Abstract
Seasonal abundance, composition and grazing rates of microzooplankton (20–200 µm) in the Zuari estuary were investigated to evaluate their importance in food web dynamics of a tropical monsoonal estuary. Average abundances of microzooplankton (organisms × 104 l−1) during the three seasons were 0.44 (southwest monsoon), 1.13 (post-monsoon) and 0.96 (pre-monsoon). Protozoan (ciliates, heterotrophic dinoflagellates and sarcodines) accounted for most (96 %) of the microzooplankton community, with micrometazoan (nauplii and copepodid stages of copepods, fish eggs, etc.). being far less abundant. Among protozoans, ciliates (loricates and aloricates) were most numerous (69 % of the total microzooplankton). Statistically significant (p < 0.001) co-variations of microzooplankton with other biological parameters such as chlorophyll a and bacterial biomass were observed. Salinity influenced microzooplankton distribution, with an optimum range of 15–20. Microzooplankton formed a large organic carbon pool, accounting for 24–40 % of the total carbon in the living matter. Seasonally averaged microzooplankton biomasses were 22.3, 36.1 and 24.6 mmol C m−3, respectively, during the southwest monsoon, post-monsoon and pre-monsoon periods, and were largely supported by non-living particulate carbon (detritus) particularly during the non-monsoon seasons. Experimental studies revealed significant microzooplankton grazing on phytoplankton standing stock, mainly (>60 %) by the pico and nano fraction (<20 µm) for most of the year. Phytoplankton growth rates (day−1) ranged between 0.69 and 1.24. Microzooplankton grazing was estimated to consume 30–82 % of the phytoplankton standing stock, and 58–97 % of the daily primary production. Results of the present study highlight the role of the microzooplankton as an important consumer of phytoplankton production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microzooplankton (MZP), fauna in 20–200 µm-size range, primarily consist of heterotrophic ciliates and dinoflagellates, with a smaller contribution from sarcodines and crustacean larval stages. They play a significant role in carbon flow in marine ecosystems (Gast 1985; Pierce and Turner 1992) by consuming 20–100 % of the primary production (Riley et al. 1965; Beers and Stewart 1970; Beers and Stewart 1970 ; Capriulo and Carpenter 1983; Frost 1991; Landry et al. 1998). Microzooplankton also directly ingest bacteria (Gast 1985; Sherr and Sherr 1987; Reid and Karl 1990), and thus being a component of in the microbial loop, act as trophic intermediaries between the bacterioplankton and larger mesozooplankton grazers (Hass 1982; Gifford and Dagg 1991) and in nutrient regeneration (Goldman et al. 1987; Probyn 1987).
It is believed that due to the close coupling between microbial and microzooplankton components of aquatic food webs, less organic carbon leaves the euphotic zone, especially where microzooplankton form the mediator route for the uptake of organic carbon, thereby influencing biogeochemical cycles (Gauns et al. 2005). Studies from the west coast of India (Madhupratap et al. 1992, 1996; Gauns et al. 1996, 2005; Jyothibabu et al. 2008; AshaDevi et al. 2010) indicate that the microzooplankton community plays a key role in the food web of the region. However, there has been no study of microzooplankton in any of the estuaries except Cochin backwaters (Jyothibabu et al. 2006) along the west coast of India. Such studies are needed for a comprehensive understanding of the role of microzooplankton in tropical estuarine systems. A year-long investigation on microzooplankton, along with other biological, chemical and physical parameters, in the Zuari estuary was carried out together with an experimental study for this purpose. The present study, carried out during the year 1996–1997 and in 2006 and 2008, tests the hypothesis that microzooplankton play a key role in maintaining higher standing stocks of carbon in tropical estuaries, possibly by efficiently linking detritus into the food web.
2 Methods
2.1 Study location
The Zuari estuary in Goa is among the major estuaries along the west coast of India (Fig. 1). The southwest monsoon (hereafter referred to as the monsoon) plays a major role in influencing the hydrographic characteristics of the Zuari estuary, as it does in other estuaries in the region. The classification of data presented in this paper has been made considering three seasons, viz. monsoon (June–September); post-monsoon (October–January) and pre-monsoon (February–May). Large freshwater influx during the monsoon is a distinguishing feature of the west coast estuaries. In the Zuari estuary, for example, salinity is as low as 0.2 psu during the monsoon, as opposed to its maximum (32.9 psu) recorded during the pre-monsoon (Qasim 1979). The water column in the estuary is generally well mixed during most parts of the year, but during the peak monsoon period, a strong salt wedge is formed, extending about 10–12 km upstream from the mouth of the estuary (Qasim 1979). Accordingly, our sampling strategy was modified during this period (see below). Further details about this estuary are given in Shetye et al. (2007).
Results of experimental studies conducted in 2006 and 2008 together with data collected monthly for 1 year (June 1996–May1997) are used here. Water samples were collected from three stations, viz., Z1, Z2 and Z3 situated about 15, 25 and 30 km upstream from the mouth of the estuary (Fig. 1). The mean depths during the high tide were about 8 m at Stn. Z1 and 4 m at Stns. Z2 and Z3. Sampling was restricted to about 1 m (1.5 m during the monsoon) below the sea surface in order to avoid the fresh water lens. All water samples, in duplicate, were obtained using 5 l Niskin samplers (General Oceanics).
Samples for microzooplankton, mesozooplankton, phytoplankton and bacteria were collected following the JGOFS Protocols (UNESCO 1994). Samples for chlorophyll a and nutrient analyses were kept in an icebox soon after collection and analyzed within 8–10 h of sampling. Temperature was noted immediately after collection using a thermometer and salinity by ATAGO S/Mill-E refractometer. Particulate organic carbon (POC) and nitrogen (PON) were analyzed by a Perkin Elmer CHN analyzer. Nitrate was estimated colorimetrically using a Skalar analyzer (Grasshoff 1976). The GF/F-filtered samples were analyzed for dissolved organic carbon (DOC) by the high temperature catalytic oxidation using a TOC-5000 analyzer (Shimadzu). Primary production (PP) and bacterial production (BP) rates were not measured during this study; instead, respective data sets on the PP and BP were obtained from Devassy and Goes (1989) and De Souza Maria-Judith (2002).
2.2 Microzooplankton
A 5-litre water sample pre-screened through 200 µm mesh was gently siphoned out using a section of PVC tubing with its cod end fitted with a 20 µm Nitex screen for retaining Microzooplankton (MZP) ≥20 µm in size. These concentrated samples were preserved in 2 % acid Lugol’s solution and 1 % hexamine buffered formaldehyde. Strontium sulphate solution (2 mg l−1) was added for preserving acantharians. Samples were also fixed separately with fresh, chilled glutaraldehyde (0.3 % final concentration) to differentiate auto and heterotrophic forms based on an autofluoroscence technique (UNESCO 1994).
A known volume of two replicates of the sample concentrate were then observed under an inverted microscope with phase contrast optics following Paranjape et al. (1985), at 100–400× magnification. Microzooplankton were identified and assigned to the following five groups: loricate ciliates (tintinnids), aloricate ciliates, heterotrophic dinoflagellates, sarcodines and micrometazoans.
Based on morphology of individual specimens, geometric shapes were assigned to each taxon and biovolumes were calculated. These volumes were then converted to carbon biomass through appropriate volume to organic carbon ratios (see below). While computing the biovolume of protozoans, additional mean cell shrinkage due to preservation was accounted for following Stoecker et al. (1994). The calculated biovolume was divided by 0.7 to make up for 30 % cell shrinkage due to preservation.
From the lorica volume (LV, µm3) the body weight carbon of a tintinnid (pg) was calculated using the equation of Verity and Langdon (1984). The cell volume of the tintinnid ciliate was assumed to be 50 % of the lorica volume (Gilron and Lynn 1989). The carbon biomass was converted from cell volume using a factor of 0.19 pg C µm−3 for aloricate ciliates (Putt and Stoecker 1989) and 0.14 pg C µm−3 for dinoflagellates (Lessard 1991). The carbon content of copepod nauplii was calculated from the body length (BL, µm) (Uye, personal communication, see also Uye et al. 1996).The amount of carbon required for microzooplankton community was then calculated based on gross growth efficiency. As protozoan microzooplankton were the dominant forms, a value of 0.4 was used for the calculation of requirement (Fenchel 1987), that is, the rate of carbon biomass production was divided by 0.4.
2.3 Heterotrophic nanoflagellate
For quantifying the abundance of heterotrophic nanoflagellates (HNF), 50 ml of water sample was fixed in 2 % glutaraldehyde. 4′-6-Diamidino-2-phenylindole (DAPI) and proflavin were added in 5–10 ml sub-samples to a final concentration of 5 µg ml−1 each, allowed to stain for 5 min (Hass 1982; Booth 1993) and filtered through 0.8 µm black Nuclepore filters (Sherr and Sherr 1983; Booth 1993). Slides were prepared and held at 5 °C in a darkened box until taken up for epifluorescence microscopy. Only unbroken well-defined individuals were counted and their biovolumes determined. The cell numbers were converted to carbon (pg C μm−3) using a factor of 0.11 (Edler 1979).
2.4 Phytoplankton cell counts
Water samples were fixed with 2 % Lugol’s iodine, preserved in 3 % formaldehyde solution and stored in the dark at room temperature until enumeration, which was done within 1 month of collection. A settling and siphoning procedure was followed to obtain 20–25 ml phytoplankton concentrates from a 250 ml sample. Two replicates of 1 ml each of these concentrates were examined with a stereoscopic binocular microscope at a magnification of 100–200× in a Sedgwick-Rafter plankton counting chamber.
2.5 Chlorophyll a
To measure the concentration of chl a (Chl a), duplicate samples (1 l) were filtered through Whatman GF/F (nominal pore size 0.7 μm) under low vacuum. The chl a pigment were extracted for 24 h in 10 ml of 90 % acetone (Qualigens AR) in dark in a refrigerator. Samples were brought to room temperature and the fluorescence measured using a precalibrated fluorometer (Turner designs). Chl a concentration was calculated from the fluorescence using an appropriate calibration factor. Phytoplankton biomass (as carbon) was calculated by multiplying the chl a concentration by 50 (Banse 1988).
A known volume of water sample (2–5 l) was passed through different pore size filters (200, 60, 20, 10 μm nylon and 0.7 μm GF/F) for the size-fractionated chl a analysis, following the above procedure.
2.6 Bacterial abundance (TDC)
Water samples (20 ml) were fixed with formaldehyde (2 %) and refrigerated in the laboratory. Sub-samples of 2 ml were stained with DAPI and filtered onto black 0.2 µm pore size Nucleopore filters (Porter and Feigh 1980), and slides were prepared. Bacteria were enumerated using UV excitation. A minimum of 20 fields were counted for each sample at 1000× in an Olympus BH2 epifluorescence microscope, and cell numbers were calculated following Parsons et al. (1984). Bacterial cell abundance was converted to carbon biomass using a value of 11 fg C cell−1 (Garrison et al. 2000).
2.7 Mesozooplankton
The mesozooplankton (ZP) samples were collected using a Heron-Tranter net (mesh size 200 µm), having a mouth area of 0.25 m2. The net was towed horizontally ~1 m below the surface for 5 min and the volume of water filtered was estimated with a flow meter (General Oceanics). Immediately after the retrieval, the ZP samples were placed on an absorbent paper to remove excess water and their volume determined by the displacement method. All samples were fixed in buffered formalin (4 % v/v) to examine their composition. Mesozooplankton biomass estimated as displacement volume was converted to dry weight (1 ml displacement volume = 0.075 g dry wt.) and to carbon (34.2 % of dry wt.; Madhupratap et al. 1992; Madhupratap and Haridas 1975).
Due to large variability, the raw data were log-transformed for normalization, and correlation between the stations and seasons was determined using the Microsoft Excel package.
2.8 Microzooplankton grazing
To determine microzooplankton grazing and growth rates of phytoplankton community, dilution experiments were carried out (Landry and Hassett 1982) at the mouth of the Zuari estuary and in the adjoining Mandovi estuary (Fig. 1) during post-monsoon, as micro-grazers attain maxima in this season. Water samples were collected from 1 m depth and pre-screened through 200 µm mesh to exclude mesozooplankton. Half of the water sample was filtered through 0.2 µm filter capsules to prepare dilution series 100, 75, 50, 25 and 10 % of the ambient concentration. Triplicates of each series were incubated for 24 h in 2-litre, acid-cleaned polycarbonate bottles. Initial and final subsamples were collected for Chl a measurement. Subsamples (1 l) were filtered onto 47 mm GF/F filter papers, extracted with 10 ml of 90 % acetone for 24 h at −20 °C, and analyzed fluorometrically with a Turner Designs fluorometer. Apparent growth rates were plotted as a function of dilution using the equation:
where N o and N t are the initial and final Chl a concentration. Regression analyses of the data yielded slope and intercept corresponding to microzooplankton grazing rate (g) and instantaneous phytoplankton growth rate (k), respectively. X is the dilution factor. Percent standing stock grazed (P i ) and potential primary production grazed (P p ) per day were calculated using the formulae (James and Hall 1998):
2.9 Nutrient enrichment experiment
The details of experimental setup are given in Sunita et al. (2013). These experiments were carried out in situ at the mouth of the Zuari estuary (Fig. 1) using clean modified Nalgene bottles (25.5 l capacity). A water sample drawn from 1 m below the surface using Niskin sampler and screened slowly through a 200 µm nylon mesh was used for experimental purpose. Utmost care was taken to avoid turbulence and damage to delicate organisms such as ciliates. Experimental bottles enriched with different nutrients (NO3, PO4, SiO4 and NH4) were deployed in situ (1 m below surface) using moored floating raft. Control (with no additional nutrients) and experimental bottles were regularly sampled over a period of 10 days to monitor nutrient levels, phytoplankton and microzooplankton population.
3 Results
3.1 Physico-chemical parameters
The temperature at the three stations did not show very large variability (Fig. 2a). The highest temperatures were observed in the month of May; which varied between 33.4 (stn Z1), 34.4 (Stn Z2) and 33.7 °C (Stn Z3) along the stretch of the estuary. Seasonally, the largest variation was observed during the pre-monsoon.
As expected, salinity exhibited large variability from a minimum of 1 at Z3, indicating nearly freshwater conditions, to a maximum of 32 at Z2 and Z1, indicating almost completely marine conditions (Fig. 2b). A general decrease in salinity from the mouth to the upper reaches of the estuary was observed during all the three seasons. With the end of the monsoon, salinity increased steadily during post-monsoon and was the highest during the pre-monsoon. During the monsoon and post-monsoon periods, average salinity at Z1 was almost twice the value of that at the upstream station Z3. Salinity variations between the Z1 and Z3 stations were minimal during the pre-monsoon.
The nitrate (NO3–N) concentration at Z1, Z2 and at Z3 stations varied within the ranges of 1.30–12.50, 0.85–14.56 and 1–11.94 µm, respectively (Fig. 2c). The highest NO3–N concentrations were recorded during the monsoon (June–July), with secondary peaks occurring during October–November (Fig. 2c).
3.2 Biological parameters
3.2.1 Microzooplankton
Ciliates [loricates (tintinnids) and aloricate forms] and heterotrophic dinoflagellates were the predominant microzooplankton. During the entire study, protozoans dominated the microzooplankton community, accounting for 96 % of total counts. Six species, Dictyocysta seshaiyai, Leprotintinnus nordequistii, Tintinnopsis beroidea, T. gracilis, T. uruguensis and Tintinnidium incertum were common among the tintinnids. Strombidium spp and Labeo spp dominated the aloricate ciliates. Protoperidinum spp and Gymnodium spp, subjugated heterotrophic dinoflagellates, acantharians, the sarcodines, and nauplii and copepodid stages of copepods constituted the micrometazoans. The average contribution of ciliates [tintinnids (31 %) and aloricate (38 %)] was more than that of heterotrophic dinoflagellates (27 %), sarcodines (1 %) and micrometazoans (4 %). Tintinnids, micro-metazoans and sarcodines exhibited maxima at stns Z1 and Z2 during monsoon and post-monsoon. The maxima in aloricate ciliates and heterotrophic dinoflagellates occurred at stns Z2 and Z3 during post-monsoon and pre-monsoon. Tintinnid diversity was higher during the non-monsoon periods and at near-mouth stations. Optimum salinity for their occurrence was found to be between 15 and 25 (see below).
In the study area as a whole, the microzooplankton density (organisms × 104 l−1) recorded during the monsoon, post- and pre-monsoon periods varied within the ranges 0.014–1.50 [0.49 (average) ± 0.55 (SD)], 0.10–7.57 (1.13 ± 2.05) and 0.19–2.66 (1.09 ± 0.76), respectively (Fig. 3a), with an annual average of 0.90 (±1.15). Spatially, they were higher at stn Z1 during monsoon and post-monsoon and at stn Z3 during the pre-monsoon. During the former two seasons, MZP were abundant at stn Z1, and at stn Z3 during the latter period. Their counts (×104) at the three stations varied from 0.19 to 7.57 at stn. Z1, from 0.014 to 7.57 at stn. Z2, and from 0.060 to 0.86 organisms l−1 at stn. Z3, with averages of 1.23 (±2.02), 0.61 (±0.51) and 0.86 (±0.91), respectively. There were significant differences in microzooplankton abundance between months (p < 0.001) as compared to stations (p > 0.01).
3.2.2 Chlorophyll a
Chl a (mg m−3) varied between 0.18 and 12.78 (0.26–8.6 at Z1; 0.36–12.74 at Z2 and 0.18–10.77 at Z3), with higher concentrations found during the pre-monsoon. The peaks were recorded in March at the upstream stations and in April at Z1 (Fig. 3b). Lowest chl a levels were observed in the month of July at all the three stations. Its variation was found to be significant (p < 0.01) between the stations, except during the pre-monsoon.
3.2.3 Phytoplankton
Analyses of phytoplankton composition showed that diatoms (Nitzchia, Ditylum, Thallassiossira sp) dominated the community. Numerically they were more abundant at the upstream station during all the three seasons. Phytoplankton cell numbers (×104) varied from 0.04 to 5.56 l−1 during monsoon, from 0.06 to 6.24 l−1 during post-monsoon, and from 0.05 to 4.01 l−1 during pre-monsoon season, with relatively higher abundance in post-monsoon. Seasonal peaks were recorded in August (monsoon), November (post-monsoon) and April (pre-monsoon) (Fig. 3c). Unlike the spatial variations, the phytoplankton cell counts showed significant seasonal variations (p < 0.001).
3.2.4 Heterotrophic nanoflagellate
The heterotrophic nanoflagellate (HNF) abundance (×107) varied from 0.29 to 11.65 l−1, with maxima in the pre-monsoon (Fig. 3d). HNFs were more abundant at the mouth (Z1 0.65–11.65 l−1) than at the upstream stations [Z2 (0.25–9.45 l−1) and Z3 (0.29–10.51 l−1)]. Peaks were recorded in March at stns Z1 and Z2 and in August at stn Z3 (Fig. 3d). Spatial variation was not significant (p > 0.05), although cell numbers decreased from stns Z1 to Z3, unlike the highly significant seasonal variation.
3.2.5 Bacterial abundance
Bacterial cell counts (TDC) ranged from 0.19 to 4.44 × 109 cells l−1 (Fig. 3e), with higher counts during post-monsoon. Cell counts (×109 l−1) at stn Z2 were slightly higher (range 0.42–4.44) than at stn Z1 (0.43–3.59) or stn Z3 (0.19–3.7). Seasonal peaks were recorded during June, December and March (Fig. 3e). Statistically, bacterial cell numbers did not show significant spatial variation (p > 0.05), as compared to the temporal (p < 0.01) change.
3.2.6 Mesozooplankton
Mesozooplankton biomass fluctuated seasonally from 0.03 ml m−3 during monsoon to 1.9 ml m−3 during pre-monsoon, with peaks in March–April (Fig. 3f). The mesozooplankton community was largely dominated by herbivores during monsoon and carnivores during other seasons. There was persistence of ctenophores during most parts of the year. Monthly variation in ZP biomass was statistically significant (p < 0.01), similar to MZP biomass.
3.2.7 Size fractionated phytoplankton biomass (Chl a)
Size-fractionated chl a analysis showed a clear seasonal shift in the autotrophic community composition. The smaller fraction (<20 µm) was dominant (>60 %) throughout the year (Fig. 4).
3.2.8 Carbon biomass
The average standing stock of MZP recorded during the study period was 15.25 mmol C m−3. The post-monsoon season was characterized by the highest average carbon biomass (21.5 mmol C m−3), followed by pre-monsoon (18.7 mmol C m−3) and monsoon (5.5 mmol C m−3). Among the individual groups, ciliates and dinoflagellates contributed substantially to MZP carbon standing stocks, followed by micrometazoans and sarcodines (Table 1). Annually, ciliates (tintinnids and aloricates) accounted for 57–84 % of the MZP biomass, whereas heterotrophic dinoflagellates, micrometazoans and sarcodines contributed 11–30, 5–26 and 0–2 %, respectively. Within the ciliated protozoans, aloricate ciliates were more abundant (11–68 %) than loricates, i.e., tintinnids (15–46 %). The average standing stock of aloricate ciliates was twofold higher than that of loricate ciliates. The carbon biomass of tintinnids and heterotrophic dinoflagellates increased with the corresponding increase in salinity from monsoon to pre-monsoon.
Carbon standing stocks of phytoplankton, bacteria, heterotrophic nanoflagellates (<20 μm) and mesozooplankton were within the ranges 5.64–24.49, 2.85–3.74, 6.64–14.74 and 0.19–0.63 mmol C m−3, respectively. Even though, biomasses of many groups were higher during the pre-monsoon, total POC was higher during the monsoon-post-monsoon period (Table 1).
3.2.9 Microzooplankton grazing
Based on the experimental studies carried out at the mouth of the Zuari estuary, microzooplankton grazing rate was found to be 0.295 day−1 and the phytoplankton growth rate was 0.904 day−1. Percent standing stock and potential primary production grazed by microzooplankton were calculated as ~34.0 and 57.6, respectively (Fig. 5). The microzooplankton grazing rate and phytoplankton growth rate were much higher in the Mandovi estuary, at 0.527 and 1.24 day−1, respectively. Percent standing stock (69) and potential primary production (97) grazed by microzooplankton were accordingly higher as well. Further, nutrient enrichment experiment carried out close to the mouth of the Zuari estuary also showed high rates of phytoplankton growth (1.6 day−1), with microzooplankton grazing on phytoplankton standing stock being around 84 % (Sunita et al. 2013). These observations clearly show that a large fraction of the autotrophic crop in the estuarine system is mobilized through microzooplankton grazing.
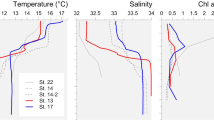
The ciliate (tintinnid) distribution with salinity is depicted in Fig. 6a. The number of tintinnid species and occurrence of their swarms were found to be at maximum within the salinity range of 15–25. At higher salinity (>25), the number of species and swarm formation also decreased. Dictyocysta sheshayaii, Dictyocysta sp, Tintinnopsis beroidea, T. gracilis, T. tubulosa, T. uruguensis, T. ventricosa, Tintinnidium incertum, and Stenosemella nucula were recorded in a wide range of salinities. The occurrence of tintinnids was higher at lower chl a concentration (≤8 mg m−3, Fig. 6b). The highest densities were at ≤2 and 6–8 mg m−3 of the chl a concentration. As many as 36 species were recorded at higher chl a, dominated by Codonellopsis ecaudata, C. shabi, Eutintinnus tennus, Leprotintinnus nordequistii, T. beroidea, T. butchii, T. dadayaii, T. directa, T. primitivum, T. tocantensis, T. tubulosa, T. uruguensis and Tintinnidium incertum. On the other hand, distribution of tintinnids indicated maximum occurrence at bacterial cell concentration of <1 × 109 l−1, while a peak in tintinnid species composition was attained at medium range (2–3 × 109 l−1). At higher bacterial cell abundance (>3 × 109 l−1), both species composition and swam formation showed a decreasing trend. Codonellopsis ostenfoidii, Dictyocysta sheshayaii, Dictyocysta sp., Leprotintinnus nordequistii, Stenosemella ventricosa, Tintinnopsis dadayaii, T. directa, T. gracilis, T. minuta, T. tubulosa, T. uruguensis, T. climacocyclis and Tintinnidium incertum had variations in bacterial cell count. The distributions of tintinnids and nanoflagellate (Fig. 6d) suggest that the tintinnid occurrence, species composition and swarm formation were highest at a nanoflagellate concentration of 2–4 × 107 l−1, beyond which a decreasing trend was observed. Codonellopsis ostenfoidii, Dictyocysta sheshayaii, Dictyocysta sp, Stenosemella ventricosa, T. beroidea, T. butchii, T. amphora, T. fimbriata, T. gracilis, T. minuta, T. tubulosa, T. uruguensis and Tintinnidium incertum occurred at a wider range of nanoflagellate cell abundance.
The relationship of microzooplankton with Chl a and bacteria was highly significant (p < 0.001) compared to HNF (p > 0.01) or ZP (p > 0.05). A similar level of significance was recorded with salinity (p < 0.05) compared to temperature. Microzooplankton abundance also varied significantly with the oxygen content and pH of water (p < 0.05).
4 Discussion
The microprotozoans, planktonic protists in the size range of approximately 20–200 µm, are known to be a major functional component in pelagic food webs (Azam et al. 1983; Strom et al. 2007; Gifford et al. 2007). These heterotrophic protists represent an important link of bacterial and microalgal biomass to higher trophic levels (Lee et al. 2007). In some systems such as Apalachicola Bay (Florida, USA), microzooplankton are known to consume on an average ten times more phytoplankton productivity than the mesozooplankton community (Putland and Iverson 2007). In the present study, based on experimental studies, potential primary production grazed by this community varied between 58 and 97 %. Comparable primary production and consumption by microzooplankton has been reported from the western Arabian Sea (Landry et al. 1998). Calbet and Landry (2004) present the global impact of microplanktonic grazers on marine phytoplankton and show that the proportion of primary production consumed by microzooplankton is about 67 % of total phytoplankton daily growth. This average is well within the range (49–77 %) reported in the review paper by Schmoker et al. (2013)
In the Zuari estuary, varying microzooplankton population with respect to relatively uniform temperature both spatially and seasonally signifies that the direct effect of temperature is not responsible for changes in microzooplankton population. Therefore, salinity (see Putland and Iverson 2007), apart from the food supply and predators, could be responsible for the observed spatio-temporal changes in abundance. The study region is subjected to heavy rainfall and land runoff during June–September every year, which results in a large decrease in salinity but an enrichment of nutrients. The maximum observed NO3–N concentration (14.6 µm) observed in the present study is higher than that observed by Devassy (1983) in the lower reaches of the Zuari estuary during monsoon (8 µm). This in turn at times supports Chl a as high as 16 mg m−3 (M. Gauns, unpublished). It is understood that salinity variation in estuaries generally controls the species composition and succession of planktonic organisms (Madhupratap and Haridas 1975). The amplitude of salinity fluctuation observed during this study (1–32) is well in agreement with those reported earlier by Dehadrai (1970) and Qasim and Sengupta (1981). Generally, higher abundance of MZP was associated with high salinity, which appears to significantly (p < 0.01) govern the MZP abundance and distribution. An optimum salinity range of 15–20 for microzooplankton (tintinnid) occurrence in the Zuari estuary, which occurs during the post-monsoon, results in high microzooplankton population and a decrease in autotrophic picoplankton biomass (Fig. 4) compared to other times of the year.
As pointed out earlier, PP and BP data were obtained from earlier studies in the study region. The PP (mg C m−2 day−1) has been found to vary from 249 to 430 (Bhattathiri et al. 1976; Devassy 1983, 1989). Devassy (1989) also recorded high surface production (79–134 mg C m−3 h−1) in the Zuari estuary during post- and pre-monsoon seasons. Phytoplankton biomass in terms of chl a in the present study was in the range of 0.2–12.8 mg m−3. Devassy and Goes (1989) also recorded wide fluctuation in chl a (0.22–3.7 mg m−3) from November to April and a sharp decline in the monsoon months. Similarly, chl a in the range of 0.56–11.86 mg m−3 was recorded by Bhargava and Dwivedi (1976) in the Mandovi-Zuari estuaries. This is again within the range recorded during the present study, indicating a cyclic variation of phytoplankton biomass in this estuary, with peaks during pre-monsoon when estuary is well mixed and the water is clear. Likewise, considerable variation in phytoplankton cell abundance has been noticed previously, ranging from 3600 to 387,500 cells l−1 (Bhattathiri et al. 1976; Devassy and Goes 1989). The range of phytoplankton cell numbers observed in the present study (400–62,000 cells l−1) is comparable to that reported by Devassy and Bhargava (1978). A combination of variability in abundance of grazers (both micro and mesozooplankton), availability of the right type of nutrients, and other factors (e.g,. clear water column) required for phytoplankton growth could produce such wide fluctuations in phytoplankton density in the estuary.
In general, microzooplankton (ciliates) are known to exert a key control over the bacterial population (Gast 1985; Sherr and Sherr 1987; Putland 2000; Sakka Hlaili et al. 2008). Ciliate contribution to the total microzooplankton in the study area was quite high (~70 %), which may play a significant role in the trophodynamics by effectively linking microbial biomass to higher (secondary/tertiary) trophic levels. Relatively high bacterial counts during post-monsoon and pre-monsoon seasons must have been supported by the higher DOC, measured during these periods (Table 1). It is likely that the blooms of Trichodesmium erythraeum, which occur every year with a marked periodicity from February to April (Devassy and Bhargava 1978), may provide DOC and promote bacterial growth during this season. Surprisingly, the bacterial counts during post-monsoon were lower than during pre-monsoon, even though the higher DOC pool occurred during the former season. We do not have experimental evidence from the Zuari estuary to support the observed mismatch. However, we feel that the observed lag may be because of the grazing pressure exerted by predators (flagellates and/or ciliates; see below), or due to the lack of labile DOC for the bacteria to take up. Devassy and Goes (1989) found viable bacterial counts in the range of 0.2–0.4 × 106 l−1. These counts are lower by an order of magnitude than those reported by Ramaiah and Chandramohan (1992) from Dona Paula Bay (near the mouth of the Zuari estuary).
Heterotrophic nanoflagellates (HNFs) form a group that has not thus far been properly investigated in the Zuari estuary. They are known to play a very important role in linking bacteria to higher trophic levels (Sanders et al. 1992). For example, in the Masan Bay (Korea), about 69 % of bacterial production is being grazed by HNF (Lee et al. 2007). By consuming bacterial production and controlling bacterial abundance, HNFs occupy a key niche within microbial food webs, and presumably impact strongly the structure and function of bacterial communities and energy fluxes. Some of the tintinnid ciliates, such as Favella sp., Tintinnopsis lobiancoi and T. kofoidii, are known to ingest flagellates (Stoecker et al. 1981). These predator species are commonly found in the Zuari estuary. Thus, investigating mechanisms that regulate abundance of HNFs is important for understanding bacterioplankton dynamics and the microbial food web. Grazing pressure exerted by ciliates (and environmental parameters like salinity) may play a regulatory role on HNFs of the Zuari estuary.
Both microplankton and nanoplankton biomasses are grazed upon by mesozooplankton (see review by Pierce and Turner 1992). Mesozooplankton biomass recorded during the present study was comparable to that reported earlier from the Zuari estuary, and also had a similar seasonal pattern with lower biomass in monsoon (Goswami and Singbal 1974; Goswami and Selvakumar 1977; Selvakumar et al. 1980; Qasim and Sengupta 1981; Padmavati and Goswami 1996). Reduction of salinity is believed to be the primary factor for lower mesozooplankton biomass during the monsoon. However, in general, copepods dominate the zooplankton community, forming as much as 66.2 % of the total annual counts, followed by decapods larvae (17.2 %). Carnivorous forms like hydromedusae, siphonophors, ctenophors and chaetognathas usually occur in the Zuari estuary during high salinity periods (Padmavati and Goswami 1996).
4.1 Microzooplankton and carbon flow in the Zuari trophodynamics
Data from the present work on microzooplankton, HNFs, bacterial abundance and mesozooplankton, and those on primary production (Devassy and Goes 1989) and bacterial production (De Souza Maria-Judith 2002), are used to evaluate the role of microzooplankton in the food web of the Zuari estuary. Mesozooplankton biomass was separated into three categories, viz., (1) herbivores (2) carnivores and (3) omnivores. This was done based on their percentage compositions as observed by Padmavati and Goswami (1996) in the Zuari estuary. Carnivorous forms such as hydromedusae, siphonophore, ctenophores and chaetognaths usually occur during high temperature and salinity period, and herbivorous-like copepods belonging to the genera Undinula, Eucalanus, Cosmocalanus, Centropages and Temora are found when the water temperature and salinity are low (Goswami and Padmavati 1996; Padmavati and Goswami 1996). Herbivorous mesozooplankton (HZ) in the study area were possibly not strictly phytoplankton feeding, but could be considered as a mixed feeding type, as they are known to graze on microzooplankton as well (Fornemann 2001).
Large variations in both living carbon (LC = chlorophyll a + bacteria + nanoflagellates + microzooplankton + mesozooplankton) and nonliving carbon components [NLC = POC-LC] were observed in the present study. POC was higher during monsoon and post-monsoon seasons (Table 1). The NLC varied from 284 to 536 mmol C m−3. The microzooplankton carbon biomass accounted for 24–40 % of living carbon component. A sizable contribution by HNFs (12–38 %) as compared to bacteria (6–13 %) and mesozooplankton (~1 %) is interesting, underlining need for further research on this group. Bacterial and HNF carbon biomass were higher during pre-monsoon seasons. As heterotrophic bacteria can utilize and grow on various kinds of organic matter in the marine ecosystem, in the Zuari estuary too, they appear to assimilate organic matter quite efficiently and are useful as food for HNFs and other members of the microzooplankton community, which, in turn, are consumed by several larval stages of mesozooplankton. Seasonal disparity in their occurrences indicates close coupling between these two microbial components (p < 0.001, n = 40, r = 0.68), as observed in the waters of the Arabian Sea (unpublished data).
In order to understand the fate of carbon in the Zuari estuary, carbon requirement to sustain the observed standing stocks of microzooplankton was calculated based on the gross growth efficiency. The highest requirement of microzooplankton was during the post-monsoon period (Table 2). The analysis revealed that even if one assumes that all organic carbon of phytoplankton, HNFs and bacteria is consumed by microzooplankton with a growth efficiency of 0.4 (Fenchel 1987), these living carbon components are individually insufficient to sustain such a high standing stock of microzooplankton, particularly during post-monsoon and pre-monsoon seasons. In all probability, POC in discrete, nano-meter sized particles might serve as alternate food for MZP, which can assimilate this particulate carbon source. For dinoflagellates, a major component of microzooplankton, a recent study by Menden-Deuer and Lessard (2000) reports ~ twofold higher carbon content per volume (0.054–0.297 pg C µm−3) than the value used in the present study (0.14 pg C µm−3). This further highlights their importance in the microzooplankton community and in the food web of the Zuari estuary.
Verity (1986) found that in the Narragansett Bay, tintinnid community growth rates increased when chl a and POC increased in the <10 and or <5 µm size ranges. In the study area, >60 % of the phytoplankton biomass (chl a) also remained in smaller fraction (<20 µm) for the most part the year (Fig. 4), thereby supporting microzooplankton community. A review by Pierce and Turner (1992) also suggests that detritus may be important for ciliates in both coastal and oceanic systems. If one assumes that about 30 % of the particulate organic carbon is either lost to the bottom or fluxed out of the estuary (Bhaskar et al. 2000), there is still enough POC in the estuary for exclusive “consumption” by MZP (Table 2). In addition, a considerable amount of the carbon required by microzooplankton might be supplied via the microbial food web, which is fueled by the in situ dissolved organic carbon pool, which was also found to be high during the high salinity periods (Table 1). Further, a significant part of the food requirements of carnivorous and herbivorous mesozooplankton might be met by microzooplankton, particularly during the post-monsoon and pre-monsoon seasons, due to the dominance of carnivores as observed in the present study as well as by others (Goswami and Padmavati 1996). The best-known predators of ciliates are planktonic copepods. For example, copepods, especially Acartia spp, have been found to feed selectively on tintinnids even when phytoplankton are abundant (Robertson 1983; Turner and Graneli 1991). In the subarctic waters of the Oyashio current, a few tintinnid species showed large fluctuations in abundance that may be controlled by the copepods (Gomez 2007). Both of these prey and predators are preponderant in the study area. The work of Last (1978) suggests that tintinnids are consumed by marine fish larvae. Later, Stoecker and Govoni (1984) confirmed that small size fish larvae of 93 µm–5 mm mostly prefer tintinnids—Favella and the dinoflagellate—Prorocentrum, whereas larger larvae feed upon copepod nauplii. At times, tintinnids form as much as 75 % of the diet of certain size classes of fish species (Jenkins 1987). The gut contents and faeces of a number of invertebrates and fish larvae show that microzooplankton form a significant portion (~31 %) of their food (Godhantaraman 2001).
In conclusion, the results of the present study from the Zuari estuary show that microzooplankton play an important role in the food web of the tropical estuarine systems. The Zuary estuary and probably all similar estuaries along the Indian west coast are largely dominated by small autotrophs for most of the year. The ability of microzooplankton to take up small food particles enables efficient linking of these autotrophs to higher trophic levels. The top-down control over food webs seems to be dominant for most of the year in the study area. Further, this study also indicates that non-living particulate carbon may be important in the nutrition of microzooplankton and overall net heterotrophy.
References
AshaDevi CR, Jyothibabu R, Sabu P, Jacob J, Habeebrehman H, Prabhakaran MP, Jayalakshmi KJ, Achuthankutty CT (2010) Seasonal variations and trophic ecology of microzooplankton in the southeastern Arabian Sea. Cont Shelf Res 30(9):1070–1084
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water column microbes in the Sea. Mar Ecol Prog Ser 10:257–263
Banse K (1988) Estimates of average phytoplankton division rates in the open Arabian Sea. Indian J Mar Sci 17:31–36
Beers JR, Stewart GL (1970) Numerical abundance and estimated biomass of microzooplankton. In: Strickland JD (ed) The ecology of plankton off La Jolla, California in the period April through September, 1967. University of California, Bull-Scripps Institute of Oceanography, California, pp 17–20
Bhargava RMS, Dwivedi SN (1976) Seasonal distribution of phytoplankton pigments the estuarine system of Goa. Indian J Mar Sci 5:87–90
Bhaskar PV, Cardozo E, Giriyan A, Garg A, Bhosle NB (2000) Sedimentation of particulate matter in the Dona Paula Bay, West Coast of India during November to May 1995–1997. Estuaries 23:722–734
Bhattathiri PMA, Devassy VP, Bharagva RMS (1976) Production at different trophic levels in the estuarine systems of Goa. Indian J Mar Sci 5:83–86
Booth BC (1993) Handbook of methods in aquatic microbial ecology. Lewis Publishers, London, p 199
Calbet A, Landry MR (2004) Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol Oceanogr 49(1):51–57
Capriulo GM, Carpenter EJ (1983) Abundance, species composition, and feeding impact of tintinnid micro-zooplankton in Central Long Island sound. Mar Ecol Prog Ser 10:277–288
Cole JJ, Finlay S, Pace ML (1988) Bacterial production in salt and fresh water ecosystems: a cross system overview. Mar Ecol Prog Ser 43:1–10
Cushing DH (1975) Marine ecology and fisheries. Cambridge University Press, Cambridge, p 278
De Souza Maria-Judith, B. D. (2002): Study of particle associated bacteria in tropical estuary. Ph.D. thesis, University of Goa, India
Dehadrai PV (1970) Observations on certain environmental features at the Dona Paula point in Mormugao Bay, Goa. Proc Indian Acad Sci Earth Planet Sci 72:56–67
Devassy VP (1983) Plankton production associated with cold water incursion into the estuarine environment. Mahasagar Bull Natl Inst Oceanogr 16:221–233
Devassy VP (1989) Red tide discolouration and its impact on fisheries. Red Tides Biol Environ Sci Toxicol, 57–60
Devassy VP, Bhargava RMS (1978) Diel changes in phytoplankton population in the Mandovi and Zuari estuaries of Goa. Mahasagar Bull Natl Inst Oceanogr 12:195–199
Devassy VP, Goes JI (1989) Seasonal patterns of phytoplankton biomass and productivity in a tropical estuarine complex (West coast of India). Proc Indian Acad Sci Earth Planet Sci 99:485–501
Edler L (1979): Recommendation for marine biological studies in the Baltic Sea: phytoplankton and chlorophyll. baltic marine biologists WG 9, Publication The National Swedish Environmental Protection Board, Malmo
Fenchel T (1982) Ecology of heterotrophic microflagellates. II Bioenergetics and growth. Mar Ecol Prog Ser 8:225–231
Fenchel T (1987) Ecology of protozoa—the biology of free-living phagotrophic protists. Springer, Berlin
Frost BW (1991) The role of grazing in nutrient-rich areas of the open sea. Limnol Oceanogr 36:1616–1630
Garrison DL, Gowing MM, Huges MP, Campbell L, Caron DA, Dennett MR, Shalapyonok A, Olson RJ, Landry MR, Brown SL, Liu Hong-Bin F, Azam GF, Steward HW, Ducklow, Smith DC (2000) Microbial food web structure in the Arabian Sea: a US JGOFS study. Deep Sea Res 47:1387–1422
Gast V (1985) Bacteria as a food source for microzooplankton in the Schlei Fjord and Baltic Sea with special reference to ciliates. Mar Ecol Prog Ser 22:107–120
Gauns M, Madhupratap M, Ramaiah N (1996) Studies on the microzooplankton from the central and eastern Arabian Sea. Curr Sci 71:874–877
Gauns M, Madhupratap M, Ramaiah N, Jyothibabu R, Fernandes V, Paul JT, Prasanna Kumar S (2005) Comparative accounts of biological productivity characteristics and estimates of carbon fluxes in the Arabian Sea and the Bay of Bengal. Deep Sea Res 52:2003–2017
Gifford DJ, Dagg MJ (1991) The microzooplankton-mesozooplankton link: consumption of planktonic protozoa by the calanoid copepods Acartia tonsa Dana and Neocalanus plumchrus Murukawa. Marine Microbial Food Webs 5:161–177
Gifford SM, Rollwangen-Bollens G, Bollens SM (2007) Mesozooplankton omnivory in the upper San Francisco Estuary. Mar Ecol Prog Ser 348:33–46
Gilron GL, Lynn DH (1989) Assuming a 50 % cell occupancy of the lorica overestimates tintinnine ciliate biomass. Mar Biol 103:413–416
Godhantaraman N (2001) Seasonal variations of taxonomic composition, abundance and food web relationship of microzooplankton in estuarine and mangrove waters, Parangipettai region, southeast coast of India. Indian J Mar Sci 30:151–160
Goldman JG, Caron DA, Dennett MR (1987) Nutrient cycling in a microflagellate food web chain: iv phytoplankton microflagellate interactions. Mar Ecol Prog Ser 38:75–87
Gomez F (2007) Trends on the distribution of ciliates in the open Pacific Ocean. Acta Oecol 32:188–202
Goswami SC, Padmavati G (1996) Zooplankton production, composition and diversity in the coastal waters of Goa. Indian J Mar Sci 25:91–97
Goswami SC, RA Selvakumar (1977) Plankton studies in the estuarine system of Goa. In: Proceedings of the symposium warm water zooplankton, NIO, Goa, UNESCO/NIO
Goswami SC, Singbal SYS (1974) Ecology of Mandovi and Zuari estuaries plankton community in the relation to hydrographic conditions during monsoon months, 1972. Indian J Mar Sci 3:51–57
Grasshoff K (1976) Methods of seawater analysis. Verlag, New York, p 317
Hass LW (1982) Improved epifluoresence microscopy for observing planktonic organisms. Annales de l’Institut Oceanographique Paris 58S:261–266
Ikeda T, Motoda S (1978) Estimated zooplankton production and their ammonia excretion in the Kuroshio and adjacent seas. Fish Bull 75:357
James MR, Hall JA (1998) Microzooplankton grazing in different water masses associated with the subtropical convergence round the South Island, New Zealand. Deep Sea Res I 45:1689–1707
Jenkins JP (1987) Comparative diets, prey selection, and predatory impact of co-occurring larvae of two flounder species. J Exp Mar Biol Ecol 110:147–170
Jyothibabu R, Madhu NV, Jayalakshmy KV, Balachandran KK, Shiyas CA, Martin GD, Nair KKC (2006) Impact of freshwater influx on microzooplankton mediated food web in a tropical estuary (Cochin backwaters–India). Est Coast Shelf Sci 69:505–518
Jyothibabu R, AshaDevi CR, Madhu NV, Sabu P, Jayalakshmy KV, Jacob J, Habeebrehman H, Prabhakaran MP, Balasubramanian T, Nair KKC (2008) The response of microzooplankton (20–200 mu m) to coastal upwelling and summer stratification in the southeastern Arabian Sea. Cont Shelf Res 28:653–671
Landry MR, Hassett RP (1982) Estimating the grazing impact of marine micro-zooplankton. Mar Biol 67:283–288
Landry MR, Brown SL, Campbell L, Constantinou J, Liu H (1998) Spatial patterns in phytoplankton growth and microzooplankton grazing in the Arabian Sea during monsoon forcing. Deep Sea Res 45:2353–2368
Last JM (1978) The food of three species of gadoid larvae in the eastern english channel and Southern North Sea. Mar Biol 48:377–386
Lee WJ, Shin K, Lee JD (2007) Studies on marine heterotrophic protists in Masan Bay, Korea. Ocean Polar Res 29:401–410
Lessard EJ (1991) The trophic role of heterotrophic dinoflagellates in diverse marine environments. Marine Microbial Food Webs 5:49–58
Madhupratap M, Haridas P (1975) Composition and variations in zooplankton abundance in the backwaters from Cochin to Alleppy. Indian J Mar Sci 4:77–85
Madhupratap M, Haridas P, Ramaiah N, Kutty CTA (1992) Zooplankton of the South West coast of India. Abundance, composition, temporal and spatial variability in 1987. In: Desai BN (ed) Oceanography of the Indian Ocean, Oxford & IBH, 177–189
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Muller H, Geller W (1993) Maximum growth rates of an aquatic ciliated protozoa. The dependence on body size and temperature reconsidered. Arch Hydrobiol 126:315–327
Padmavati G, Goswami SC (1996) Zooplankton ecology in the Mandovi-Zuari estuarine system of Goa, West coast of India. Indian J Mar Sci 25:268–273
Paranjape MA, Conover RJ, Harding GC, Prouse NH (1985) Micro-and macrozooplankton on the Nova Scotian shelf in the prespring bloom period: a comparison of their potential resource utilization. Can J Fish Aquat Sci 42:1484–1492
Pierce RW, Turner JT (1992) Ecology of Planktonic ciliates in marine food webs. Rev Aquat Sci 6:139–181
Porter K, Feigh YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Probyn TA (1987) Ammonium regeneration by microplankton in an upwelling environment. Mar Ecol Prog Ser 37:53–64
Putland JN (2000) Microzooplankton herbivory and bacterivory in Newfound land coastal waters during spring, summer and winter. J Plankton Res 22:253–277
Putland JN, Iverson RL (2007) Microzooplankton: major herbivores in an estuarine planktonic food web. Mar Ecol Prog Ser 340:173–187
Putt M, Stoecker DK (1989) An experimentally determined carbon: volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limnol Oceanogr 34:1097–1103
Qasim SZ (1979): Changing ecology of Cochin backwaters. In: contributions to marine sciences, dedicated to Dr. CV Kurian, University of Cochin, 137–142
Qasim SZ, Sengupta R (1981) Environmental characteristics of the Mandovi-Zuari estuarine system in Goa. Est Coast Shelf Sci 13:557–578
Ramaiah N, Chandramohan D (1992) Ecology and biology of luminous bacteria in the Arabian Sea. In: Desai BN (ed) Oceanography of the Indian Ocean, Oxford & IBH, 11–24
Ramaiah N, Chandramohan D, Nair VR (1995) Autotrophic and heterotrophic characteristics in a polluted tropical estuarine complex. Est Coast Shelf Sci 40:45–55
Reid DF, Karl D (1990) Bacterial growth, abundance, and loss due to protozoan grazing during the 1989 spring bloom. Antarct J USA 25:156–157
Riley GA, Van Hemert D, Wangersky PJ (1965) Origin of aggregates in surface and deep waters of the Sargasso Sea. Limnol Oceanogr 10:354–363
Robertson JR (1983) Predation by estuarine zooplankton on tintinnid ciliates. Est Coast Shelf Sci 16:27–36
Sakka Hlaili A, Grami B, Niquil N, Gosselin M, Hamel D, Troussellier M, Hadj Mabrouk H (2008) The planktonic food web of the Bizerte lagoon (south-western Mediterranean) during summer: I Spatial distribution under different anthropogenic pressures. Est Coast Shelf Sci 78:61–77
Sanders RW, Caron DA, Berninger UG (1992) Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Mar Ecol Prog Ser 86:1–14
Sarma VVSS, Dileep Kumar M, Manerikar M (2001) Emission of carbon dioxide from a tropical estuarine system, Goa, India. Geophys Res Lett 28(7):1239–1242
Schmoker C, Herna´ndez-Leo´n S, Calbet A (2013) Microzooplankton grazing in the oceans:impacts, data variability, knowledge gaps and future directions. J Plankton Res 35(4):691–706
Selvakumar RA, Nair VR, Madhupratap M (1980) Seasonal variations in secondary production of the Mandovi-Zuari estuarine systems of Goa. Indian J Mar Sci 9:7–9
Sherr BF, Sherr EB (1983) Enumeration of heterotrophic microprotozoa by epifluroscence microscopy. Est Coast Shelf Sci 16:1–7
Sherr EB, Sherr BF (1987) High rates of consumption of bacteria by pelagic ciliates. Nature 325:710–711
Shetye SR, Kumar MD, Shankar D (2007) The Mandovi and Zuari estuaries. National Institute of Oceanography, Goa, p 145
Stoecker DK, Govoni JJ (1984) Food selection by young larval gulf menhaden (Brevoortia patronus). Mar Biol 80:299–306
Stoecker DK, Guillard RL, Kavee RM (1981) Selective predation by Favella ehrenbergii (Tintinina) on and among dinoflagellates. Biol Bull 160:136–145 (marine biological laboratory, woods hole)
Stoecker DK, Sieracki ME, Verity PG, Michaels AE, Haugen E, Burkill PH, Edwards ES (1994) Nanoplankton and protozoan microplankton during the JGOFS North Atlantic bloom experiment. J Mar Biol Ass U K 74:427–443
Strom SL, Macri EL, Olson MB (2007) Microzooplankton grazing in the coastal Gulf of Alaska: variations in top-down control of phytoplankton. Limnol Oceanogr 52:1480–1494
Sunita M, Pratihary A, Gauns M, Roy R, Babasaheb T, Pai IK, Naqvi SWA (2013) Response of phytoplankton to nutrient enrichment with high growth rate in a tropical monsoonal estuary (Zuari estuary), west coast of India. Indian J Geo Mar Sci 42(3):314–325
Turner JT, Graneli E (1991) Zooplankton feeding ecology: grazing during enclosure studies of phytoplankton blooms from the west coast of Sweden. J Exp Mar Biol Ecol 157:19–31
UNESCO, 1994. Protocols for the Joint Global Ocean Flux Study (JGOFS) core measurements, IOC manuals and guides 29 UNESCO, Paris, 170
Uye SI, Nagano N, Tamaki H (1996) Geographical and seasonal variations in abundance, biomass and estimated production rates of micro-zooplankton in the Inland Sea of Japan. J Oceanogr 52:689–703
Uye SI, Nagano N, Shimazu T (1998) Biomass, production and trophic roles of micro- and net-zooplankton in Dokai Inlet, a heavily eutrophic inlet, in summer. Plan Biol Ecol 45:171–182
Venogradov AP (1953). Mem Sears Fdn Mar Res, 647
Verity PG (1986) Growth rates of natural tintinnids populations in Narragansett Bay. Mar Ecol Prog Ser 29:117–126
Verity PG, Langdon C (1984) Relationships between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay. J Plankton Res 6:859–868
Acknowledgments
We thank Director-CSIR, NIO for the encouragement and facilities. Survey work was carried out as a part of Ph D. thesis (under the Indian-JGOFS programme), and experimental studies were carried out under the programmes NWP 0014 and PSC0108. This is NIO’s contribution no. 5748.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gauns, M., Mochemadkar, S., Patil, S. et al. Seasonal variations in abundance, biomass and grazing rates of microzooplankton in a tropical monsoonal estuary. J Oceanogr 71, 345–359 (2015). https://doi.org/10.1007/s10872-015-0292-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-015-0292-6