Abstract
The composite nanofibers from Au nanoparticles/carbon nanofibers (Au/CNFs) were fabricated through electrospinning, chemical reduction and high-temperature calcination methods. The polyacrylonitrile acted as the carbon precursor polymer. A series of characterization methods, which include UV–Vis diffuses reflectance spectra, UV–Vis spectra, Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, and X-ray diffraction respectively, were used to investigate the morphology and properties of catalysts. The result showed that the gold nanoparticles were well-distributed in/on the CNFs. In the end, the composite material was applied to epoxidation of styrene to investigate its catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold nanoparticles (Au NPs) are a kind of stable noble metal nanoparticles. In view of its electronic, magnetic and optical properties [1–3], as well as the applications in biology and catalysis, the Au NPs have attracted considerable attention [4]. In recent years, many reachers are studying the composite material that contains gold nanoparticles and many kinds of supporters, such as Au/SiO2, Au/ZrO2, Au/CeO2, Au/CNTs and Au/TiO2 [5–9], etc. In the preparation of this material, the high-quality nanomaterials with various compositions and dimensions have been fabricated successfully through different physical and chemical ways [10], which commonly include homogeneous deposition precipitation [11, 12], deposition precipitation [13, 14] and impregnation. The activity of the catalyst mainly dependents on the size, morphology and crystalline phase of nanoparticles, in addition, there are other factors, such as the content of metal, the kinds of carrier, etc., are also very crucial [15].
Olefin epoxidation is significant to the valuable materials for the synthesis of a wide variety of chemical products [16]. For example, styrene is a subject of great interest from both academic and industrial points of view [17]. The oxirane group is an essential group of organic intermedium in the reaction which makes epoxides [8]. On the other hand, the benzaldehyde which is a product of styrene epoxidation is an important fine chemical product and it can be widely used in many fields, such as medicine, dyes, flavors and resin additives [18].
So, there are more and more researchers to pay more attention to styrene epoxidation and study it. Traditionally, during this reaction, there are many kinds of oxidants, such as tert-butyl hydroperoxide (TBHP), O2, KMnO4, H2O2, NaClO, etc. [19]. The solvent of the reaction contains acetonitrile, isopropanol and other organic solvent. In addition, the molar ratio of the styrene and oxidants, reaction temperature, and many others are the investigated factors.
In this work, combined the electrospinning technique and high temperature calcination technology, the compound catalyst which contained gold nanoparticles and carbon nanofibers named Au/CNFs was manufactured and it was applied to styrene epoxidation in order to explore the property of the catalyst.
Experimental
Materials
Polyacrylonitrile (PAN, average Mw = 80000, AR) was bought from Kun Shan Hongyu Plastic Co., Ltd. Nitrogen–nitrogen dimethylformamide (DMF, 99.5 %, AR) was purchased from the Tianjin Guangfu Technology Development Co. The chloroauric acid tetrahydrate (AuCl3·HCl·4H2O, Au ≥ 47.8 %, AR), Absolute ethyl alcohol (C2H5OH, ≥99.7 %, AR) and Acetonitrile (CH3CN, ≥99.0 %, AR) were purchased from Sinopharm Chemical Reagent Co. Ltd. Styrene (C8H8, ≥99.0 %, AR) was bought from Beijing InnoChem Science &Technology Co., Ltd. tert-butyl hydroperoxide [(CH3)3COOH, 70 %, AR] was purchased from Tianjin Alfa Aesar Chemical Co., Ltd. The isopropanol, dichloromethane, and ethyl acetate were purchased from the Tianjin Fuyu Fine Chemical Co. All the chemicals were used as received without further purification.
Catalyst Preparation
The PAN powders were dissolved in DMF, which the concentration of PAN was kept in 8 wt%. After continuous powerfully magnetic stirring 12 h at room temperature, the solution became homogeneous and transparent, then the AuCl3·HCl·4H2O was added into the above system, in which the mole ratio of AN (which is monomer of PAN) and AuCl3·HCl·4H2O is 50, and the mixture was continued stirring 12 h. During the process, the solution was treated by supersonic wave for several times to ensure it was fully dissolved. After that, the above solution was used as the precursor of electrospinning (the installation is composed of three sections: The high voltage power supplied as a source of electric field with an applied voltage of 15 kV; The fixed aluminum foil served concurrently as counter electrode and the collector; The glass tube wrapped with copper wire that connects with high voltage power supply as a container of precursor. In this work, the distance between collector and the thin nozzle of glass tube was 16 cm [20]). By this way, the nanofibers containing cation Au3+ was got, which was named Au3+/PAN.
Afterwards the Au3+/PAN nanofibers membrane was put into the high pressure reaction vessel, in which it was full of H2. With a stationary temperature, pressure and a period of time, the Au/PAN nanofibers were obtained.
Next, the Au/PAN was put into a porcelain boat (the nanofibers membrane were tiled in the central of the boat), then it was placed the middle of high temperature vacuum tube furnace in the nitrogen atmosphere, at 600 °C kept for 2 h, the carbon nanofibers load nano-gold was obtained and named Au/CNFs.
Characterization
UV–Vis diffuse reflectance spectra (DRS) (UV-3600, Shimadzu Corporation) with a variable range from 190 to 800 nm of wavelength, which was used to investigate if the trivalent Au was reduced to zerovalent Au completely or not, in which the barium sulfate was a standard type reference material for background corrections. However, the UV–Vis spectrophotometer (UV-1800, Mapada) provided an evidence for the characteristic absorption of gold ion. Fourier transform infrared spectroscopy (FT-IR, 670, Thermo Nicolet Corporation) confirmed the characteristic functional groups of PAN, Au3+/PAN, Au/PAN and Au/CNFs. After the samples were pasted on the conducting resin, scanning electron microscopy (SEM, Hitachi S-3400N, Japan) was used to observe the morphology of nanofibers of Au/PAN and Au/CNFs. The feature of gold nanoparticles was examined by Transmission electron microscopy (TEM) (TEM, F20 S-TWIN, Tecnai). The crystal form of gold nanoparticles was tested by X-ray diffraction (XRD, Rigaku Ultima IV, Japan) in the range of 2θ angles from 10° to 90°. In this process, the nanofibers films were made to the tablet which size was 20 mm × 20 mm × 1 mm, and the carbon nanofibers were grinded to a powder.
Catalytic Styrene Epoxidation Reaction
In order to test the catalytic activity of the catalyst, the samples were placed into the system which contained 1 mL styrene, 5 mL TBHP, 5 mL solvent, which was applied to the reaction of styrene epoxidation. The reaction was carried out under conditions of circulating condensate with continuous stirring at the rate of 200 r/min for 5 h, and the whole process was in the atmosphere of nitrogen.
When the reaction was finished, the products were analyzed by Gas Chromatograph (Shimadzu, GC-2010 Plus) with FID using an Rtx-5 column and N2 as the carrier gas. On the other side, the Agilent (5975C) gas chromatography mass spectrometer (GC–MS) was also used to ensure the component of the product. The conversion of substrate and the relative amount of other component in the products were calculated through an area normalization method. The computational formula as following [21]:
Results and Discussion
The preparation method of the Au/CNFs nano heterogeneous composite material is shown in Fig. 1, during which the technology of electrospinning, chemical reduction and high-temperature calcination were combined. The PAN and AuCl3·HCl·4H2O were added into the DMF. By the electrospinning, the Au3+/PAN were got. After reduced by H2, the Au/PAN was obtained. Finally the Au/PAN was calcinated to get the Au/CNFs. The Fig. 2 reflects about the result of reaction styrene epoxidation. In this process, styrene, TBHP and Au/CNFs acted as substrate, oxygen source and catalyst respectively. In nitrogen atmosphere, the reaction was finished after 5 h. The major products are not only styrene oxide and benzaldehyde, but also others, such as phenylacetaldehyde, and so on.
A comparison of the UV–Vis spectra of Au3+/PAN (Fig. 3b), Au/PAN (Fig. 3c) and PAN (Fig. 3a) nanofibers is displayed in Fig. 3. As shown in Fig. 3, the characteristic absorption peak of Au3+ or \({\text{AuCl}}_{{ 4}^{\text{-}}}\) ions is at 323 nm (Fig. 3b) and disappears after reducing by the hydrogen in the reaction progress (Fig. 3c), which reveal that trivalent Au was reduced to zerovalent Au completely in/on nanofibers. On the other hand, an absorption band at 535 nm is the characteristic SPR absorption by Au NPs [22, 23]. The absorption in the visible region is due to the band structure of metal nanoparticles increasing, which indicates the Au NPs were formed.
In order to explain the absorption peak at 323 nm in Fig. 3b, AuCl3·HCl·4H2O was dissolved in DMF and the characteristic absorption peak of Au3+ or \({\text{AuCl}}_{{ 4}^{\text{-}}}\) at 326 nm (the DMF act as the reference solution) was got, which coincides with the Fig. 3b. The result is shown in Fig. 4.
The FT-IR spectra of precursor PAN, Au3+/PAN, Au/PAN, and Au/CNFs are recorded in wavenumber range between 500 and 4000 cm−1 and shown in Fig. 5 respectively. In Fig. 5a, b and c, the stretching vibration of C–N bonds forms peaks at 1360 cm−1, and alkyl of PAN nanofibers generates bending vibration appears at 1454 cm−1. The stretching vibration peak of nitrile group (–CN) appears at 2239 cm−1 and C–H appears at 2933 cm−1, however these organic functional groups disappeared after the process of carbonization(in Fig. 5d) [24]. Finally the vibration peak at 1726 cm−1 might derive from the C=O bonds formed in the hydrolyzed PAN nanofilms [25] or the residuary solvent DMF [26].
The Fig. 6 is the SEM image of Au nanoparticles combines with PAN nanofibers (A, B) and carbon nanofibers (C, D). They indicate that the smooth surface and uniform diameter nanofibers were prepared in this experiment successfully [27]. But there are many bright spots in these pictures, which was the presence of gold nanoparticles. In the whole picture, that bright spots are bigger than in else regions may be because the gold nanoparticles are close to each other, which might be because the nanoparticles agglomerated in the process of reduction by H2 and high temperature sintering.
The TEM images are exhibited in Fig. 7 and the inset in Fig. 7b is the histogram size distribution of Au nanoparticles loaded on the carbon nanofibers. They suggest that metal nanoparticles distributed on the fibers uniformly. From the inset of the Fig. 7b, it shows that the average grain diameter of Au nanoparticles is 6.64 nm. Furthermore, it shows the spacing of lattice of the Au is 0.248 nm in Fig. 7d, which demonstrates the main lattice plane is (111) of Au crystals [28].
In Fig. 8, the peaks located at 2θ = 38.184°, 44.392°, 64.576°, 77.547° and 81.721° were assigned to the (111), (200), (220), (311) and (222) crystal planes of face-centered-cubic (fcc) of Au (JCPDS, Card no. 7440-57-5), respectively [8, 29–31]. In addition, the value at 2θ = 17.13° of absorption peak is the crystal of polyacrylonitrile monomer.
In this work, TBHP was used as the oxygen source to investigate the activity of catalyst. The influence of solvent included acetonitrile, ethanol, isopropanol, dichloroethane and ethylacetate was compared. Moreover, the amount of catalyst in its optimum solvent which was previous used was investigated, and then the recycling of catalyst was also inspected.
Table 1 shows the effect of different solvents on styrene conversion and products distribution. It is clear that isopropanol is more favorable for the conversion of styrene and the formation of benzaldehyde, so isopropanol was chosen as the solvent of the system.
The effect of catalyst amount on styrene oxidation was investigated in the range of 0.01–0.05 g at 82 °C for 5 h in the reaction which solvent is isopropanol. In Table 2, the result shows that the optimal mount is 0.04 g, in which the conversions reach 78 %, and the selectivity of benzaldehyde is 52 %.
The time dependence of styrene epoxidation within 24 h is investigated (Fig. 9). It was found that the conversion of styrene increased gradually to 100 % with prolongation of reaction time. When the reaction time is 3 h, the selectivity of styrene oxide is 50 %, instead it decreases constantly as the reaction continues. The selectivity of benzaldehyde is creasing, throughout which the rate of increase from 1 to 2 h is the highest, and after the rate of increase reduces gradually. On the other hand, the other products began to appear after 4 h.
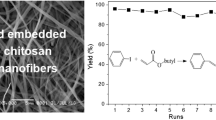
The stability of catalyst is important for its application. Herein, the stability of Au/CNFs was investigated. After the reaction, the catalyst was easily separated by filtration, and then it subjected to the second run under the same conditions. Cyclic experiment result is shown in Fig. 10. The conversion changed slightly after five runs, but the selectivity of benzaldehyde and styrene oxidation was reduced respectively. And the others such as benzyl benzoate and others starts occurrence and their rate is on the rise. The results show that the catalyst has stability and recyclable applicability for the oxidation of styrene to a certain degree.
Conclusions
In this work, the composite nanofibers Au/CNFs were structured and then characterized by DRS, FT-IR, SEM, TEM and XRD. In order to test the catalyst property, it was applied in the styrene epoxidation. The result shows that the optimal solvent is isopropanol in this experiment and its most reasonable amount of catalyst is 0.04 g in it, in which the conversion reach 78 % at 5 h, and the selectivity of benzaldehyde and styrene oxide is 52 and 48 % respectively. Then the catalyst was carried out recycling test, which it was found that the catalyst had got certain stability and recycling. However, as the increase of cycling times, the content of other products also increased gradually.
References
C. C. Han, L. N. Wu, L. Ge, Y. J. Li, and Z. Zhao (2015). AuPd bimetallic nanoparticles decorated graphitic carbon nitride for highly efficient reduction of water to H2 under visible light irradiation. Carbon 92, 31–40.
Y. C. Wei, J. Q. Jiao, Z. Zhao, J. Liu, J. M. Li, G. Y. Jiang, Y. J. Wang, and A. J. Duan (2015). Fabrication of inverse opal TiO2-supported Au@CdS core–shell nanoparticles for efficient photocatalytic CO2 conversion. Appl. Catal. B 179, 422–432.
L. Collado, A. Reynal, J. M. Coronado, D. P. Serrano, J. R. Durrant, and V. A. de la Pena O’Shea (2015). Effect of Au surface plasmon nanoparticles on the selective CO2 photoreduction to CH4. Appl. Catal. B 178, 177–185.
J. Bai, Y. X. Li, S. T. Yang, J. S. Du, S. G. Wang, J. F. Zheng, Y. Z. Wang, Q. B. Yang, X. S. Chen, and X. B. Jing (2007). A simple and effective route for the preparation of poly(vinylalcohol) (PVA) nanofibers containing gold nanoparticles by electrospinning method. Solid State Commun. 141, 292–295.
L. S. Guczi, K. Frey, A. Beck, G. B. Peto, C. S. Daroczi, N. Kruse, and S. Chenakin (2005). Iron oxide overlayers on AuSiO2 Si(1 0 0) promoting effect of Au on the catalytic activity of iron oxide in CO oxidation. Appl. Catal. A 291, 116–125.
X. Zhang, H. Shi, and B. Q. Xu (2007). Comparative study of AuZrO2 catalysts in CO oxidation and 1,3-butadiene hydrogenation. Catal. Today 122, 330–337.
M. Anandkumar, C. H. Ramamurthy, C. Thirunavukkarasu, and K. S. Babu (2015). Influence of age on the free-radical scavenging ability of CeO2 and AuCeO2 nanoparticles. J. Mater. Sci. 50, 2522–2531.
J. H. Liu, F. Wang, T. Xu, and Z. G. Gu (2010). Styrene epoxidation over carbon nanotube-supported gold catalysts. Catal. Lett. 134, 51–55.
A. Pandikumar, S. Manonmani, and R. Ramaraj (2012). TiO2–Au nanocomposite materials embedded in polymer matrices and their application in the photocatalytic reduction of nitrite to ammonia. Catal. Sci. Technol. 2, 345–353.
H. W. Liang, W. J. Zhang, Y. N. Ma, X. Cao, Q. F. Guan, W. P. Xu, and S. H. Yu (2011). Highly active carbonaceous nanofibers a versatile scaffold for constructing multifunctional free-standing membranes. ACS Nano. 5, 8148–8161.
R. Zanella, S. Giorgio, C. R. Henry, and C. Louis (2002). Alternative methods for the preparation of gold nanoparticles supported on TiO2. J. Phys. Chem. B 106, 7634–7642.
N. S. Patil, B. S. Uphade, P. Jana, R. S. Sonawane, S. K. Bhargava, and V. R. Choudhary (2004). Epoxidation of styrene by anhydrous t-butyl hydroperoxide over Au/TiO2 catalysts. Catal. Lett. 94, 89–93.
G. J. Hutchings (2007). A golden future for green chemistry. Catal. Today 122, 196–200.
D. K. Dumbre, V. R. Choudhary, N. S. Patil, B. S. Uphade, and S. K. Bhargava (2014). Calcium oxide supported gold nanoparticles as catalysts for the selective epoxidation of styrene by t-butyl hydroperoxide. J. Colloid Interface Sci. 415, 111–116.
H. Zhu, M. L. Du, M. Zhang, M. L. Zou, T. T. Yang, L. N. Wang, J. M. Yao, and B. C. Guo (2014). Probing the unexpected behavior of AuNPs migrating through nanofibers a new strategy for the fabrication of carbon nanofiber–noble metal nanocrystal hybrid nanostructures. J. Mater. Chem. A 2, 11728–11741.
B. Wang, J. Zhang, X. Zou, H. G. Dong, and P. J. Yao (2015). Selective oxidation of styrene to 1, 2-epoxyethylbenzene by hydrogen peroxide over heterogeneous phosphomolybdic acid supported on ionic liquid modified MCM-41. Chem. Eng. J. 260, 172–177.
F. Wang, J. Zhang, C. Liu, and J. H. Liu (2015). Pd–palygorskite catalysts preparation, characterization and catalytic performance for the oxidation of styrene. Appl. Clay Sci. 105, 150–155.
X. D. Cai, H. Y. Wang, Q. P. Zhang, and J. H. Tong (2014). Selective oxidation of styrene efficiently catalyzed by spinel. J. Sol-Gel Sci. Technol. 69, 33–39.
Y. Y. Fang, Y. Z. Chen, X. Z. Li, X. C. Zhou, J. Li, W. J. Tang, J. W. Huang, J. Jin, and J. T. Ma (2014). Gold on thiol-functionalized magnetic mesoporous silica sphere catalyst for the aerobic oxidation of olefins. J. Mol. Catal. A 392, 16–21.
H. Liu, J. Bai, S. Wang, C. P. Li, L. P. Guo, H. O. Liang, T. Xu, W. Y. Sun, and H. Q. Li (2014). The preparation of silver nanoparticlescarbon nanofibers as catalyst in the styrene epoxidation. Colloids Surf. A 448, 154–159.
X. S. Hu, J. Bai, J. Z. Wang, C. P. Li, and W. Xu (2015). Preparation of 4A-zeolite-based Ag nanoparticle composite catalyst and research of the catalytic properties. RSC Adv. 5, 2968–2973.
X. B. Ke, S. Sarina, J. Zhao, X. G. Zhang, J. Chang, and H. Y. Zhu (2012). Tuning the reduction power of supported gold nanoparticle photocatalysts for selective reductions by manipulating wavelength of visible light irradiation. Chem. Commun. 48, 3509–3511.
L. Y. Wang, J. Li, W. Dai, Y. Lv, Y. Zhang, and S. Gao (2014). Facile and efficient gold-catalyzed aerobic oxidative esterification of activated alcohols. Green Chem. 16, 2164–2173.
L. P. Guo, J. Bai, J. Z. Wang, H. O. Liang, C. P. Li, W. Y. Sun, and Q. R. Meng (2015). Fabricating series of controllable-porosity carbon nanofibers-based palladium nanoparticles catalyst with enhanced performances and reusability. J. Mol. Catal. A 400, 95–103.
Y. C. Chou, C. L. Shao, X. H. Li, C. Y. Su, H. C. Xu, M. Y. Zhang, P. Zhang, X. Zhang, and Y. C. Liu (2013). BiOCl nanosheets immobilized on electrospun polyacrylonitrile nanofibers with high photocatalytic activity and reusable property. Appl. Surf. Sci. 285, 509–516.
Y. D. Liu, H. G. Chae, and S. Kumar (2011). Gel-spun carbon nanotubes/polyacrylonitrile composite fibers. Part I: effect of carbon nanotubes on stabilization. Carbon 49, 4466–4476.
H. Liu, J. Bai, C. P. Li, W. Xu, W. Y. Sun, T. Xu, Y. R. Huang, and H. Q. Li (2014). An effective approach to preparing MgO–Ag NPs–CNFs and Al2O3 –Ag NPs–CNFs for styrene epoxidation action. RSC Adv. 4, 3195–3200.
D. Nepak and S. Darbha (2015). Selective aerobic oxidation of alcohols over Au–Pd sodium titanate nanotubes. Catal. Commun. 58, 149–153.
J. Li, Y. H. Ni, C. Liu, and L. Zhang (2015). Noncovalent assembly of the carbon nanofibers/Au nanocomposite and its application in 4-nitrophenol reduction. J. Cluster Sci. 26, 1547–1556.
S. Das and T. Asefa (2012). Core–shell–shell microsphere catalysts containing Au nanoparticles for styrene epoxidation. Top. Catal. 55, 587–594.
R. J. Hobson and A. H. Windle (1993). Crystallization and shape emulation in atactic poly(vinyl chloride) and polyacrylonitrile. Polymer 34, 3582–3596.
Acknowledgments
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (21266016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, Y., Li, C., Bai, J. et al. The Construction of Au/Carbon Nanocomposite Material, Characterization and Their Application in Catalytic Reaction of Styrene Epoxidation. J Clust Sci 27, 1147–1158 (2016). https://doi.org/10.1007/s10876-016-0979-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-0979-x