Abstract
Objective
This case-controlled study was designed to correlate urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8) levels, with renal involvement in a cohort of systemic lupus erythematosus (SLE) patients to examine their diagnostic performance.
Patients and Methods
In 73 SLE patients, and in 23 healthy volunteers, urinary levels of TWEAK, OPG, MCP-1, and IL-8 levels were measured. Disease activity was assessed by total SLE disease activity index, and renal activity by renal activity index (rSLEDAI), and both were correlated with urinary biomarkers. Sensitivity, specificity, and predictive values of individual biomarkers to predict lupus nephritis were also calculated.
Results
Significantly higher levels of urinary biomarkers were observed in SLE patients with lupus nephritis (LN) compared with those without LN (TWEAK, p < 0.001; MCP-1, p < 0.001; OPG, p < 0.001; IL-8, p < 0.032). Other significantly higher levels were observed in SLE patients with LN compared with control subjects (TWEAK, MCP-1, OPG, and IL-8 p < 0.001). Positive correlations were observed between rSLEDAI and TWEAK (r = 0.612 and p < 0.001), MCP-1 (r = 0.635 and p < 0.001), and OPG (r = 0.505 and p < 0.001).
Conclusions
Urinary levels of TWEAK, OPG, and MCP-1 positively correlate with renal involvement as assessed by rSLEDAI with reasonable sensitivity, specificity, and predictive values to detect lupus nephritis while IL-8 was not significantly associated with global or rSLEDAI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal involvement in systemic lupus erythematosus (SLE) is common with 50% of patients developing lupus nephitis (LN) in the first year of diagnosis and is associated with an adverse outcome [1]. Despite treatment, it has been reported that the 5-year renal survival rate ranges from 46% to 95% [2]. A number of biochemical markers are currently used to clinically assess SLE renal disease activity, such as anti-double-stranded DNA antibodies and complement component levels. Nevertheless, the correlation between these markers and LN is imperfect, and their utility in reflecting disease activity and predicting outcome remains controversial [3].
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNF ligand super family (TNFRSF) [4]. Similar to TNF, TWEAK is cleaved into a circulating trimeric form that is thought to mediate its biologic effects. The TNFRSF member Fn14 was, conclusively, identified as a receptor for TWEAK in 2001 [5]. Binding of TWEAK to the Fn14 receptor induces a strong proinflammatory response in various cell types, including macrophages, fibroblasts, and synoviocytes. Interestingly, several of the cytokines/chemokines induced by TWEAK are major contributors to the pathogenesis of LN [6]. In a mouse model of SLE, the absence of Fn14 or treatment with an anti-TWEAK antibody reduces renal inflammation and the severity of proteinuria [7]. In a recent report, Schwartz et al. [8] supported a crucial role of TWEAK in the pathogenesis of LN and provided strong evidence for urinary TWEAK (uTWEAK) as a candidate clinical biomarker for LN. Preliminary studies showed that uTWEAK is a novel and accurate method used to repeatedly assess kidney disease in SLE [9] and can induce mesangial cells, podocytes, and endothelial cells to secrete proinflammatory chemokines, which are crucial in the pathogenesis of LN [10]. Recently, higher levels of uTWEAK are indicative of LN, as opposed to non-LN SLE, with better reflection of renal disease activity in longitudinal follow-up [8].
Monocyte chemoattractant protein-1 (MCP-1) is a leukocyte chemotactic factor that is involved in mediating inflammation and injury in LN [11]. In patients with SLE, urine monocyte chemoattractant protein-1 (uMCP-1) levels are elevated in those with active nephritis and reduced after immunosuppressive treatment [12, 13]. Nevertheless intrarenal expression of other proinflammatory mediators, such as MCP-1, interleukin-8 (IL-8), and ostoprotegrin (OPG) has been shown to play an important role in the pathogenesis of chemokine-mediated renal injury in SLE [8, 14–18].
These new lines of evidence inspired us to measure these novel urinary biomarkers (uTWEAK, uMCP-1, uOPG, and uIL-8) in a cohort of SLE patients, with and without LN, in order to correlate findings with other disease variables, laboratory investigations, and global and renal disease activity indices to examine their diagnostic performance in relation to LN.
Subjects and Methods
The study comprised 73 patients with systemic lupus erythematosus (70 females and three males). All patients fulfilled the 1982 revised criteria of the American Rheumatism Association for the classification of SLE [19]. Their mean age was 28.54 ± 8.1 years, and the mean disease duration was 5.8 ± 3.8 years. The patients’ group was further divided into two groups: group I (those with active lupus nephritis, n = 50) and group II (those with inactive or nonrenal patients, n = 23). The control group, group III, consisted of 23 healthy volunteers (22 females and one male), with a mean age of 31.57 ± 9.2 years. All subjects were recruited from the Rheumatology and Rehabilitation Department and the Internal Medicine Department, Faculty of Medicine, Cairo University. All participants gave informed written consent in accordance with the Declaration of Helsinki.
SLE Disease Activity Index Assessment
The global disease activity was assessed by total SLEDAI (tSLEDAI). A SLEDAI score of ≥4 was taken as an indicator of high levels of disease activity [20].
For renal involvement, renal SLEDAI (rSLEDAI) was used to assess kidney disease activity. The score consists of the four kidney-related parameters: hematuria, pyuria, proteinuria, and urinary casts. Scores for the renal SLEDAI can range from 0 (inactive renal disease) to a maximum of 16. Active lupus nephritis was those with an rSLEDAI score of 4 or more [21].
Renal biopsy specimens were classified according to the World Health Organization (WHO) criteria: minimal changes (class I), mesangial alterations (class II), focal proliferative (class III), diffuse proliferative (class IV), and membranous (class V) glomerulonephritis [22].
Laboratory investigations included CBC, serum chemistry, ESR, serum C3, C4, and anti-dsDNA, complete urine analysis, 24-h urinary proteins excretion, and urinary biomarkers of nephritis (uTWEAK, uMCP-1, uOPG, and uIL8). All available laboratory values were used in further analysis.
Urinary Biomarkers Measurements
Freshly voided urine specimens were collected early in the morning from patients and controls. Urine samples were placed at 4°C and transported directly to the laboratory where they were centrifuged to remove sediment and frozen in aliquots at −80°C for further analysis. No other manipulations were done as urinary chemokines are stable in long-term frozen storage [10]. The levels of urinary markers (TWEAK, OPG, MCP-1, and IL-8) were measured by specific ELISA, according to the manufacturer’s directions (Bender MedSystems, Vienna, Austria; Biovendor Laboratory Medicine, Brno, Czech Republic; Quantikine; R&D systems, Minneapolis; USA, Pierce Endogen, Rockford, USA, respectively). Urinary TWEAK levels were determined by ELISA, as described previously [10], with the intra- and inter-assay coefficients of variation being 7.9% and 9.2%, respectively, and an average urine recovery of 88%. The lower range of the uTWEAK assay is 15.6 pg/ml [23]. Urinary MCP-1 had intra- and inter-assay coefficients of variation of 5.9% and an average urine recovery of 92%. The lower range of the uMCP-1 assay is 5 pg/ml. The intra- and inter-assay coefficients of variation for uOPG were 4.9% and 9%, respectively. The lower range of the OPG assay is 0.1 pg/ml. The IL-8 had intra- and inter-assay coefficients of variation of 10% and an average urine recovery of 120%. The lower range of the IL-8 ELISA is 25 pg/ml. Urine values below this were considered undetectable. Urinary biomarkers levels were standardized to urine creatinine measured in the same spot urine and expressed as pg/mg Cr.
Statistics
Data were coded and summarized using SPSS version 12.0 for Windows. Quantitative variables were described using mean ± standard deviation and categorical data by using frequency and percentage. Nonparametric testing was performed by Mann–Whitney U test and used to compare uTWEAK and other urinary biomarker levels among the studied groups of patients and normal control subjects. Spearman’s rho correlation test was used to correlate urinary biomarkers with other disease variables. In all tests, p value of <0.05 was inferred as statistically significant. Regression analysis was performed to examine the cumulative effect of all urinary biomarkers (uTWEAK, uMCP1, uOPG, and uIL-8) in the occurrence of LN, and one-way ANOVA was used to examine the differences of individual urinary biomarker values among different classes of renal biopsy. Receiver operating characteristic curves were used to identify the ability of the measured biomarkers in order to distinguish between patients' group and to obtain sensitivity, specificity, positive, and negative predictive values.
Results
SLE patients (n = 73) were recruited into two groups: SLE patients with active lupus nephritis (group I) and those patients with inactive or nonrenal involvement (group II). Statistically, significant differences were found between both groups in the following variables; systolic blood pressure (mmHg) (SBP), diastolic blood pressure (mmHg) (DBP), tSLEDAI, rSLEDAI, serum creatinine level (mg/dl), 24-h urinary proteins (g/24 h), C3 (u/ml), and C4 (u/ml). Detailed comparisons between both groups are presented in Table I.
Regarding urinary biomarkers measured, a significant difference was observed in uTWEAK (pg/mg Cr), uMCP-1(pg/mg Cr), and uOPG (pg/mg Cr) levels, being higher in SLE patients with LN (group I) compared with those without (group II) and normal control (p < 0.001). Other detailed results are presented in Table II while statistical differences, median and interquartile ranges of individual urinary biomarkers are presented in Table II and Fig. 1.
Boxplot showing individual urinary biomarkers evaluated (TWEAK, MCP-1, OPG, and IL-8). The lines inside the boxes indicate the median; the outer borders of the boxes indicate 25th and 75th percentiles; the bars extending from the boxes indicate the 10th and 90th percentiles. TWEAK, MCP-1, and OPG in group I vs. group II and normal control (p < 0.001). IL-8 in group I vs. group II (p = 0.032); IL-8 in group I vs. normal control (p < 0.001)
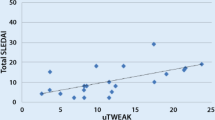
Spearman’s rho test was used to measure possible linear relationships between urinary biomarkers measured and other diseases variables in terms of demographic, clinical, and important laboratory investigations used to assess renal involvement in SLE groups of patients. Positive correlations were observed between uTWEAK (pg/mg Cr.) vs. SBP (r = 0.255 and p = 0.029), hematuria (r = 0.254 and P = 0.03), pyuria (r = 0.281 and p = 0.01), urinary casts (r = 0.552 and p < 0.001), 24-h urinary proteins (r = 0.247 and p = 0.03), tSLEDAI (r = 0.577 and p < 0.001), and rSLEDAI (r = 0.612 and p < 0.001). Other important details of correlations are presented in Table III and Fig. 2.
a The positive correlations between urinary TWEAK and renal disease activity index (rSLEDAI; r = 0.612 and p = <0.001). b The negative correlation between urinary TWEAK levels in SLE patients (n = 73) and renal biopsy grading (r = −0.06 and p > 0.05). c, d The negative correlation between TWEAK levels vs. C3 (r = −0.544 and p < 0.001) and C4 (r = −0.409 and p < 0.001)
Relative to renal involvement, uTWEAK showed sensitivity of 89%, specificity of 56%, a positive predictive value of 93%, and a negative predictive value of 66.7%. Other detailed sensitivity, specificity, positive predictive, and negative predictive values for other urinary biomarkers are presented in Table IV.
Renal biopsies grading were available for 44 patients, all in group I, with LN. According to the WHO classification, four patients classified as class I, 12 patients classified as class II, 14 patients with class III, six patients with class V, and eight patients with class IV. No statistical differences were observed regarding urinary biomarkers levels among different classes of renal biopsies by one-way ANOVA (TWEAK, F = 0.521; MCP-1, F = 0.696; OPG, F = 1.178; IL-8, F = 0.289).
Furthermore, multivariate analysis was carried out to examine the power of all urinary biomarkers together (TWEAK, MCP-1, OPG, and IL-8), in the occurrence of LN as a dependent variable, and results showed a highly significant effect on LN combined together (F = 33.462).
Discussion
The current cross-sectional study was designed to evaluate the diagnostic performance of uTWEAK and other urinary biomarkers (uOPG, uMCP-1 and uIL-8), in a cohort of SLE patients with and without LN, as well as to correlate findings with other disease variables, and standard laboratory investigations were used to assess renal function and disease activity indices.
Although many novel biomarkers have been studied in lupus nephritis, none have been rigorously validated in large-scale longitudinal cohorts of patients. It was recommended that future directions in SLE biomarker research should focus on a combination of novel markers with conventional clinical parameters in order to enhance the sensitivity and specificity for the prediction of renal flares and prognosis in LN [24].
Our findings clearly show that urinary levels of TWEAK were significantly higher in SLE patients with LN compared to those without or with inactive renal disease and normal healthy subjects (p < 0.001). Moreover, uTWEAK levels positively correlate with tSLEDAI and rSLEDAI scoring systems. Similar to our findings, Schwartz et al. [10] showed that in SLE patients, uTWEAK levels were higher in patients with active compared with never or nonactive nephritis (p = 0.001) and levels of uTWEAK correlated with the rSLEDAI (p < 0.001). In this study, uTWEAK levels were higher in patients undergoing a flare as compared with patients with chronic stable disease (p = 0.036), and uTWEAK levels were significantly higher in patients undergoing a renal flare, as opposed to a nonrenal flare. In their most recent work, Schwartz et al. [8] observed that uTWEAK was better at distinguishing between LN and non-LN SLE patients than anti-DNA antibodies and complement levels. In another work, El-Gendi and El-Sherif [25] reported that TWEAK correlated with traditional disease activity parameters (C3, C4, anti-dsDNA, and tSLEDAI) as well as rSLEDAI.
In our study, uTWEAK positively correlated with SBP (p = 0.029), and descriptors of rSLEDAI, e.g., hematuria (p = 0.03), pyuria (p = 0.01), urinary casts (p < 0.001), 24-h urinary proteins (p = 0.03), and negatively correlated C3 (p < 0.001) and C4 (p < 0.001). In our opinion, the later findings have important clinical implications, although complement components (C3 and C4), known to be consumed in SLE patients with LN, yet in SLE complement components can be consumed in other immune-complex-mediated lesions, such as vasculitis with a stable renal function. Given that, uTWEAK could be the novel and more specific biomarker of potential diagnostic and prognostic roles to assess LN. In our study of relative to renal involvement, uTWEAK showed sensitivity of 89%%, specificity of 56%, a positive predictive value of 93%, and a negative predictive value of 66.7% (95% CI = 0.7–0.9).
In animal models, Molano et al. [26] have shown that the interaction of TWEAK with its receptor Fn14 is instrumental in the pathogenesis of nephritis in the chronic graft-versus-host-induced model of lupus. Using a different approach, Zhao et al. [7] observed that kidney cells displayed the TWEAK receptor Fn14 and TWEAK stimulation of mesangial cells and podocytes induced a potent proinflammatory response and upregualtion of several cytokines. In this study, lupus-induced mice treated with an anti-TWEAK neutralizing mAb had a significantly diminished kidney expression of IL-6, MCP-1, IL-10 as well as proteinuria. Given that, a TWEAK blockade may be a novel therapeutic approach to reduce renal damage in SLE [23].
OPG is produced by the heart, lungs, kidney, and bone while MCP-1 is a chemotactic cytokine that is involved in the progression of glomerular and tubulointerstitial injury [17]. Several cross-sectional studies have confirmed that levels of uMCP-1 are elevated in patients with active LN compared with those with inactive renal disease or healthy controls [17, 23, 27, 28]. In our study, urinary levels of OPG and MCP-1 positively correlate with rSLEDAI (p < 0.001) and other descriptors of rSLEDAI. Consistent with our findings, Alzawawy et al. [29] observed that uMCP-1 was significantly higher in SLE patients with active LN compared with those with inactive LN, and normal healthy controls positively correlate with proteinuria. In an early report, Norris et al. [13] showed that in patients with active LN, uMCP-1 was significantly higher than in lupus patients in the inactive phase of the disease or in healthy volunteers, and high doses of i.v. methylprednisolone significantly lowered uMCP-1 in patients with active LN. Tucci et al. [30] presented evidence that uMCP-1 promotes renal disease in experimental glomerulonephritis while its increased urinary levels reflect the severity of kidney disease in humans. Nevertheless, Rovin et al. [27] have shown that the mean uMCP-1 level at the time of renal flares was significantly higher than that of healthy controls, patients with inactive renal disease and patients with active or inactive nonrenal SLE. In addition, uMCP-1 was also found to be a sensitive indicator for renal flare, with 73% of the flare values above the 95th percentile of disease controls. uMCP-1 levels were higher in patients with proliferative (WHO class III or IV) than membranous (class V) nephritis. In our study, we did not observe a significant difference in uMCP-1 levels among different classes of renal biopsy. Moreover, Rovin et al. [27] in a longitudinal follow-up of 12 patients, uMCP-1 increased as early as 2 to 4 months before renal flares and remained high for at least 4 months after treatment of flares. In patients who improved clinically, uMCP-1 levels fell to control levels, whereas in patients who were refractory to treatment, uMCP-1 remained high. Similar findings of uMCP-1 in LN were reported by Tian et al. [31]. In this study, 73 patients with diffuse proliferative lupus nephritis were followed. After an observation of 2 years, 22 patients experienced renal flares, and those experiencing renal flares showed higher levels of uMCP-1. Using a different approach, Chan et al. [32] examined the expression of chemokine mRNAs in urinary sediments of nine patients with active LN for 24 weeks. The authors demonstrated that urinary mRNA expression of MCP-1 and other chemokines correlated significantly with SLE disease activity scores and anti-dsDNA titers during the course of immunosuppressive treatment.
In an early study, Kiani et al. [17] reported that uOPG concentrations were strongly associated with tSLEDAI and renal disease activity descriptors of the Safety of Estrogens in Lupus Erythematosus-National Assessment (SELENA) SLEDAI. Furthermore, in their study uMCP-1 was also associated with the renal disease activity descriptors of SELENA SLEDAI, including hematuria (p = 0.027), and with a positive anti-dsDNA (p = 0.016).
IL-8 is a potent neutrophil chemokine that has been implicated in the pathogenesis of renal inflammation in human glomerulonephritis. In SLE, it was postulated that the promoter region of the IL-8 gene contains polymorphic residues that influence the level of IL-8 expression in response to immune-complex deposition, and thereby affects the severity of renal injury [33]. Moreover, the local production of IL-8 in diseased glomeruli might be involved in the pathogenesis of glomerular diseases, and a measurement of IL-8 in the urine might be useful for monitoring the glomerular diseases [34]. In our study, positive correlation was observed between Il-8 vs. 24-h urinary proteins (p = 0.02) and negative correlation with both C3 (p = 0.032) and C4 (p = 0.023). In a previous work, Tsai et al. [35] have shown that proinflammatory cytokines IL-6 and IL-8 can reflect the renal inflammatory activity in patients with lupus tubulointerstitial nephritis as well as in those with lupus glomerulonephritis. In our study, uIL-8 was not associated with either global disease activity or rSLEDAI compared with other urinary biomarkers evaluated.
Importantly in our study, multivariate analysis was carried out to examine the power of all urinary biomarkers together (TWEAK, MCP-1, OPG, and IL-8) in the occurrence of LN as a dependent variable, with results showing a highly significant effect on LN when combined (F = 33.462). Furthermore, in our study all urinary biomarkers except for IL-8 showed positive correlations with tSLEDAI and rSLEDAI and negative correlation with C3 and C4, illustrating central roles and close association of these urinary biomarkers in cytokines-mediated renal injury in SLE. Although the strength of the association is weak, though “statistically significant,” we recommended re-evaluating such findings in large cohorts of SLE patients with different disease severity to validate the clinical importance of these urinary biomarkers in diagnosis and prognosis of renal disease in SLE. These observations in our study, and in the previous studies presented, give cumulative experience and more solid evidence of important roles of urinary biomarkers, and this in turn supports potential therapeutic target in chemokines-mediated renal injury in SLE.
Conclusions
The current knowledge supports that urinary TWEAK and other urinary biomarkers (OPG, MCP-1, and IL-8) play central roles in the pathogenesis of LN. Adoption of new therapeutic strategies such as monoclonal antibodies antagonizing the effects of these cytokines could be the missing thread for a reasonable and appropriate approach for the treatment of cytokine-dependent renal injury in SLE.
References
Seshan SV, Jennette JC. Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med. 2009;133:233–48.
Korbet SM, Lewis EJ, Schwartz MM. Factors predictive of outcome in severe lupus nephritis. Lupus Nephritis Collaborative Study Group. Am J Kidney Dis. 2000;35:904–14.
Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum. 2004;50(7):2048–65.
Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, et al. TWEAK a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–10.
Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, et al. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–46.
Campbell S, Michaelson J, Burkly LC, Putterman C. The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front Biosci. 2004;8:2273–84.
Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, et al. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179:7949–58.
Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11(5):R143.
Gao HX, Campbell S, Burkly LC, Jarchun I, Banas B, Schlondorff D, et al. TNF-like weak inducer of apoptosis (TWEAK) stimulates human kidney cells to elaborate chemokines central to the pathogenesis of lupus nephritis [Abstract]. Arthritis Rheum. 2006;54(Suppl):S602.
Schwartz N, Su L, Burkly LC, Mackay M, Aranow C, Kollaros M, et al. Urinary TWEAK and the activity of lupus nephritis. J Autoimmun. 2006;27(4):242–50.
Rovin BH, Birmingham DJ, Nagaraja HN, Yu CY, Hebert LA. Biomarker discovery in human SLE nephritis. Bull NYU Hosp Jt Dis. 2007;65(3):187–93.
Wada T, Yokoyama H, Su SB, Mukaida N, Iwano M, Dohi K, et al. Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int. 1996;49(3):761–7.
Noris M, Bernasconi S, Casiraghi F, Sozzani S, Gotti E, Remuzzi G, et al. Monocyte chemoattractant protein-1 is excreted in excessive amounts in the urine of patients with lupus nephritis. Lab Invest. 1995;73(6):804–9.
Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–76.
Kelley VR, Rovin BH. Chemokines: therapeutic targets for autoimmune and inflammatory renal disease. Springer Semin Immunopathol. 2003;24:411–21.
Rovin BH, Phan LT. Chemotactic factors and renal inflammation. Am J Kidney Dis. 1998;31:1065–84.
Kiani AN, Johnson K, Chen C, Diehl E, Hu H, Vasudevan G, et al. Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J Rheumatol. 2009;36(10):2224–30.
Tesar V, Masek Z, Rychlík I, Merta M, Bartůnková J, Stejskalová A, et al. Cytokines and adhesion molecules in renal vasculitis and lupus nephritis. Nephrol Dial Transplant. 1998;13:1662–7.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7.
Bombardier C, Gladman D, Urowitz M. Committee on prognosis studies in systemic lupus erythematosus. The derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40.
Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91.
Churg J, Sobin LH. Renal disease: classification and atlas of glomerular disease. Tokyo: Igaku-Shoin; 1982.
Campbell S, Burkly LC, Gao H, Berman JW, So L, Browning B, et al. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol. 2006;176:1889–98.
Mok CC. Biomarkers for lupus nephritis: a critical appraisal. J Biomed Biotechnol. 2010;2010:638413.
El-Gendi SS, El-Sherif WT. Anti-C1q antibodies, sCD40L, TWEAK and CD4/CD8 ratio in systemic lupus erythematosus and their relations to disease activity and renal involvement. Egypt J Immunol. 2009;16(1):135–48.
Molano A, Lakhani P, Aran A, Burkly LC, Michaelson JS, Putterman C. TWEAK stimulation of kidney resident cells in the pathogenesis of graft versus host induced lupus nephritis. Immunol Lett. 2009;125(2):119–28.
Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16(2):467–73.
Tucci M, Barnes EV, Sobel ES, Croker BP, Segal MS, Reeves WH, et al. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004;50(6):1842–9.
Alzawawy A, Zohary M, Ablordiny M, Eldalie M. Estimation of monocyte-chemoattractantprotein-1 (Mcp-1) level in patients with lupus nephritis. Int J Rheum Dis. 2009;12(4):311–8.
Tucci M, Calvani N, Richards HB, Qatar C, Silvestris F. The interplay of chemokines and dendritic cells in the pathogenesis of lupus nephritis. Ann N Y Acad Sci. 2005;1051:421–32.
Tian S, Li J, Wang L, Liu T, Liu H, Cheng G, et al. Urinary levels of RANTES and M-CSF are predictors of lupus nephritis flare. Inflamm Res. 2007;56(7):304–10.
Chan RW, Lai FM, Li EK, Tam LS, Chow KM, Li PK, et al. The effect of immunosuppressive therapy on the messenger RNA expression of target genes in the urinary sediment of patients with active lupus nephritis. Nephrol Dial Transplant. 2006;21(6):1534–40.
Rovin BH, Lu L, Zhang XA. Novel interleukin-8 polymorphism is associated with severe systemic lupus erythematosus nephritis. Kidney Int. 2002;62(1):261–5.
Wada T, Yokoyama H, Tomosugi N, Hisada Y, Ohta S, Naito T, et al. Detection of urinary interleukin-8 in glomerular diseases. Kidney Int. 1994;46(2):455–60.
Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85(3):207–14.
Conflicts of Interest Statement
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-shehaby, A., Darweesh, H., El-Khatib, M. et al. Correlations of Urinary Biomarkers, TNF-Like Weak Inducer of Apoptosis (TWEAK), Osteoprotegerin (OPG), Monocyte Chemoattractant Protein-1 (MCP-1), and IL-8 with Lupus Nephritis. J Clin Immunol 31, 848–856 (2011). https://doi.org/10.1007/s10875-011-9555-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9555-1