Abstract
Alkaline earth metals act as dominating acid-neutralizing species in atmosphere and hence, regulate the rain water chemistry significantly. In this contribution, concentrations of these metals (Mg, Ca, Sr and Ba) and other major ions in rain water samples, collected during south-west monsoon of year 2017, from a coastal location (Berhampur) in eastern part of India have been analyzed to trace their provenances and controlling factors. The chemical compositions of rain water reveal oceanic and continental supply of Mg and Sr to the site, whereas Ca and Ba are pre-dominantly supplied through continental sources. The dominancy of continental fluxes at this coastal site is mainly due to particulate fluxes from regional lithologies and favorable wind pattern for long-range transport from south-western/western directions. An inverse model involving chemical mass balance between rain water composition and its possible sources have been adopted in this study to quantify the source(s) contributions. These model results show that the continental Mg is mainly derived from long-range transport of mafic minerals from Deccan Traps (40 ± 21%) with sub-ordinate contribution (15 ± 6%) from regional lithologies. On average, about 70% of rain water Ca at Berhampur is derived from carbonates, whereas most of the Ba (~95%) is supplied from regional silicates (charnockites and khondalites). Owing to faster dissolution kinetics of these silicates with higher Ba content, the silicates contribute most of the rain water Ba concentration over this region. The median Ba content (29 nM) at this location is systematically higher than available literature Ba data for rain water worldwide (1-22 nM). The observed higher concentrations of Ba, a micronutrient, in rain water emphasize important role of regional lithology in the biogeochemical cycling of nutrients over the region via wet deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rainfall is an efficient pathway for removing the atmospheric gaseous and particulate pollutants, which are supplied through both natural (e.g. sea-salt aerosols, eolian dusts) and anthropogenic (e.g. industrial emission, biomass burning, transport, agricultural activities) sources (Berner and Berner 2012; Jacob 1999). Interaction of these pollutants with water vapors during cloud formation and washout processes regulates rain water chemistry, which influences the ecosystems and biogeochemical cycling of major nutrients (Vet et al. 2014). A large number of studies on chemical composition of rain water (RW) has already been carried out worldwide to understand the intensity of and processes related to RW acidity and its effect on ecosystems, historical monuments, terrestrial as well as oceanic life (Cong et al. 2010; Lara et al. 2001; Likens and Bormann 1974; Negrel and Roy 1998; Pascaud et al. 2016; Rastogi and Sarin 2005; Rhode et al. 2002; Sequeira and Kelkar 1978). The pH of rain varies significantly at regional scales and is regulated by the balance between abundance of acidic (sulfuric and nitric acids formed from SO2 and NO2 gases respectively) and alkaline (e.g. Ca and Mg) components in the atmosphere. The occurrence of acid rain events is more frequent in regions with significant anthropogenic emissions, whereas rains with higher pH values are peculiar for regions with higher dust loads (Vet et al. 2014). Interestingly, despite of significant increase in SO2 and NO2 emissions in India, the RW is mostly reported to be alkaline in nature (Bhaskar and Rao 2017; Budhavant et al. 2011; Chatterjee and Singh 2012; Das et al. 2010; Jain et al. 2000; Kulshrestha et al. 2005; Rastogi and Sarin 2005; Rajeev et al. 2016; Roy et al. 2016; Tiwari et al. 2007). This alkaline nature has mainly been attributed to an efficient neutralization of the acidic components of RW by abundant base cations, mainly the alkaline earth metals, present in the mineral dusts (Bisht et al. 2015; Budhavant et al. 2011; Rao et al. 2016; Safai et al. 2004; Tiwari et al. 2012).
The abundance of these alkaline earth elements in RW is predominantly regulated by provenances of the mineral dust, their composition and relative contribution at the site. Forward model calculations based on major ions data from an urban site in western India (Pune) show that continental sources supply most of the Ca but only ~37–68% of Mg in RW with oceanic source accounting for the remaining amount of Mg (Budhavant et al. 2011). However, provenance studies based on Sr isotopes of RW from the western part of India (Ahmedabad) have suggested supply of these cations from nearby litho-units (Deccan basalts and carbonates) with occasional supply through long-range transportation (African silicates; (Chatterjee and Singh 2012)). Although barium serves as an important bio-essential element in oceans, there have been only limited efforts to investigate sources and deposition of barium present in the atmosphere. Available only a few studies on rain water Ba indicate its dominant supply from continental sources with insignificant oceanic input (Jickells et al. 1992; Özsoy and Örnektekin 2009). The present study focuses on the abundances and sources of selected alkaline earth metals (Mg, Ca, Sr and Ba) in RW from a coastal, semi-urban location (Berhampur) from the eastern part of India. We have employed an inversion approach to apportion the sources and their relative contribution to the RW chemistry. Outcomes of this study show the dominance of regional lithology (charnockites and carbonates) in regulating Ca and Ba concentrations, whereas significant Mg is transported from the Deccan basalts to the eastern India.
2 Sampling and analysis
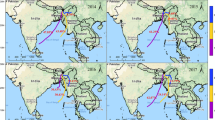
The present study site, Berhampur (19° 20’N; 84° 50′ E; Elevation ~ 26 m) is a semi-urban coastal location from the eastern part of India (Fig. 1). It is about 10 km away from the Gopalpur port, which is often hit by severe cyclones (with wind speed reaching upto 270 km/h) resulted from tropical depressions developed over the Bay of Bengal. The total population of this location is about 350,000. The temperature at this location varies between 22 and 40 °C. This site falls in the core Indian monsoon zone (Gadgil 2003) and receives an average (for 2001–2016) rainfall of 1267 ± 231 mm (Fig. 2; India Meteorological Department (IMD) dataset) annually. About 70% of the annual rainfall at Berhampur occurs during the south-west monsoon period. This study was carried out during 2017 and the rainfall pattern of this year matches well with the average rainfall pattern at this location for last two decades (Fig. 2). The annual rainfall amount for the year 2017 was 1363 mm. Air mass backward trajectory analyses using HYSPILT model (https://www.ready.noaa.gov/HYSPLIT.php) show that the air parcel to Berhampur during the sampling period mainly comes from the south-west or westerly direction with minimal change in the wind direction at different heights (Fig. 1b). The wind originates from the Arabian sea and travels through continental landmasses, mainly the Deccan basalts before reaching the location. Geologically, this site is located on the Eastern Ghats, which is mainly composed of granulites, khondalites and charnockites (Ramakrishnan and Vaidyanadhan 2010).
a Location map of rain water samples collected during the monsoon period of the year 2017. For reference, spatial distribution of annual rainfall (for 2017; Data source: https://trmm.gsfc.nasa.gov/) and wind pattern during the monsoon period (reanalysis data from http://www.ncep.noaa.gov/) are also shown. b Wind back-trajectory plot (from HYSPILT model) for the sampling period show long-range transport of particulates from the western India to the studied location. The plot shows both average and associated errors in the wind trajectory at different altitudes (500, 1000 and 5000 m)
Monthly distribution of rainfall at Berhampur during the year 2017. Average monthly rainfall amount at this location for last 17 years is also shown, for comparison. Data source: IMD data archive (http://www.imd.gov.in/)
Samples from individual rain events (n = 25) were collected at Berhampur during the south-west monsoon period of 2017. The collection of samples was carried out following the method adopted by Chatterjee and Singh (2012). The sampling site is located in a residential area from the western edge of the town. Samples were collected at ~ 5 m above the ground using a plastic container. The pre-cleaned container was opened after starting of the rainfall to avoid any dry deposition, whereas the sample collection was immediately stopped after ending of rainfall to ensure no evaporation loss. The collected samples were filtered using 0.2 μm nylon filters and stored in a polycarbonate bottle. Prior to sample storing, these bottles were rinsed 2–3 times with the samples itself to avoid any contamination. These filtered aliquots were used for their chemical analyses. Major ions (Na, K, NH4, Ca, Mg, Cl, NO3 and SO4) concentrations of the samples were measured using an ion chromatograph (Compact IC plus 882, Metrohm) instrument. Analytical detail of the methodology adopted in this study is provided in Tripathy et al. (2010). Briefly, the major cations were analyzed by passing the samples through a C4 150/4.0 column with methane sulfonic acid as eluent to separate individual species. Concentrations of these species were quantified using a standard calibration approach. Sampling aliquots for the anion measurement could not be acidified after its collection due to logistic issues, so the analyses were carried out using the unacidified aliquot of the sample. Analyses of the anions (Cl, NO3 and SO4) were carried out after their separation using a Metrosep A Supp 5–250/4.0 column with Na2CO3/NaHCO3 solution as eluent. Chemical analyses of four filtered aliquots of de-ionised water were carried out to constrain the procedural blank for these measurements. The blank levels were significantly lower than the sample signals. Several samples were measured in replicates to constrain the precision of these major ion analyses. The precision of these analyses were found to be different for different ions (0.2–4%) and this information is provided in the supplementary material (Table S1). The accuracy of the analyses were constrained by measuring a Merck© multi-element standard solutions with known concentrations. The average accuracy of the measurements was found to be ~6% (cf. Table S1). The concentrations of two trace elements, Ba and Sr of the samples were measured using a quadrupole-inductive coupled plasma mass spectrometer (Q-ICPMS). Analytical details on these mass spectrometric analyses are provided in Singh et al. (2013). Briefly, the intensities of 137Ba and 88Sr isotopes were measured and the concentrations were calculated using a standard calibration approach. These analyses were carried out using the Kinetic Energy Discrimination (KED) mode of the mass spectrometer to minimize the isobaric interferences. The background counts for these trace elements (~125 cps) were insignificant compared to the average counts (~56, 000 cps) observed for Ba and Sr measurements. The precision of the Ba and Sr analyses was ±5% (n = 6). The Ba and Sr concentrations of an international reference solution of natural waters (SRM 1640a) were measured to constrain the accuracy. Accuracy of these two elemental analyses was found to be ~2–4% (cf. Table S1).
We have also carried out the air-mass backward trajectory analyses for the sampling period at Berhampur using the HYSPILT model from http://www.arl.noaa.gov. The cumulative wind direction (with associate spread) for three different height levels (500, 1000 and 5000 m) is depicted in Fig. 1b. In this study, we have also calculated the marine contribution for individual species using Na-normalized elemental ratios of the samples and average seawater compositions (Das et al. 2005a). The non-marine component of a given species [X (= Ca, Mg, Sr, Ba)], known as non-sea salt (nss) part, is calculated using the following equation (Rastogi and Sarin 2005):
where, the subscript, rw and sw stand for rain and sea water compositions respectively.
3 Results
Chemical data for the RW samples from Berhampur, India are provided in the supplementary material (Table S2). Among these samples, one anomalous sample (BRW17–01), which was collected during May and after a rain-break episode, shows extremely high (~3–10 times) concentrations for all chemical species when compared to those of other samples; this outlier sample is not considered in constraining the general trend of RW chemistry for this region.
The total cations [TZ+ = Na++NH4++K++Ca+2 + Mg+2; all in equivalent units] of these samples vary from 43 μEq to 682 μEq, with a median value of 185 μEq (Table S2). The cationic chemistry of these RW samples mostly follow Mg+2 < K+ < NH4+ < Na+ < Ca+2 trend. Calcium dominates the cation budget and accounts for nearly half of the total cation content (in equivalent units). The average Na (62 ± 51 μEq), K (10 ± 7 μEq), NH4 (36 ± 26 μEq), Ca (70 ± 58 μEq) and Mg (9 ± 6 μEq) concentrations at this semi-urban site are higher compared to corresponding median values reported earlier for an urban city (Bhubaneswar) from the eastern India (Das et al. 2005a; Norman et al. 2001). The Cl concentrations of the samples vary widely from 12 to 223 μEq, but linearly with their corresponding Na content. The slope of the Na-Cl regression line (1.02) is marginally lower than the seawater Cl/Na molar ratio (~1.17; (Drever 1997)). The lower Cl/Na ratio can be attributed either to additional supply of Na from silicates (cf. section 4) or depletion of chloride from sea-salt aerosols (Sarin et al. 2010). The SO4/NO3 equivalent ratios for the samples vary between 0.9 and 4.2, with an average value of 2.3 ± 0.9. This average SO4/NO3 ratio is comparable with that reported earlier for other parts of India (Rastogi and Sarin 2005; Chatterjee and Singh 2012; Bisht et al. 2015; Rao et al. 2016) and indicates the dominant role of sulphuric acid compared to nitric acid as an acidifying agent in the atmosphere.
The Sr concentrations of the samples vary from 7 nM to 234 nM, with a median value of 34 nM. This Sr content is intermediate to that reported earlier for Bangaladesh (23 nM; (Galy et al. 1999)) and Ahmedabad (83 nM; (Chatterjee and Singh 2012)). Based on these three location (Berhampur, Bangladesh and Ahmedabad) data, the Sr concentrations seem to be about two times lower for coastal regions than for the continental location (Ahmedabad). The Ba concentrations of the RW vary between 3 nM and 114 nM, with an average value of 38 ± 29 nM. Prior to this, there was lack of data on rain water Ba from Indian region. Furthermore, only a few studies have reported Ba content in RW at other global locations (Berg et al. 1994; Freydier et al. 2002; Jickells et al. 1992; Özsoy and Örnektekin 2009). The reported Ba concentrations at global locations vary from 1 nM to 22 nM, with the lowest value for Bermuda whereas highest value for Africa. The median Ba value (29 nM) observed for Berhampur during this study is the highest compared to existing literature rain water data.
4 Discussion
Chemical composition of rain water is mainly regulated by relative solute contribution from its possible sources, i.e. sea spray, continental and anthropogenic inputs. Sea-salt aerosols are produced through breaking of waves and mostly have Na-Cl composition. In contrast to Na, other major cations, calcium and magnesium in RW are mainly supplied through dissolution of suspended particles present in the atmosphere. These particles are either eroded products of regional lithology and/or long-range transported from distant continental sources. Regional lithology of the present location is dominated by Precambrian silicate rocks (charnockite and khondalite) with some exposures of carbonates (Ramakrishnan and Vaidyanadhan 2010). In addition to regional sediments, long-range transport of particulates from the Deccan basalts is also a potential continental source at the studied site (Fig. 1a). Average chemical compositions of all these possible natural sources are listed in Table 1. The anthropogenic sources are another possible source of solutes to the RW and these sources contribute to the SO4 and NO3 abundances via emission of SO2 and NO2 gases.
Ternary diagram of major cations (in equivalent units) depicts that most of the data from Berhampur fall close to the Ca Apex (Fig. 3). On average, Ca+2 and Mg+2 account for ~91% of the cationic budget. The alkali elements (Na+ and K+) together contribute only (9 ± 3%) of the total cation content. These chemical compositions indicate minimal solute supply from the sea-salts and the dominancy of continental sources at this coastal location. One possible explanation for this is favorable westerly wind direction (Fig. 1b) for transportation of more continental than oceanic particulates to the study site. To evaluate this, we have computed the non-sea salt (nss) component for the alkaline earth metals using Eq. (1) (cf. section 2) and the average seawater composition provided in Table 1. The average non-oceanic components of Ca (98 ± 1%), Mg (42 ± 17%; excluding five samples with negative values), Sr (74 ± 17%) and Ba (~100%) for these samples indicate almost insignificant marine supply for Ca and Ba. The estimated nss-Ca components at Berhampur agrees well with that reported for an east-coast urban site (Das et al. 2005a) and also, those from other parts of India (Budhavant et al. 2011; Rastogi and Sarin 2005; Safai et al. 2004). Consistent with earlier studies, a significant fraction of rain water Mg is supplied by oceanic sources. It is interesting to note here that estimated nss-Mg values are found to be negative for five samples. These negative values are estimated involving Na-normalized ratios and hence, are attributable to possible supply of Na, in addition to the oceanic source, through dissolution of silicate minerals (e.g. Plagioclase feldspar, augite). In case of supply of Na from silicate minerals, the computed nss-component in this study will only be a lower limit. However, the estimated nss component for Ca and Ba are found almost 100%, pointing to minimal supply of rain water Na through dissolution of silicate minerals.
The nss-Sr values for most of the samples vary between 3 and 91 nM, excluding two anomalous samples (BRW-11 and 12) with extremely high values (143 nM and 203 nM; Table S2). The exact cause for these two outliers is not known as no major change in wind trajectory (from HYSPILT model) was observed for these two dates. However, extremely higher concentrations of oceanic (Na and Cl) and continental (Ca, Ba) species in these samples with no major change in SO4 and NO3 (Tables 1) indicate removal of higher load of atmospheric pollutants, possibly derived from a local dust event resulted from regional convection. Excluding these two outliers, the estimated nss-Sr component accounts for 74 ± 17% of the total Sr. This average nss-Sr value confirms supply of appreciable (~25%) amount of Sr through sea salt aerosols. This observation is also evident from a significant correlation between Sr and Cl (Fig. 4a). In addition to Cl, rain water Sr also exhibits good correlation with their corresponding Ca concentrations (Fig. 4b), which is pre-dominantly derived through continental sources. Interestingly, Sr concentrations also co-vary with the SO4 values (Fig. 4c). Sulphate in RW can come from both anthropogenic and sulfur-rich minerals (e.g. gypsum (CaSO4)). In order to ascertain the anthropogenic supply of Sr, correlation between Sr and NO3 is also evaluated. A good correlation is observed between these two parameters (figure not shown). Figure 4 depicts good correlations of Sr with oceanic (Cl), continental (Ca) and anthropogenic (SO4 and NO3) proxies, confirming appreciable supply of Sr to the atmosphere from all the three sources.
The nss-component of Ba varies between 3 and 114 nM, which on average accounts for ~100% of the total Ba in these RW samples. This indicates insignificant supply of Ba from sea-salt aerosols. To ascertain the possible source(s) of Ba, correlation between Ba and Ca is evaluated. Figure 5 depicts good co-variation between Ba and Ca, confirming their dominant supply from continental sources. For reference, typical Ba/Ca ratios for various possible sources are also shown in Fig. 5. The average Ba/Ca ratio for these samples are intermediate to that of the seawater, carbonates, charnockites and Deccan basalts. Figure 6 shows a mixing plot of Ca-normalized elemental ratios of Mg and Ba which further corroborates the minimal supply of Ba from oceanic sources. The relative contributions from each of the possible sources with the given compositions are expected to yield the RW composition. The mixing plot clearly shows that the contributions from carbonates, charnockites and Deccan basalts can explain the observed abundances of alkaline earth metals at Berhampur. Furthermore, it can be concluded that the carbonate is the dominant contributor of Ca with subordinate supplies from the other two continental sources (Fig. 6).
4.1 Quantification of relative solute supply from various sources
An inversion approach has been adopted in this study to estimate the relative contributions of selected alkaline earth metals in RW at Berhampur, India. We have employed an inverse model used successfully earlier by Tripathy and Singh (2010) to compute the contributions from various sources to the Ganga river chemistry. The details on the algorithm and computational code are provided elsewhere (Tripathy and Das 2014). Briefly, this model relies on a set of chemical mass balance equations (Eq. (2–3)) involving Na-normalized ratios of elements (X) in RW and those of their possible sources.
where, (X/Na)i stands for average elemental ratio of X (i.e. Mg, Ca and Ba) for the source i (oceanic, carbonates, Deccan basalts and charnockites) and fi reflects the fractional solute contribution of rain water Na from source i. The parameters provided in the left-hand side of Eq. (2) are the measured composition of the samples. The algorithm starts from a given set of a-priori source compositions with associated uncertainties (Table 1) and then iterates to find a set of a-posteriori solution that explains the mass balance equations (Eq. (2–3)) with least residuals. We have used only four possible sources, viz. oceanic (sea-spray aerosols), carbonates, regional silicates and Deccan basalts in the present study. The regional silicates are mainly composed of granulites, charnockites and khondalites. We although have used charnockite composition for modeling, the inversion can efficiently converge to a representative composition for regional silicates. In addition to these four sources, anthropogenic sources can also contribute to the concentrations of alkaline earth metals in RW. However, this source has not been included in this model work. Exclusion of this source is mainly due to lack of representative chemical composition for this source which is complicatedly driven by gas-particulate phase transformation and oxidation reactions. We recognize that anthropogenic input can be a source of uncertainty for the model results. However, to minimize this effect, efforts were made to use elements with least anthropogenic influence in the model. The abundance of NO3 can serve as a proxy for anthropogenic sources and hence, correlations of the individual alkaline earth metals with NO3 are assessed. The correlation with NO3 was found to be significantly higher for Sr (r2 = 0.42), compared to Ca (0.31), Mg (0.33) and Ba (0.28). We, therefore, have not used the Sr mass balance equations in the model.
Figure 7 depicts results from the inverse model on the average contribution from various sources to the alkaline earth metals in RW at Berhampur. The average contribution of sea-salt aerosols on Ca (2 ± 1%) and Ba (~almost close to zero) is insignificant. The oceanic input contributes about half of rain water Mg at this location. These estimates (on sea-spray supply) from inverse model are consistent with the nss-component of these constituents (cf. above discussion). Among the continental sources, the rain water Mg is mainly derived from the Deccan basalts (40 ± 21%). Basalts are mainly characterized with mafic minerals (Subbarao and Hooper 1988) and hence, are likely to be a potential source for Mg. Interestingly, the local lithologies, viz. carbonates (8 ± 4%) and regional silicates (6 ± 3%), do not contribute significant amount of Mg (Fig. 7) at Berhmapur. In contrast to Mg, Ca and Ba although are mainly supplied through local lithologies, their dominant source rocks are different. The dominant continental source of Ca (69 ± 12%) is found to be carbonates with minimal supply from regional silicates (12 ± 6%). Considering faster weathering kinetics of carbonates, it is less likely that these minerals have been long-range transported. Further, lithology of the Deccan Traps does not contain significant carbonate exposures (Das et al., 2015b). In contrast, rain water Ba at Berhampur is derived mainly from local silicate rocks (95 ± 4%). This observation is consistent with distinctly higher Ba abundances reported in river sediments from this part (eastern) of India compared to other large river systems (Chakrapani and Subramanian 1990). The average Ba concentration in charnockites (Rao and Rao 1988) is about 10 times higher than that reported for Deccan basalts (Das and Krishnaswami 2007). Charnockite exposures are mostly limited to the eastern part of India and not to the Western India, confirming regional supply from Ba. Above discussion shows that both long-range and short-range transportation play crucial role in regulating the abundance of alkaline earth metals. The regional lithologies (charnockites and carbonates) predominantly regulate Ca and Ba content of RW, whereas mafic minerals derived through long-range transportation account for most of the continental Mg at this coastal site in eastern India.
5 Conclusions
The chemical compositions of RW, including alkaline earth metals and major ions, from a semi-urban, coastal site (Berhampur) in eastern India have been investigated to constrain their sources and controlling factors. Although chemistry of these samples are typical of earlier reported data from this region, Ba content of RW from this region is found to be systematically higher than reported for any location worldwide. The alkaline metals at Berhampur are supplied through both marine and oceanic sources, however relative source contributions for different elements vary significantly. Inverse modeling of this dataset estimates that sea-salt spray, on an average, contributes about half of Mg and 25% of Sr, whereas Ca and Ba has negligible marine supply at this coastal site. The continental Mg at this site is supplied largely through long-range transport of mafic minerals from the Deccan Traps from the western India. The rain water Ca at Berhampur is largely supplied through dissolution of carbonate minerals, whereas Ba is derived through chemical alteration of regional silicates (charnockites, khondalites). The observed elevated Ba content at this site is largely due to faster dissolution kinetics of these regional rocktypes, which are characterized with higher Ba abundances. Outcomes of this study point towards the importance of regional lithology and their weathering kinetics in driving the wet deposition of atmospheric barium.
References
Berg, T., Royset, O., Steinnes, E.: Principal component analysis of data for trace elements and main components in precipitation falling on Norway. Environ. Monit. Assess. 31, 259–273 (1994)
Berner, E.K., Berner, R.A.: Global Environment: Water, Air and Geochemical Cycles, 2nd edn. Princeton University Press, New Jersey (2012) 488 pp
Bhaskar, V.V., Rao, P.S.P.: Annual and decadal variation in chemical composition of rain water at all ten GAW stations in India. J. Atmos. Chem. 74, 23–53 (2017)
Bisht, D.S., Tiwari, S., Srivastava, A.K., Singh, J.V., Singh, B.P., Srivastava, M.K.: High concentration of acidic species in rainwater at Varanasi in the indo-Gangetic plains, India. Nat. Hazards. 75, 2985–3003 (2015)
Budhavant, K.B., Rao, P.S., Safai, P.D., Ali, S.K.: Influence of local sources on rainwater chemistry over Pune region, India. Atmos. Res. 100, 121–131 (2011)
Chakrapani, G.J., Subramanian, V.: Preliminary studies on the geochemistry of the Mahanadi river basin, India. Chem. Geol. 81, 241–253 (1990)
Chatterjee, J., Singh, S.K.: 87Sr/86Sr and major ion composition of rainwater of Ahemdabad, India: sources of base cations. Atmos. Environ. 63, 60–67 (2012)
Cong, Z., Kang, S., Zhang, Y., Li, X.: Atmospheric wet deposition of trace elements to central Tibetan plateau. Appl. Geochem. 25, 1415–1421 (2010)
Dalai, T.K., Krishnaswami, S., Sarin, M.M.: Barium in the Yamuna river system in the Himalaya: sources, fluxes, and its behavior during weathering and transport. Geochem. Geophys. Geosyst. 3, 1–23 (2002)
Das, A., Krishnaswami, S.: Elemental geochemistry of river sediments from the Deccan traps, India: implications to sources of elements and their mobility during basalt-water interaction. Chem. Geol. 242, 232–254 (2007)
Das, R., Das, S.N., Misra, V.N.: Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmos. Environ. 39, 5908–5916 (2005a)
Das, A., Krishnaswami, S., Sarin, M.M., Pande, K.: Chemical weathering in the Krishna basin and Western Ghats of the Deccan traps, India: rates of basalt weathering and their controls. Geochim. Cosmochim. Acta. 69, 2067–2084 (2005b)
Das, N., Das, R., Chaudhury, G.R., Das, S.N.: Chemical composition of precipitation at background level. Atmos. Res. 95, 108–113 (2010)
Dash, B., Sahu, K.N., Bowes, D.R.: Geochemistry and original nature of Precambrian khondalites in the eastern Ghats, Orissa, India. Trans. R. Soc. Edinb. Earth Sci. 78, 115–127 (1987)
Drever, J.: Geochemistry of Natural Waters: Surface and Groundwater Environments, 3rd edn. Prentice hall, New Jersey (1997)
Freydier, R., Dupre, B., Dandurand, J., Fortune, J., Sigha-Nkamdjou, L.: Trace elements and major species in precipitation at African stations: concentrations and sources. Bull. Soc. Geol. Fr. 173, 129–146 (2002)
Gadgil, S.: The Indian monsoon and its variability. Annu. Rev. Earth Planet. Sci. 31, 429–467 (2003)
Galy, A., France-Lanord, C., Derry, L.A.: The strontium isotopic budget of Himalayan rivers in Nepal and Bangladesh. Geochim. Cosmochim. Acta. 63, 1905–1925 (1999)
Jacob, D.J.: Introduction to Atmospheric Chemistry. Princeton university Press, New Jersy (1999) 280 pp
Jain, M., Kulshrestha, U.C., Sarkar, A.K., Parashar, D.C.: Influence of crustal aerosols on wet deposition at urban and rural sites in India. Atmos. Environ. 34, 5129–5137 (2000)
Jickells, T., Chruch, T., Scudlark, J., Dehairs, F.: Barium in North Atlantic rain water: a reconnaissance. Atmos. Environ. 26, 2641–2646 (1992)
Kulshrestha, U.C., Granat, L., Engardt, M., Rodhe, H.: Review of precipitation monitoring studies in India – a search for regional patterns. Atmos. Environ. 39, 7403–7419 (2005)
Lara, L., Artaxo, P., Martinelli, L., Victoria, R., Camargo, P., Krusche, A., Ayers, G., Ferraz, E., Ballester, M.: Chemical composition of rainwater and anthropogenic influences in the Piracicaba river basin, Southeast Brazil. Atmos. Environ. 35, 4937–4945 (2001)
Likens, G.E., Bormann, F.H.: Acid rain: a serious regional enviormental problem. Science. 184, 1176–1179 (1974)
Millot, R., Gaillardet, J., Dupre, B., Allegre, C.J.: Northern latitude chemical weathering rates: clues from the Mackenzie river basin, Canada. Geochim. Cosmochim. Acta. 67, 1305–1329 (2003)
Negrel, P., Roy, S.: Chemistry of rainwater in the massif central (France): a strontium isotope and major element study. Appl. Geochem. 13, 941–952 (1998)
Norman, M., Das, S.N., Pillai, A., Granat, L., Rodhe, H.: Influence of air mass trajectories on the chemical composition of precipitation in India. Atmos. Environ. 35, 4223–4235 (2001)
Özsoy, T., Örnektekin, S.: Trace elements in urban and suburban rainfall, Mersin, northeastern Mediterranean. Atmos. Res. 94, 203–219 (2009)
Pascaud, A., Sauvage, S., Coddeville, P., Nicolas, M., Croise, L., Mezdour, A., Probst, A.: Contrasted spatial and long-term trends in precipitation chemistry and deposition fluxes at rural stations in France. Atmos. Environ. 146, 28–43 (2016)
Rajeev, P., Rajput, P., Gupta, T.: Chemical characteristics of aerosol and rain water during an El Nino and PDO influenced Indian summer monsoon. Atmos. Environ. 145, 192–200 (2016)
Ramakrishnan, M., Vaidyanadhan, R. Geology of India, volume 1, Geological Society of India, 556 pp. (2010)
Rao, M., Rao, V.D.: Chemical constraints on the origin of the charnockites in the Eastern Ghat Mobile belt, India. Chem. Geol. 69, 37–48 (1988)
Rao, P.S.P., Tiwari, S., Matwale, J.L., Pervez, S., Tunved, P., Safai, P.D., Srivastava, A.K., Bisht, D.S., Singh, S., Hopke, P.K.: Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two megacities in India and dominant source region of secondary aerosols. Atmos. Environ. 146, 90–99 (2016)
Rastogi, N., Sarin, M.M.: Chemical characteristics of individual rain events from a semi-arid region in India: three-year study. Atmos. Environ. 39, 3313–3323 (2005)
Rhode, H., Dentener, F., Schulz, M.: The global distribution of acidifying wet deposition. Environ. Sci. Technol. 36, 4382–4388 (2002)
Roy, A., Chatterjee, A., Tiwari, S., Sarkar, C., Das, S.K., Ghosh, S.K., Raha, S.: Precipitation chemistry over urban, rural and high altitude Himalayan stations in eastern India. Atmos. Res. 181, 44–53 (2016)
Roy-Barman, M., Jeandel, C.: Marine Geochemistry: Ocean Circulation, Carbon Cycle and Climate Change. Oxford University Press, UK (2016) 398 pp
Safai, P.D., Rao, P., Momin, G.A., Chate, D., Praveen, P.S.: Chemical composition of precipitation during 1984-2002 at Pune, India. Atmos. Environ. 38, 1705–1714 (2004)
Sarin, M.M., Kumar, A., Srinivas, B., Sudheer, A.K., Rastogi, N.: Anthropogenic sulphate aerosols and large cl-deficit in marine atmospheric boundary layer of tropical bay of Bengal. J. Atmos. Chem. 66, 1–10 (2010)
Sequeira, R., Kelkar, D.: Geochemical implications of summer monsoonal rainwater composition over India. J. Appl. Meteorol. 17, 1390–1396 (1978)
Singh, S.P., Singh, S.K., Bhushan, R.: Internal cycling of dissolved barium in water column of the bay of Bengal. Mar. Chem. 154, 12–23 (2013)
Subbarao, K., Hooper, P.: Reconnaissance map of Deccan basalt Group in the Western ghats, India. In: (Ed. K V Subbarao) Deccan Flood Basalts pp. 69–90. Geological Society of India, (1988)
Tiwari, S., Kulshrestha, U., Padmanabhamurty, B.: Monsoon rain chemistry and source apportionment using receptor modeling in and around national cpital region (NCR) of Delhi, India. Atmos. Environ. 41, 5595–5604 (2007)
Tiwari, S., Chate, D., Bisht, D., Srivastava, M., Padmanabhamurty, B.: Rainwater chemistry in the north western Himalaya region, India. Atmos. Res. 104-105, 128–138 (2012)
Tripathy, G.R., Das, A.: Modeling geochemical datasets for source apportionment: comparison of least square regression and inversion approaches. J. Geochem. Explor. 144, 144–153 (2014)
Tripathy, G.R., Singh, S.K.: Chemical erosion rates of river basins of the ganga system in the Himalaya: reanalysis based on inversion of dissolved major ions, Sr and 87Sr/86Sr. Geochem. Geophys. Geosyst. 11(Q03013), 1–20 (2010)
Tripathy, G.R., Goswami, V., Singh, S.K., Chakrapani, G.J.: Temporal variations in Sr and 87Sr/86Sr of the ganga headwaters: estimates of dissolved Sr flux. Hydrol. Process. 24, 1159–1171 (2010)
Vet, R., Artz, R., Carou, S., Shaw, M., Ro, C., Aas, W., Bakr, A., Bowersox, V., Dentener, F., Galy-Lacaux, C., Hou, A., Pienaar, J., Gillett, R., Forti, M., Gromov, S., Hara, H., Khodzher, T., Mahowald, N., Nickovic, S., Rao, P., Reid, N.: A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 93, 3–100 (2014)
Acknowledgments
This research work was carried out as a part of SM’s summer internship at IISER, Pune. The authors sincerely thank Ms. Sarala Das for her kind help and enthusiasm in collecting the samples. Help of Dr. Sumita Kedia in preparing the location map is thankfully acknowledged. Thanks are also due to Mr. Anupam Samanta for guidance during the IC analyses. Rainfall Data for the figures are taken from TRMM and IMD websites, whereas wind pattern data shown is taken from the NCEP data archive. The wind backward trajectory analyses were carried out from http://www.arl.noaa.gov. Financial support for this work is provided by DST-SERB through the Early Career Research grant to GRT (ECR/2016/001884). KR acknowledges support from ECR/2015/000490.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Tripathy, G.R., Mishra, S., Danish, M. et al. Elevated Barium concentrations in rain water from east-coast of India: role of regional lithology. J Atmos Chem 76, 59–72 (2019). https://doi.org/10.1007/s10874-019-9387-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-019-9387-6