Abstract
Autophagy is an important cell activity which is the process of formation of autophagosomes, docking with lysosomes and degradation. The intrinsic pathway of apoptosis involves mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release followed by caspase activation. Many molecules, e.g., Ca2+ and mTOR, and different stresses such as endoplasmic reticulum (ER) stress and nutritional stress take part in these two processes. However, the mechanism of how they work together so as to determine cell fate decisions remains to be clarified. Here, we present a computational model for cell fate decisions based on intertwined dynamics with autophagy and apoptosis involving Ca2+, mTOR, and both ER stress and nutritional stress. In agreement with experimental observations, the model predicts that both Ca2+ and the stresses play critical roles in regulating the choice between autophagy and apoptosis in a combinatorial way. The model presented here might be a good candidate for providing the qualitative mechanism of cell fate decisions mediated by Ca2+, mTOR, and two kinds of stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Autophagy is an important process which degrades macromolecules and also eliminates organelles and unwanted structures in response to diverse stress stimuli [1]. The induction of autophagy involves a complex signal transduction pathway [2]. It is not a completely passive cellular function, but an active biological process which helps cells to maintain homeostasis through a series of intracellular signal transduction pathways stimulated by internal or external stresses. Scholars have been studying autophagy for many years [3, 4]. They gave various explanations about autophagy on some uncertain questions. It has been shown that there is a basic level of autophagy in mammalian cells. In normal process of cell metabolism, the basic level of autophagy helps cells survive longer in the face of diverse stresses. During autophagy, cells will first form a subcellular organelle with two or more membranes, called autophagosomes, which contain damaged organelles or proteins to be degraded. Then autophagosomes will dock with lysosomes to form autolysosomes which degrade the encapsulated substances and produce the energy needed for cell survival.
It is known that autophagy can affect many physiological processes, such as cell cycle, cell apoptosis, pathogen clearance, aging, and especially apoptosis, which are related to the occurrence of diseases in various systems [5]. Apoptosis has been extensively studied by many scholars over the past decades. Both intrinsic and extrinsic apoptotic pathways have been studied at the cellular and transcriptional levels. As we know, autophagy and apoptosis share some common regulatory proteins and stimulus factors such as Ca2+, ER, and nutritional stresses. However, their triggered manifestations are different [6]. Ca2+ is an important messenger molecule in the process of apoptosis. There are still many disputes about the role of Ca2+ in apoptosis. At present, one unanimous conclusion is that the loss of Ca2+ in the endoplasmic reticulum can induce the apoptosis of ER stress pathway cells, and the increase of Ca2+ concentration in cytoplasm can provide important information in intrinsic apoptotic pathway. The increase of Ca2+ concentration in cytoplasm is also a powerful factor of macrophage autophagy [7], but its exact role remains ambiguous.
It is known that mTORC1 is the target of rapamycin [8]. The mechanistic target of rapamycin (mTOR) is a critical protein in the regulation of cell fate decisions, especially in cancer cells [9]. Understanding the mechanism of how mTOR influences these processes has far-reaching implications in pharmacology. A recent study suggests that mTOR signaling is inhibited during autophagy initiation, but reactivated upon prolonged starvation [10]. Under the signal of nutritional stress, mTOR can also inhibit apoptosis through acting on ULK1 [2]. Apoptosis may begin with autophagy, and autophagy can often end with apoptosis [11]. Inhibition or a blockade of caspase activity may lead a cell to default into Type II programmed cell death, i.e., autophagic cell death, from Type I programmed cell death, i.e., apoptosis [12].
It is worth noting that there are various stress stimuli, and they act on the network at different locations in the choice between autophagy and apoptosis. The pathogenesis of many diseases is related to the apoptosis induced by ER stress, such as Alzheimer’s, Parkinson’s, and other neurodegenerative diseases [13]. For DNA damage and ER stress, they both affect complexes composed of Bcl2 in endoplasmic reticulum. The accumulation of damaged or not properly folded proteins in the ER lumen leads to harmful ER stress. Normally, Bcl2 forms complexes with Bax and Beclin1, which inhibit the activity of Bax and Beclin1. Colored Bcl2 inhibits both Beclin1 and Bax by forming inhibitory complexes with them. Taking DNA damage and ER stress as input, when input is activated, both complexes will be dissociated, and then Bax and Beclin1 can be expressed. Depending on the severity of ER stress, either autophagy-controlled survival or apoptosis can be induced.
But when the input signal is the nutritional stress, it acts on mTOR. Similarly, under normal conditions, mTOR inhibits the development of autophagy. In the same way, when nutritional stress enters the network as an input, it inhibits mTOR and the strength of autophagy will be increased. In addition to the above stress categories, there are also oxidative stress [14], growth factor, etc. In this paper, we study the effects of Ca2+ and two types of stresses individually or in a combinatorial way.
The purpose of this article is to present a computational model cell fate decisions based on intertwined dynamics with autophagy and apoptosis involving the Ca2+, mTOR, caspases, ER stress, and nutritional stress. Not surprisingly, mathematical models of autophagy and apoptosis, with different levels of sophistication, have been proposed [5, 7, 13,15,17]. Although cell fate decisions under multiple perturbations are performed [15], the model is so comprehensive that it is difficult to analyze its dynamics from the viewpoint of network topology. In addition, most of the previous models do not include an essential characteristic of the network, i.e., the choice between autophagy and apoptosis under multiple input signals, especially, the combinatorial effects of Ca2+, mTOR, and both ER stress and nutritional stress. Thus, a new model is needed which incorporates both autophagy and apoptosis, and which can account for their mechanisms, relevance, and potential implications in cell fate decisions. In agreement with experimental observations, the model presented here can account for the choice between autophagy and apoptosis, depending on cooperation between the multiple signals. We used numerical methods to calculate steady state solutions and bifurcation. All the analysis and figures were done using MATLAB and XPPAUT.
The paper is organized as follows. We first study the models of autophagy and apoptosis separately, and then integrate them to study how they interplay to affect cell fate decisions. After that, by using bifurcation theory, we try to consider how autophagy and apoptosis interact with each other as well as the importance of Ca2+ and mTOR. We make a further bifurcation analysis under ER and nutritional stresses in a combinatorial way. The paper ends with discussion in the final section.
2 Main results
2.1 The core network
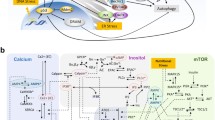
The core network which regulates the crosstalk between autophagy and apoptosis pathway is shown in Fig. 1. The new model is presented on the basis of [16, 17], in which they proposed a theoretical framework to analyze the interplay of autophagy and apoptosis. Among the Bcl2 family proteins, Bcl2 is an anti-apoptotic protein, while Bax and Bid are pro-apoptotic proteins. Different expression levels of Bcl2 lead to different cell dynamics. It is also shown that Bax plays an important role in apoptosis. Bax is often used as a marker of apoptosis. The suicidal cascade typically involves the activation of a family of cysteine proteases called caspases. In addition, there are several kinds of caspases in the mammalian cells. In [18], they confirmed that the intrinsic pathway of apoptosis is activated by effector caspases. Here, we take caspases (Casp) as the marker of apoptosis.
Normally, Bcl2 binds to Bax to form Bax-Bcl2 complex, thus inhibiting the expression of Bax. The ER stress may cause the phosphorylation of Bcl2 [17], which will dissociate the complex and make Bax express normally. Active Bax causes cytochrome c into the cytosol and the caspases could be activated. Highly expressed caspases will eventually lead to cell apoptosis. A mathematical model related to apoptosis was established [19]. With the tool of dynamical analysis, they showed that apoptosis acted as a bistable switch, and Bax was an important factor affecting the apoptosis. A simplification of the network including only the core nodes was made [5]. In addition, a model with a logistic growth was developed to study the role of autophagy in yeast cells, and it was confirmed that autophagy was valuable for maintaining cell populations [20]. In this paper, a new model is developed by integrating the models of autophagy and apoptosis so as to study the interplay of these two processes, as shown in Fig. 2. The model consists of several ODEs derived from the conservation law and mass action kinetics for the species.
2.2 The apoptosis module is bistable
In order to get a better understanding of the core network, we firstly study the apoptotic network shown in Fig. 3. The signal-response curve of apoptosis inducer, i.e., Casp, with respect to ER stress variation, is shown in Fig. 4. The activation of caspases under higher ER stress represents the initiation of apoptosis. The mathematical model of the apoptosis module is shown in Eq. (1–3) in appendix. Caspases are able to switch between the high and low steady states. When the ER stress is at a low level, the caspases are at the low steady states. By the increase of ER stress level, the system switches to the high steady states corresponding to the occurrence of apoptosis.
2.3 The model of autophagy
The regulatory network of autophagy is shown Fig. 5, base on Fig. 1. The Bcl2 family of proteins have more than a dozen members and are important regulators of autophagy and apoptosis. Among them, Bcl2 is an anti-apoptotic protein. Beclin1, the mammalian orthologue of yeast Atg6, has a central role in the formation of autophagosome, which is increased during periods of cell stress. In our model, we take Beclin1 as a marker of autophagy. When stress is first induced, Bcl2 can be phosphorylated by JNK, and the Bcl2-Beclin1 complex is dissociated. Then, Beclin1 can be expressed.
The kinase mTOR is a major evolutionarily conserved sensor in the autophagy signal transduction pathway of eukaryotic organisms [21]. Under normal condition, mTOR inhibits the formation of ULK1, ATG13, and FIP200 complexes by phosphorylating ULK1. When pressure occurs, it causes mTOR to become inactive and the complex will form, and the complex is a key substance in the formation of autophagosome membranes. Beclin1 is involved in the formation of autophagosomes.
Under the condition of ER stress, Bcl2 also interacts with inositol 1,4,5-trisphosphate receptor (IP3R) which is a stress-activated Ca2+ channel that releases Ca2+ from the ER into the cytoplasm. Elevated concentration of Ca2+ activates CaMKKβ, which promotes AMPK, and AMPK has an inhibitory effect on mTOR to induce autophagy.
2.4 Autophagy as a rheostat
The mathematical model of the autophagic module shown in Fig. 5 is given in Eq. (4–8) in the appendix. The response to both kinds of stress is to activate autophagy, in an attempt to relieve the stress. If the stress becomes too large, autophagy cannot rescue cells, and then the cells tend to die.
The stress-response curves which plot the final expression level of the marker, i.e., active Beclin1, as a function of ER or nutritional stress, are shown in Fig. 6. When there is no any stress, autophagy stays at its basal level. By the increasing ER stress or nutritional stress, the expression level of autophagy is increased, which manifests that autophagy works as a rheostat. Due to not taking the apoptosis pathway into consideration, we cannot simulate the real situation that the autophagy should be inhibited when the stress becomes too large, which means it is necessary to study the coupled model.
3 The coupled model of autophagy and apoptosis
We then construct a coupled model of autophagy and apoptosis based on the core network shown in Fig. 1. For simplification, only important nodes are taken into consideration, as shown in Fig. 2. The full model is given in Eqs. (9–18) in the appendix. By studying the computational model of intertwined dynamics with autophagy and apoptosis involving the Ca2+, mTOR, Casp, ER stress, and nutritional stress, we can show how cells determine the choice between autophagy and apoptosis, depending on cooperation between multiple signals.
3.1 Both stresses can induce state transitions in the coupled model
In order to systematically explore the interplay of apoptosis and autophagy under different types of stress, here we investigate whether and how the transition between the autophagic and apoptotic state occurs under diverse perturbations.
To understand the crosstalk between autophagy and apoptosis, time series under different nutritional stresses are shown in Fig. 7. As we can see, the expression level of caspases is in a particularly low state under the low stress. On the contrary, the Beclin1 level stays at a relatively high state, which means that autophagy occurs. With the increase of nutritional stress, the level of caspases will eventually stay at a highly expressed state and Beclin1 is inhibited by caspases. Such results are in consistent with the experimental findings in [22]. Under nutritional stress, autophagy of MC3T3-E1 may antagonize apoptosis in the first 2 h by inhibiting the activity of caspase-3. While the autophagy level reduces after the first 2 h, leading to the increase of caspases and thus the occurrence of apoptosis due to the inhibition of caspases by Beclin1, which was also experimentally validated [23].
The signal-response curves of the coupled network under ER or nutrition stress are shown in Fig. 8. There are two stable branches, i.e., lower and higher Casp levels, corresponding to autophagy and apoptosis, respectively. Comparing the bifurcation graphs of Fig. 8 with Fig. 4, we find that the bifurcation value for the couple network at which the cell apoptosis occurs is greater than that of the apoptosis module alone due to the mutually exclusive mechanism of autophagy and apoptosis, which also emphasizes the role of autophagy in normal mammalian cells as a protective mechanism. Our model also realizes the irreversibility of apoptosis.
Actually, at a low ER stress, cells stay in the autophagy state. With increasing ER stress, caspases will increase through Bcl2 and Bax. After caspases reach their threshold, a bifurcation occurs and apoptosis will happen. Although increased ER stress induces the increase of Beclin1 through Bcl2, increased ER stress also induces the increase of caspases, which inhibits Beclin1. When integrating these two regulations, at a higher ER stress, Beclin1 stays at a lower state and caspases stay at a higher state; thus, cells stay at the apoptotic state. More exactly, at a lower ER stress, the pathway including Bcl2 and Beclin1 plays a decisive role to keep Beclin1 at a higher state and cells at the autophagy state. While at a higher ER stress, the pathway including Bcl2 and Bax plays a decisive role to increase the caspases level and keep cells at the apoptotic state.
3.2 Ca2+ fine-tunes the bistable switch
Signalosomes play an important role in maintaining cell dynamical balance to achieve normal physiological activities. Ca2+ is one of them. So it is necessary to consider the effects of Ca2+ on the dynamics of the coupled network. Under normal physiological conditions, Ca2+ remains at a relatively low concentration in the cytoplasm to ensure the stability of various intracellular activities. As we can see from Fig. 9, the variation of Ca2+ can affect the system dynamics significantly. Under different Ca2+, the system is always bistable. However, variation of the inhibition of Ca2+ by Bcl2 affects the transition between autophagy and apoptosis. For example, for a larger inhibitory rate of Ca2+ by Bcl2, i.e., kin, a stronger stress is needed to achieve cell fate transition, as in Fig. 9.
The role of Ca2+ in coupled model under ER stress and nutritional stress. a Signal response curves of caspases with respect to ER stress at different rates of inhibition of Ca2+ by Bcl2. Green line: kin = 0.1; red line: kin = 1; blue line: kin = 2. b Signal responses curve of caspases with respect to nutritional stress at different rates of inhibition of Ca2+ by Bcl2. Green line: kin = 0.1; red line: kin = 1; blue line: kin = 2. Casp is the marker of apoptosis
In order to further develop this hypothesis, we tried to decrease the value of kin; the bistability does not disappear; however, the low-state bifurcation points move to the left. In other words, for a stronger inhibitory rate, i.e., kin, a lower stress can induce cell apoptosis. As summarized above, Ca2+ functions as a rheostat which fine-tunes the transition between autophagy and apoptosis which matches the results in [15]. Similar results can also be obtained by varying the promotion of caspases by Ca2+, i.e., by increasing the parameter value of kacp′ ′ ′ (data not shown).
3.3 The effects of Ca2+ mediated by mTOR on cell fates
The inhibition of mTOR by Ca2+ can enhance the expression of autophagy and also remodel the intracellular Ca2+ signaling mechanism. We know that through IP3R, Ca2+ in the endoplasmic reticulum is released into the cytoplasm.
As we cut off the mTOR inhibition by Ca2+, we find that the the system dynamics changes significantly with respect to the two kinds of stresses. Especially, under ER stress, cells will not enter the apoptotic state even under a high ER stress level. On the other hand, for the case of nutritional stress, with the decreasing inhibition of mTOR by Ca2+, the original irreversible switch becomes reversible. It seems that the inhibition of mTOR by Ca2+ can be a key regulation to control cell fate decisions under the nutritional stress. More exactly, the transition of cell fates from apoptosis to autophagy can be realized by decreasing the inhibition of mTOR by Ca2+, as shown in Fig. 10. When we enhance the inhibitory effect of Ca2+ on mTOR, we find that the low-state bifurcation point shifts to the left. In other words, the initiation of apoptosis only requires a lower level of stress, which is consistent with the experimental observations in [21].
Effects of Ca2+ mediated by mTOR in the coupled model under the ER stress and nutritional stress. a Signal response curves of caspases with respect to ER stress at different rates of inhibition of mTOR by Ca2+. Green line: kimtor′ = 0; red line: kimtor′ = 4; blue line: kimtor′ = 8. b Signal response curves of caspases with respect to nutritional stress at different rates of inhibition of mTOR by Ca2+. Green line: kimtor′ = 0; red line: kimtor′ = 4; blue line: kimtor′ = 10. Casp is the marker of apoptosis
In summary, the effects of Ca2+ mediated by caspases or mTOR show different effects on cell fates. More exactly, Ca2+ mediated by mTOR can determine if state transition can occur and whether the switch is reversible, depending on the strength of the stresses. On the other hand, Ca2+ mediated by caspases can only determine when the state transition occurs.
3.4 Effects of combinatorial perturbations
As mentioned above, the perturbation of either ER or nutritional stress can induce apoptosis. But in real situations, multiple types of stress can interact with each other. For example, it has been shown that EBSS-triggered starvation activates ER stress in APRE-19 cells [24]. Thus, we need to examine how multiple perturbations affect the choice of cell fates in a combinatorial way. Bifurcation under combinatorial perturbations has been used to study synergism and antagonism in biological systems [25]. In addition, scholars have also examined drug or target combinations [26].
The bifurcation diagram with ER stress and nutritional stresses as control parameters is shown in Fig. 11, which reflects the relationship between the two types of stresses. For a larger nutritional stress, a smaller ER stress can induce apoptosis and vice versa, which indicates that apoptosis is perhaps produced by multiple stresses in a combinatorial way. The coupled model can be in the autophagy or apoptotic state, depending on the level of both ER stress and nutritional stress.
The bifurcation diagram with ER and nutritional stresses as control parameters at kimtor′ = 4. Regions of different dynamical behaviors in ER stress and nutritional stress plane. Mono-stability corresponding to apoptosis occurs in the region labeled II. While the system is bistable in the region labeled I
Similar results can be also obtained for the combination of Ca2+ and one kind of stress, as shown in Fig. 12. We can see that nutritional stress and Ca2+ cooperate in an approximately linear way to induce the occurrence of apoptosis. On the other hand, ER stress and Ca2+ cooperate in a nonlinear way. When mTOR inhibition by Ca2+ is weak, ER stress alone cannot induce occurrence of apoptosis. When mTOR inhibition by Ca2+ is strong enough, they can cooperate in a combinatorial way.
The two-parameter bifurcation diagrams. The red curves are the saddle-node bifurcation curves. a Bifurcation diagram with ER stresses and kimtor′ as control parameters. b Bifurcation diagram with nutritional stress and kimtor′ as control parameters. Mono-stability corresponding to apoptosis occurs in the region labeled II, while the system is bistable in the region labeled I
4 Discussion
In this paper, we presented a computational model cell fate decisions based on intertwined dynamics with autophagy and apoptosis involving the Ca2+, mTOR, caspases, ER stress, and nutritional stress. Autophagy and apoptosis are important biological processes in mammalian cells. Cells maintaining homeostasis in mammals need such two processes. The crosstalk between them involves many signaling pathways. In previous researches, people mainly consider one kind of stress or perturbation as a single input. In our paper, we consider two different kinds of stresses, ER and nutritional stresses, targeting different nodes. Both signals can switch the state between autophagy and apoptosis in a combinatorial way.
Since the crosstalk between autophagy and apoptosis is very complex, we only focus on the importance of Ca2+ pathway and mTOR pathway. Ca2+ can regulate caspases directly or indirectly through mTOR. These two pathways have different effects on cell fate decisions. More exactly, Ca2+ mediated by mTOR can determine if state transition can occur and whether the switch is reversible, depending on the strength of the stresses. On the other hand, Ca2+ mediated by caspases can only determine when apoptosis occurs.
Finally, we explore the impact of multiple perturbations on cell fate decisions in a combinatorial way. We found that for a larger nutritional stress, a smaller ER stress can induce apoptosis and vice versa, which indicates that apoptosis is perhaps produced by multiple stresses in a combinatorial way. Similar results can be also obtained for the combination of Ca2+ and each of the two stresses. Interestingly, we find that nutritional stress and Ca2+ cooperate in an approximately linear way to induce the occurrence of apoptosis. On the other hand, ER stress and Ca2+ cooperate in a nonlinear way.
In summary, the model presented here incorporates both autophagy and apoptosis and can be used to clarify the mechanisms, relevance, and potential implications of Ca2+ and the ER and nutritional stresses in cell fate decisions individually or in a combinatorial way. In agreement with some experimental observations, the model can account for the choice between autophagy and apoptosis, depending on cooperation of the multiple signals.
References
Mizushima N, Autophagy: process and function. Genes Dev. 21(22), 2861–2873 (2007)
Kundu, M., Kosmatka, M., Bardeesy, N., Hurley, R.L., Witters, L.A., DePinho, R.A., Cantley, L.C.: Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112, 1493–1502 (2008)
Loos, B., Engelbrecht, A.M.: Cell death: A dynamic response concept. Autophagy 5(5), 590–603 (2009)
Loos, B., Engelbrecht, A.M., Lockshin, R.A., et al.: The variability of autophagy and cell death susceptibility. Autophagy 9(9), 1270–1285 (2013)
Tyson, J.J., Baumann, W.T., Chen, C., et al.: Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Cell Commun. Signal. 11(7), 523–532 (2011)
Kapuy, O., Vinod, P.K., Mandl, J., et al.: A cellular stress-directed bistable switch controls the crosstalk between autophagy and apoptosis. Mol. BioSyst. 9(2), 296–306 (2013)
Sun, F., Xu, X., Wang, X., et al.: Regulation of autophagy by Ca2 +. Tumor Biol. 37(12), 15467–15476 (2016)
Kapuy, O., Vinod, P.K.: mTOR inhibition increases cell viability via autophagy induction during endoplasmic reticulum stress-an experimental and modeling study. FEBS Open Bio 4, 704–713 (2014)
Dorvash, M., Farahmandnia, M., Tavassoly, I.: A systems biology roadmap to decode mTOR control system in cancer. Interdiscip. Sci. 9, (2019). https://doi.org/10.1007/s12539-019-00347-6
Nikoletopoulou, V., Markaki, M., Palikaras, K., et al.: Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 1833(12), 3448–3459 (2013)
Bhutia, S.K., Dash, R., Das, S.K., et al.: Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 70(9), 3667–3676 (2010)
Booth, L.A., Tavallai, S., Hamed, H.A., et al.: The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell. Signal. 26(3), 549–555 (2014)
Kaneko, M., Imaizumi, K., Saito, A., et al.: ER stress and disease: Toward prevention and treatment. Biol. Pharm. Bull. 40(9), 1337–1343 (2017)
Orsolya, K., Papp, D., Tibor, V., et al.: Systems-level feedbacks of NRF2 controlling autophagy upon oxidative stress response. Antioxidants 7(3), 39 (2018)
Liu, B., Oltvai, Z.N., Bayr, H., et al.: Quantitative assessment of cell fate decision between autophagy and apoptosis. Sci. Rep. 7(1), (2017). https://doi.org/10.1038/s41598-017-18001-w
Tavassoly, I., Shajahan, A.N., Parmar, J., et al.: Dynamical modeling of the interaction between autophagy and apoptosis in mammalian cells: a systems pharmacology framework. CPT Pharmacometrics Syst. Pharmacol. 4(4), 263–272 (2013)
Tavassoly, I.: Dynamics of cell fate decision mediated by the interplay of autophagy and apoptosis in cancer cells. Springer International Publishing, Switzerland (2015)
Schleich, K., Lavrik, I.N.: Mathematical modeling of apoptosis. Cell Commun. Signal. 11(1), 44–44 (2013)
Zhang, T., Brazhnik, P., Tyson, J.J.: Computational analysis of dynamical responses to the intrinsic pathway of programmed cell death. Biophys. J. 97(2), 415–434 (2009)
Jin, H., Lei, J.: A mathematical model of cell population dynamics with autophagy response to starvation. Math. Biosci. 258, 1–10 (2014)
Kim, J., Kundu, M., Viollet, B.: AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13(2), 132–141 (2011)
Zhu, W., Chen, Q., Liu, Q., Zhou, Y., Yan, Y., et al.: Study on the interaction between autophagy and apoptosis of MC3T3-E1 at starvation state. J North Sichuan Medical College 2, 206–211(2015) (2015)
Wang, Y., Wang, H., Zuo, L., et al.: Role of autophagy in hepatic stellate cell apoptosis induced by endoplasmic reticulum stress. Acta Univ. Med. Anhui 51(8), 1115–1119 (2016)
Zhang, Y., Ren, S., Liu, Y., et al.: Inhibition of starvation-triggered endoplasmic reticulum stress, autophagy, and apoptosis in ARPE-19 cells by taurine through modulating the expression of calpain-1 and calpain-2. Int. J. Mol. Sci. 18(10), 2146 (2017)
Liu, Y.W., et al.: Bifurcation-based approach reveals synergism and optimal combinatorial perturbation. J. Biol. Phys. 42(3), 399–414 (2016)
Luo, M., Jiao, J., Wang, R.: Screening drug target combinations in disease-related molecular networks. BMC Bioinformatics 20(S7), (2019). https://doi.org/10.1186/s12859-019-2730-8
Funding
This research is supported by the National Natural Science Foundation of China (Grant No. 11971297) and the National Science Foundation of Shanghai (Grant No. 17ZR1410800).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
We briefly describe the mathematical models used to study the crosstalk between the autophagy and apoptosis modules. Such a coupled network can be translated into a set of differential equations that describe how each component in the network changes with time. The rate of change of each component is described by ordinary differential equation based on either the law of mass action or Michaelis-Menten kinetics [5, 7, 14, 15].
1.1 Apoptosis
1.2 Autophagy
1.3 The coupled model
Rights and permissions
About this article
Cite this article
Ge, Z., Wang, R. Fate decisions mediated by crosstalk of autophagy and apoptosis in mammalian cells. J Biol Phys 46, 133–149 (2020). https://doi.org/10.1007/s10867-020-09542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-020-09542-9