Pd NPs were confirmed by UV–Visible spectroscopy, FT-IR, XRD and TEM analysis. UV–Visible spectra clearly showed the absorption peak at 280 nm, which is due to the surface plasmon resonance on Pd NPs. XRD analysis exhibit the crystalline nature of Pd NPs with face centered cubic structure. The TEM images clearly showed the spherical shape with average particles size in the range from 10 to 20 nm. The synthesized Pd NPs were investigated by antibacterial, antifungal and larvicidal activities. Different treatments of Pd NPs exhibit better activity in gram positive and gram negative bacterial strains. By contrast, antifungal activity of Pd NPs has not showed any significant activity due to its nontoxicity. The evaluation of larvicidal activity, the maximum efficacy was observed in synthesized Pd NPs against the larvae of Aedes aegypti (LC50 = 27.36%; LC90 52.50%) and M. azedarach leaf extract for (LC50 = 93.96%; LC90 182.63%), respectively. The synthesis of Pd NPs by this method offers the rapid, facile, nontoxic, single step method and control the larval population of the mosquito vector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoscience and technology is a multidisciplinary area of frontier research. Eco-friendly synthesis of noble metal nanoparticle is one of the emerging fields in material science. Ag, Au, Ru, Pt and Pd were 4d transition metals, which belongs to the platinum group. Palladium nanoparticles (Pd NPs) have a significant role of physicochemical properties that depends on the size and shape as well as the high surface to volume ratio. Pd NPs used for versatile applications such as catalytic [1], sensor [2], fuel cell [3], polymeric membrane [4], antioxidant [5], textile, antibacterial [6], antifungal [7], anticancer [8], larvicidal [9] and so on. Recently, the synthesis of metal nanoparticles have been reported using extraction of plants such as Aloe vera [10], Azadirachta indica [11, 12], Bougainvillea glabra [13], Carissa carandas [14], Couroupita guianensis [15], Cymbopogon citrates [16], Moringa oleifera [17], Musa paradisiaca [18], Naregamia alata [19], Nicandra physalodes [20], Phyllanthus niruri [21], Pteridium aquilinum [22], Sargassum muticum [23] and Ulva lactuca [24].
Nowadays, green chemistry route of Pd NPs was synthesized by leaf extract such as Anacardium occidentale [25], Cacumen platycladi [26] and Piper betle [7]. In addition to that the Pd NPs was synthesized by peel extract of M. paradisiac [27], bark extract of Cinnamomum zeylanicum [28] and fruit extract of Terminalia chebula [29]. The small adaptations in this method offered an effective and alternate way to minimize the physical and chemical synthesis of nanoparticles. Recently, very few works have been done in the biomedical field and nano-mosquitocidals activity about Pd NPs. In this regard, the curiosity of Pd NPs researchers has focused on the developmental aspects for several applications. In our attempt, we carried out the synthesis of Pd NPs using Melia azedarach leaf extract as a bio-reductant and stabilizer. Previously, a significant work has been reported on the use of M. azedarach leaf extract for metal nanoparticles synthesis [30].
The Zika virus belongs to the genus Flavivirus, and is spread by Aedes mosquito. Aedes aegypti can vector several arboviruses causing dengue, chikungunya, zika and yellow fever, which represents major public health concerns nowadays [31]. In addition to that, 3900 million people are at risk of infection with dengue viruses in 128 countries [32]. Past two decades, several insecticides are used to control the mosquito vectors. Conversely, it stimulates the holistic development of insecticidal resistant strains and it also indirectly affected the ecosystem to reach at bioaccumulation and bio magnification route [33]. Currently, bio-control agents and conventional method are used but not fully arrested the mosquito populations [34, 35]. In this condition, we need to develop the new technologies to limit the mosquito vectors as well as insecticide behaviors consist with low cost, high efficiency and less toxicity to the environments [36]. Recently, nanotechnology have attracted considerable attention in controlling mosquito vectors using biological mediated metal and metal oxide nanoparticles such as Ag, Pd, ZnO, CeO2 and TiO2 NPs [9, 37–40].
Melia azedarach L. (Meliaceae) is a semi-evergreen tree grown up to 20–30 m height and commonly known as Mahaneem or Malaveppu. It is native of India and mainly found in the forest of North-West Indo-Himalayan tracts, Pakistan, China and tropical countries. Leaves and fruits contain two major bioactive compounds namely triterpenoids and limonoids. The various plant parts like leaves, seeds, barks and roots have been used for skin diseases, rheumatic pains, diuretic and astringent. It has a wide spectrum of biological properties such as anticancer, antiviral, antidiabetic, antioxidant, larvicidal, antimicrobial and ovicidal activities [30, 41].
In the present investigation, we carried out the environment friendly synthesis of Pd NPs using M. azedarach leaf extract. Synthesized Pd NPs were used at different concentrations to evaluate the antibacterial, antifungal and larvicidal activities. To the best of our knowledge, we report for the first time the Pd NPs synthesis using M. azedarach leaf extract as well as three dimensional views of applications.
Materials and Methods
Chemicals
Palladium(II) chloride, [PdCl2 (99.9%)] from Alfa Aesar (India) was used. Microbiological media were obtained from Himedia Laboratories, (India), double distilled water, analytical grade chemical and reagents were used for the experiments.
Collection of Plant Material and Synthesis of Pd NPs
Melia azedarach leaves were collected from Bharathiar University Campus, Coimbatore, Tamil Nadu, India. The 10 g of leaves were added with 100 mL of double distilled water and boiled with stirring at 45–60 °C for 15 min. The obtained extraction was filtered using Whatman No.1 filter paper and the collected leaf extract was stored at room temperature for further usage. The 1 mL of leaf extract was added into 100 mL of 1 mM PdCl2 solution stirred at 100 °C for 20 min. The colour of the mixture gradually changed from light yellow to dark brown within the next 20 min, which indicates the formation of Pd NPs.
Microbes
Bacterial strains of Bacillus subtilis—ATCC 6633, Staphylococcus aureus—MTCC 96, Streptococcus pneumoniae—MTCC 1936, Escherichia coli—MTCC 40, Proteus vulgaris—MTCC 7299, Pseudomonas aeruginosa—MTCC 2642 and fungal strains of Aspergillus niger, Fusarium solani, Nigrospora oryzae and Trichoderma viride were collected from PG and research Department of Plant Biology and Plant Biotechnology, Ramakrishna Mission Vivekananda College, Chennai.
Characterization
The synthesized Pd NPs were analysis by UV–Visible spectroscopy in the wavelength range from 200 to 600 nm using Elico spectrophotometer (Model SL-210) operation at a resolution of 1 nm. The functional group vibrations of the plant extract and synthesized Pd NPs are analyzed using Bruker–Tensor 27 series spectrophotometer by the KBr pellet technique in the range of 400–4000 cm−1. XRD analysis recorded by Cu Kα radiation with the wavelength of 1.54060 Å using PANalytical X-PERTO PRO diffractometer system in the range of 2θ from 10° to 80°. The average crystalline size was calculated from the width of the XRD peaks using Scherrer’s formula D = 0.9λ/βcosθ. Synthesized Pd NPs was subjected to HR-TEM analysis by the instrument Tecnai F20 model operated at an accelerating voltage of 200 kV.
Antibacterial Activity of Pd NPs
The antibacterial activity of the synthesized Pd NPs was examined against three gram-positive (B. subtilis, S. aureus, S. pneumoniae) and three gram-negative bacteria (E. coli, P. vulgaris, P. aeruginosa) by disc diffusion method. These four bacterial strains were grown in nutrient broth at 37 °C until the bacterial suspension has reached 1.5 × 108 CFU/mL. Approximately, 20 mL of molten nutrient agar was poured into the Petri dishes and cooled. All the bacterial suspension was swapped over the medium, the disc loaded with 10, 30 and 50 µL of Pd NPs were placed over the medium using sterile forceps. Plant leaf extract used as negative control and ofloxacin as a positive control. Plates were incubated for 24 h at 37 °C. The inhibition zone of each disc was measured and each experiment was carried out in triplicates.
Antifungal Activity of Pd NPs
The antifungal activity of Pd NPs was evaluated against the following human pathogenic fungal strains of A. niger, F. solani, N. oryzae and T. viride were grown in Potato Dextrose Agar (PDA) slants and sub-cultured before use. The plates were left overnight at room temperature to check for contamination. The fungal strains 1.5 × 108 CFU/mL grown in Potato Dextrose Broth (PDB) were seeded over the plates using sterile cotton swabs. The disc loaded with 10, 30 and 50 µL of Pd NPs was placed over the medium using sterile forceps. Plant leaf extract used as negative control and gentamicin as a positive control. Plates were incubated for 48 h at 37 °C. The zone of inhibition was measured and expressed in millimeter (mm). Each experiment was performed in triplicates.
Mosquitoes
The mosquitoes of Aedes aegypti were reared in the vector control laboratory, Department of Zoology, Annamalai University, The larvae were fed on dog biscuits and yeast powder in the 3:1 ratio. Adults were fed blood through a parafilm membrane and provide with 10% sucrose solution. Mosquitoes were held at 28 ± 2 °C for 75–85% relative humidity, with 12 h photoperiod at a photosynthetic flux of 12.6 µ mol m−2 s−1 provided by cool daylight fluorescent lamps.
Toxicity Against Mosquito Larvae
Larvicidal activity of the aqueous extract and Pd NPs from M. azedarach was evaluated according to WHO protocol [37]. Different test concentration of aqueous leaf extract 4, 8, 12, 16 and 20% and Pd NPs was tested at 1.2, 2.4, 3.6, 4.8 and 6% in 100 mL deionized water were prepared. A. aegypti (n = 20) late III instar larvae were introduce into each tested concentration, five replicates were performed. Larval mortality rate was recorded at 24 h after exposure, during no food was given to the larvae.
Statistical Analysis
All the data were expressed as mean ± SE. Data from antibacterial experiments were analyzed using ANOVA followed by Tukey’s HSD test (P ≤ 0.05). The larval mortality was observed, and the average mortality data were subjected to Probit analysis for calculating LC50 and, lower and upper confidence limit values at 95% using the SPSS software package 16.0 version. Results with p value of <0.05 were considered to be statistically significant.
Result and Discussion
Characterization of Pd NPs
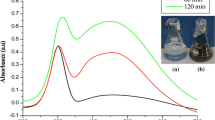
The mixture of leaf extract with PdCl2 solution was subjected to UV–Visible spectroscopy analysis during the rapid reduction process to reveal the formation of Pd NPs. The recorded spectrum showed an absorbance peak at 280 nm, which corresponds to the wavelength of the surface plasmon resonance of Pd NPs. It clearly indicates the reduction of PdII to Pd NPs synthesis (Fig. 1). Similar results are coherent with the previous reports [1, 42]. FT-IR analysis was carried out for leaf extract. The bands which are appeared at 3422 and 2919 cm−1 correspond to –OH stretching and aliphatic –C–H– stretching, respectively. The bands at 1637 and 1390 cm−1 are due to the –C=C– stretching and –C–O stretching. The IR bands observed at 1390 and 592 cm−1 may be ascribed to –C–O–C stretching and C–Cl stretching modes, respectively (Fig. 2) [7]. Hence, as an important bioactive compound such as triterpenoids and limonoids present in the leaf extract of M. azedarach [41]. It plays an important role for the reduction process and formation of Pd NPs. The two new strong bands recorded at 2339 and 1721 cm−1 in the spectra of the synthesized Pd NPs were assigned to CO2 stretching, C=O carbonyl stretching respectively. These peaks may be raised due to the reduction of palladium chloride to palladium nanoparticles [43]. X-ray diffraction peaks at 40.11°, 46.66° and 68.12° were indexed with the planes (111), (200) and (220) for the face centered cubic palladium as per the JCPDS card No. 89-4897 (Fig. 3). The well resolved and intense XRD pattern clearly showed that high crystalline nature. The average crystalline size was estimated using Scherrer’s equation and it was found to be 26.91 nm. The HR-TEM images further ascertain that the Pd NPs are predominantly spherical in morphology with their sizes ranging from 10 to 20 nm and has an average size about 10 nm (Fig. 4a–c). The selected area electron diffraction (SAED) pattern shows well define spotty ring which implies the good crystalline nature of the Pd NPs (Fig. 4d).
Effects of Pd NPs in Antibacterial Activity
The antibacterial activity of Pd NPs performed against three gram positive and gram negative bacterial human pathogens using three different loading concentrations at 10, 30 and 50 µL of Pd NPs (Fig. 5). The inhibition zones with significant effect shown by the Pd NPs 50 µL concentration of B. subtilis (8.33 ± 0.33 mm). In addition, S. aureus, E. coli, P. vulgaris (7.33 ± 0.33 mm) and followed by S. pneumoniae and P. aeruginosa (5.33 ± 0.33 mm) (Table 1). The 30 and 10 µL concentration exhibited the modulated and minimum zone of inhibition when compared to 50 µL of Pd NPs treatments. In this activity depends upon the concentration of Pd NPs. The actual mechanism of antibacterial activity, metal nanoparticles bind with the bacterial cell membrane due to the electrostatic attraction towards the mesomoal cell organelle. Generally, mesomoal cell organelle is indirectly involved in an important role in cellular respirations, DNA replication, cell division and controls the surface area of the bacterial membrane. The crucial actions of Pd NPs reduce the mesomoal functions and increase the reactive oxygen species (ROS) generation which leads to cell death [44]. With this ability of Pd NPs makes a cavity on surface of the bacterial cell membrane and cases the structure changes take place due to cell expiration. Recently, Pd NPs showed that maximum zone of inhibition against S. aureus when compared to E. coli, interestingly Pd NPs as useful antibacterial agents specifically for gram positive bacteria [45]. Similarly, aqueous extract of S. muticum synthesized Ag NPs 50 ppm concentration exhibited zone of inhibition at 5 mm in Bacillus subtilis, Klebsiella pneumoniae and Salmonella typhi bacteria stains, while inhibition zones were increase more than 10 mm when increase the concentration of Ag NP at 150 ppm. In this activity doses dependent of nanoparticles treatment [23].
Effect of Pd NPs in Antifungal Activity
The antifungal activity of Pd NPs did not show any activity against all fungal strains at the three tested concentration as well as positive and negative controls (Fig. 6). Generally, for almost all fungi the central core of the cell wall is a branched β-1, 3, 1, 6 glucan that is linked to chitin via a β-1, 4 linkages. Nevertheless, Pd NPs bonds to the fungal surface due to the surface charges after that it’s uptake by the active transport system of fungal cell and enters to the cytoplasm. They may not kill the fungal cell due to the nontoxic nanoparticles at the treatments of least concentrations. Recently, Mallikarjuna et al. [7] have been reported green synthesized Pd NPs against A. niger fungal strain exhibited the maximum zone inhibition observed at 150 µL concentration and this antifungal activity based on the dose dependent as well as particles sizes and shapes [7]. In our study, the antifungal activity of Pd NPs could not be collapse the membrane and intercellular communication. Since the fungus as a well-developed eukaryotic microorganism and sustained with nanoparticles treatments at the minimum does.
Effect of Pd NPs in Larvicidal Activity
In order to evaluate the toxicity experiments clearly showed the leaf extract of M. azedatach against the larva of A. aegypti LC50 value 93.96% and synthesized Pd NPs was highly effective against A. aegypti larvae LC50 27.36% (Table 2).The Pd NPs showed three times higher mortality rate when compare to M. azedarach leaf extract treatment alone. The smaller particles size increase high surface to volume ratio and thus plays an important role on larvicidal activity. Bio-fabricated nanoparticles showed a better larvicidal activity against A. aegypti mosquito vector. Similarly, P. niruri leaf and M. oleifera seed extract based synthesized Ag NPs were highly effective against A. aegypti larva showed LC50 of 6.68 ppm and young (III) instars at LC50 of 13.84 ppm [17, 21]. In addition to that extract of C. carandas, N. alata and N. physalodes synthesized Ag NPs were highly toxic against A. aegypti larvae LC50 value at 15.69, 13.57 and 13.61 µg/mL [14, 19, 20]. Notably, C. citratus leaf extract synthesized Au NPs were highly toxic against A. aegypti young instars at low dosages LC50 of Au NPs 20.27, 23.24, 8.63, 35.09 and 41.52 ppm [16]. The exact mechanism of larvicidal activity is not known clearly till now, even though the obtained results suggest that the positive charged Pd NPs interact with negative charged A. aegypti cell membrane, due to the electrostatic interaction. In this regards, Pd NPs penetrated through the cytosol and interference with intracellular cell signaling. In addition to that it bounds with sulphur contain proteins they act as a sulfa drugs which cause the cell death. Typically, metal and metal oxide NPs reach the midgut epithelial membrane then the enzymes become inactivation, and peroxide leading cell death [39]. Only one attempt had been done in the synthesized Pd NPs using Eclipta prostrata leaf extract and it exhibited 78.13% of antimalarial potential in Plasmodium berghei parasites [9]. We demonstrated that notable effect at low dosages to strongly reduced larval population of dengue mosquito vector.
Conclusions
In this endeavor, Pd NPs were synthesized by M. azedarach leaf extract. FT-IR spectra clearly indicated the interactions of bio active compounds are involved in nanoparticle synthesis. XRD pattern showed the presence of crystalline nature of Pd NPs with face centred cubic structure. The spherical shaped morphology of Pd NPs with sizes ranging from 10 to 20 nm was also demonstrated by TEM analysis and SAED pattern emphasizes the polycrystalline nature of nanoparticles, which is good agreement with the XRD results. Eco-friendly synthesized Pd NPs showed their potent antibacterial activity in all the tested strains, especially in gram positive bacteria. The antifungal activity of Pd NPs treatment did not arrest the fungal growth due to its nontoxicity nature. Larvicidal activity of Pd NPs exhibit three times higher than the M. azedarach leaf extract treatments and it’s an alternative tool for effectively controlled the invasive dispersal of dengue vector. In this synthesis protocol it offers a simple, reliable, cost effective, environment safe approach and it can also be extended to synthesize other metal NPs. Finally, it is suggested that can be used for other kind of application such as biosensor, catalytic, antimicrobial and nano-mosquitocidal agents.
References
M. Nasrollahzadeh and S. M. Sajadi (2014). J. Colloid Interf. Sci. 462, 243–251.
S. Kundu, P. K. Warran, S. M. Mursalin, and M. Narjinary (2015). J. Mater. Sci. 26, 9865–9872.
I. F. Romos, B. C. Montes, M. M. G. Maldonado, C. L. Menendez, A. R. Mayol, L. M. D. Vazquez, and C. R. Cabrera (2015). J. Chem. Educ. 92, 360–363.
H. Mahdavi, A. Rahimi, and T. Shahalizade (2016). J. Polym. Res. 23, 39.
A. J. Kora and L. Rastogi (2016). Ind. Crop. Prod. 81, 1–10.
S. Y. Park, J. W. Chung, R. D. Priestley, and S. Y. Kwak (2012). Cellulose 19, 2141–2151.
K. Mallikarjuna, N. John Sushma, B. V. Subba Reddy, G. Narasimha, and B. Deva Prasad Raju (2013). Int. J. Chem. Anal. Sci. 4, 14–18.
A. Dumas and P. Couvreur (2015). Chem. Sci. 6, 2153–2157.
G. Rajakumar, A. A. Rahuman, I. M. Chung, A. V. Kirthi, S. Marimuthu, and K. Anbarasan (2015). Parasitol. Res. 114, 1397–1406.
D. Dinesh, K. Murugan, P. Madhiyazhagan, C. Panneerselvam, M. Nicoletti, W. Jiang, G. Benelli, B. Chandramohan, and U. Suresh (2015). Parasitol. Res. 114, 1519–1529.
K. Murugan, C. Panneerselvam, C. M. Samidoss, P. Madhiyazhagan, M. Roni, J. Subramaniam, D. Dinesh, R. Rajaganesh, M. Paulpandi, H. Wei, A. T. Aziz, M. S. Alsalhi, S. Devanesan, M. Nicoletti, R. Pavela, A. Canale, and G. Benelli (2016). Res Vet. Sci. 106, 14–22.
B. Chandramohan, K. Murugan, C. Panneerselvam, P. Madhiyazhagan, R. Chandirasekar, D. Dinesh, P. Mahesh Kumar, K. Kovendan, U. Suresh, J. Subramaniam, R. Rajaganesh, A. T. Aziz, B. Syuhei, M. S. Alsalhi, S. Devanesan, M. Nicoletti, H. Wei, and G. Benelli (2016). Parasitol. Res. 115, 1015–1025.
S. Vincent, K. Kovendan, B. Chandramohan, S. Kamalakannan, P. Mahesh Kumar, V. Vasugi, C. Praseeja, J. Subrmaniam, M. Govindarajan, K. Murugan, and G. Benelli (2016). J. Clust. Sci. doi:10.1007/s10876-016-1038-3.
M. Govindarajan and Benelli (2016). J. Clust. Sci. doi:10.1007/s10876-016-1035-6.
J. Subramaniam, K. Murugan, C. Panneerselvam, K. Kovendan, P. Madhiyazhagan, D. Dinesh, P. Mahesh Kumar, and B. Chandramohan (2016). Environ. Sci. Pollut. Res. doi:10.1007/s11356-015-6007-0.
K. Murugan, G. Benelli, C. Panneerselvam, J. Subramaniam, T. Jeyalalitha, D. Dinesh, M. Nicoletti, S. Hwang, U. Suresh, and P. Madhiyazhagan (2015). Exp. Parasitol. 153, 129–138.
V. Sujitha, K. Murugan, M. Paulpandi, C. Panneerselvam, U. Suresh, M. Roni, M. Nicoletti, A. Higuchi, P. Madhiyazhagan, J. Subramaniam, D. Dinesh, C. Vadivalagan, B. Chandramohan, A. A. Alarfaj, M. A. Munusamy, D. R. Barnard, and G. Benelli (2015). Parasitol. Res. 114, 3315–3325.
P. Anbazhagan, K. Murugan, A. Jaganathan, V. Sujitha, C. M. Samidoss, S. Jayashanthani, P. Amuthavalli, A. Higuchi, S. Kumar, M. Nicoletti, A. Canale, and G. Benelli (2016). J. Clust. Sci. doi:10.1007/s10876-016-1047-2.
R. M. S. T. Azarudeen, M. Govindarajan, A. Amsath, U. Muthukumaran, and G. Benelli (2016). J. Clust. Sci. doi:10.1007/s10876-016-1067-y.
M. Govindarajan, H. F. Khater, C. Panneerselvam, and G. Benelli (2016). Res. Vet. Sci. 107, 95–101.
U. Suresh, K. Murugan, G. Benelli, M. Nicoletti, D. R. Barnard, C. Panneerselvam, P. Mahesh Kumar, J. Subramaniam, D. Dinesh, and B. Chandramohan (2015). Parasitol. Res. 114, 1551–1562.
C. Panneerselvam, K. Murugan, M. Roni, A. T. Aziz, U. Suresh, R. Rajaganesh, P. Madhiyazhagan, J. Subramaniam, D. Dinesh, M. Nicoletti, A. Higuchi, A. A. Alarfaj, M. A. Munusamy, S. Kumar, N. Desneux, and G. Benelli (2016). Parasitol. Res. 115, 997–1013.
P. Madhiyazhagan, K. Murugan, A. Naresh Kumar, T. Nataraj, D. Dinesh, C. Panneerselvam, J. Subramaniam, P. Mahesh Kumar, U. Suresh, M. Roni, M. Nicoletti, A. A. Alarfaj, A. Higuchi, M. A. Munusamy, and G. Benelli (2015). Parasitol. Res. doi:10.1007/s00436-015-4671-0.
K. Murugan, C. M. Samidoss, C. Panneerselvam, A. Higuchi, M. Roni, U. Suresh, B. Chandramohan, J. Subramaniam, P. Madhiyazhagan, D. Dinesh, R. Rajaganesh, A. A. Alarfaj, M. Nicoletti, S. Kumar, H. Wei, A. Canale, H. Mehlhorn, and G. Benelli (2015). Parasitol. Res. 114, 4087–4097.
D. S. Sheny, D. Philip, and J. Mathew (2012). Spectrochim. Acta Part A. 91, 35–38.
G. Zhan, J. Huang, M. Du, L. A. Rauf, Y. Ma, and Q. Li (2011). Mater. Lett. 65, 2989–2991.
A. Bankar, B. Joshi, A. R. Kumar, and S. Zinjard (2010). Mater. Lett. 64, 1951–1953.
M. Sathishkumar, K. Sneha, I. S. Kwak, J. Mao, S. J. Tripathy, and Y. S. Yun (2009). J. Hazard. Mater. 171, 400–404.
K. M. Kumar, B. K. Mandal, K. S. Kumar, P. S. Reddy, and B. Sreedhar (2013). Spectrochim. Acta Part A. 102, 128–133.
R. Ramanibai and K. Velayutham (2015). Res. J. Vet. Sci. 98, 82–88.
G. Benelli (2015). Parasitol. Res. 114, 2801–2805.
G. Benelli and H. Mehlhorn (2016). Parasitol. Res. 115, 1747–1754.
U. Muthukumaran, M. Govindarajan, M. Rajeswary, and S. L. Hoti (2015). Parasitol. Res. 114, 1817–1827.
G. Benelli (2016). Parasitol Res. 115, 23–34.
G. Benelli (2016). Enzym. Microb. Technol. doi:10.1016/j.enzmictec.2016.08.022.
K. Murugan, D. Dinesh, K. Kavithaa, M. Paulpandi, T. Ponraj, M. S. Alsalhi, S. Devanesan, J. Subramaniam, R. Rajaganesh, H. Wei, S. Kumar, M. Nicoletti, and G. Benelli (2015). Parasitol. Res. 115, 1085–1096.
M. Govindarajan, M. Rajeswary, U. Muthukumaran, S. L. Hoti, H. F. Khater, and G. Benelli (2016). J. Photochem. Photobiol. B. 161, 482–489.
N. Bala, S. Saha, M. Chakraborty, M. Maiti, S. Das, R. Basu, and P. Nandy (2014). RSC adv. doi:10.1039/C4RA12784F.
K. Gopinath, V. Karthika, C. Sundaravadivelan, S. Gowri, and A. Arumuagm (2015). J. Nanostruct. Chem. 5, 295–303.
T. Y. Suman, R. R. S. Ravindranath, D. Elumalai, P. K. Kaleena, R. Ramkumar, P. Perumal, L. Aranganathan, and P. S. Chitrarasu (2015). Asian Pac. J. Trop. Dis. 5, 224–230.
M. F. Khan, A. K. Rawat, B. Pawar, S. Gautam, A. K. Srivastava, and D. S. Negi (2014). Fitoterapia 98, 98–103.
M. Nasrollahzadeh, S. M. Sajadi, E. Honarmand, and M. Maham (2015). New. J. Chem. 39, 4745–4752.
A. Kalaiselvi, S. M. Roopan, G. Madhumitha, C. Ramalingam, and G. Elango (2015). Spectrochim. Acta Part A. 135, 116–119.
K. Gopinath, S. Gowri, and A. Arumuagm (2013). J. Nanostruct. Chem. 3, 68.
C. P. Adams, K. A. Walker, S. O. Obare, and K. M. Docherty (2014). PLoS ONE 9, e85981.
Acknowledgements
One of the authors K. Gopinath is highly grateful to thank School Physics Alagappa University, Karaikudi for extending the XRD facility. The authors also thank Mr. C. Karthikeyan, Jamal Mohamed College, Trichy, Tamil Nadu, India, for helping to analyze the XRD result with standard fitting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhakyaraj, K., Kumaraguru, S., Gopinath, K. et al. Eco-Friendly Synthesis of Palladium Nanoparticles Using Melia azedarach Leaf Extract and Their Evaluation for Antimicrobial and Larvicidal Activities. J Clust Sci 28, 463–476 (2017). https://doi.org/10.1007/s10876-016-1114-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1114-8