Abstract
Roughly speaking, restitution is the dependence of recovery time of cardiac electrical activity on heart rate. Increased restitution slope is theorized to be predictive of sudden death after heart injury such as from coronary artery occlusion (ischemia). Adrenaline analogs are known to increase restitution slope in normal hearts, but their effects in failing hearts are unknown. Twenty-six rabbits underwent coronary ligation (n = 15) or sham surgery (n = 11) and implantation of a lead in the heart for recording electrocardiograms. Eight weeks later, unanesthetized rabbits were given 0.25–2.0 ml of 1 μmol/L isoprenaline intravenously, which increased heart rate. Heart rate was quantified by time between QRS peaks (RR) and heart activity duration by R to T peak time (QTp). Ligated rabbits (n = 6) had lower ejection fraction than sham rabbits (n = 7, p < 0.0001) indicative of heart failure, but similar baseline RR (269 ± 15 vs 292 ± 23 ms, p = 0.07), QTp (104 ± 17 vs 91 ± 9 ms, p = 0.1), and isoprenaline-induced minimum RR (204 ± 11 vs 208 ± 6 ms, p = 0.4). The trajectory of QTp vs TQ plots displayed hysteresis and regions of negative slope. The slope of the positive slope region was >1 in ligated rabbits (1.27 ± 0.66) and <1 in sham rabbits (0.35 ± 0.14, p = 0.004). The absolute value of the negative slope was greater in ligated rabbits (− 0.81 ± 0.52 vs − 0.35 ± 0.14, p = 0.04). Isoprenaline increased heart rate and slopes of restitution trajectory in failing hearts. The dynamics of restitution trajectory may hold clues for sudden death in heart failure patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The electrocardiographic QT interval plotted against heart rate or, more commonly, its reciprocal, the beat to beat interval [1, 2], is called QT dynamicity by physicians [1, 3]. Its in vitro (tissue or experimental level) correlate, the duration of action potential plotted against the preceding beat to beat interval or, alternatively, the preceding diastolic interval (beat to beat interval minus the preceding action potential duration) is called the restitution curve. There is a belief that the slope of QT dynamicity could be used to predict sudden cardiac death, based on what is called the restitution hypothesis [4]. Computer simulations and theoretical analyses from nonlinear dynamics determined in the 1980s and early 1990s that a steep (>1) restitution curve slope was conducive to alternans (alternating electrical behavior), reentrant arrhythmias (a type of abnormal heart rhythm), and chaotic behavior [5–8]. An animal study showing that the slope of the restitution curve during ventricular fibrillation was >1 [9] and two animal studies showing that reducing the restitution slope converted ventricular fibrillation to ventricular tachycardia [10, 11] gave further support to the idea that restitution slope was an important factor in the generation of arrhythmias.Footnote 1
There exist human studies partially consistent with the restitution hypothesis. That is, patients going on to suffer sudden cardiac death [12, 13] and patients with long QT syndrome [14] have been found to have steeper slopes of their QT dynamicity curves compared to control subjects. However, theoretical predictions have not been met in human studies in that they have failed to demonstrate QT dynamicity slope values remotely close to 1. One partial explanation is that QT dynamicity plots QT against RR interval rather than TQ interval, which, under most conditions, results in a shallower slope. However, published values of QT/RR slope are too shallow for the difference in choice of independent variable to be the sole reason for producing a slope far smaller than 1. For instance, in the Chevalier study of patients 9–14 days after their myocardial infarction (coronary artery occlusion), median QTe/RR slope was a mere 0.13 [12]. This failure to observe a sufficiently steep slope may explain why some clinical studies find that a shallower, not steeper, QT/RR slope predicts sudden cardiac death [15, 16].
The goal of the present study was to see if QT/TQ slopes greater than the theoretical threshold value of 1 could be observed during beta-adrenergic stimulation in a rabbit model of ischemia-induced congestive heart failure.Footnote 2 Arrhythmia incidence is increased in heart failure, so if the restitution hypothesis were correct, one would expect the restitution curve slope to be increased in heart failure. Contrary to expectation, the restitution curve slope is flattened in experimentally induced congestive heart failure [17], which argues against the restitution hypothesis. However, there are studies in semi-isolated rabbit heart and man that show that stimulation of beta-adrenergic receptors via sympathetic nerve stimulation or adrenaline analogs increases the slope of the restitution curve in normal hearts [18, 19]. Indeed, consistent with the restitution hypothesis, increased sympathetic activity increases ventricular tachyarrhythmias [20, 21] and high parasympathetic activity protects against them [22, 23]. Therefore, we hypothesized that it might be possible in the setting of sympathetically mediated heart rate increase to observe QT/TQ slope >1 in heart failure, despite the known presence of a lower slope in baseline conditions in heart failure. Sudden transient increases in sympathetic nerve activity and adrenaline release from the adrenal gland occur in man throughout the day from postural change, startle, physical exertion, etc. To simulate sympathetically mediated heart rate increase, we used isoprenaline, an adrenaline analog that stimulates only the beta-adrenergic receptors of the heart. We observed restitution both during the departure from and return to steady state and called this the restitution trajectory.
In our study, we deliberately refrained from electrically pacing the heart in order to study truly dynamic, as opposed to static, restitution slope in conscious animals. Standard [24] and so-called dynamic restitution [9] curve slopes are both measured during pacing protocols with a fixed heart rate at their core and are, therefore, essentially static measures. In such static restitution experiments, the pacemaker tissue of the heart has been removed or destroyed so that the experimenter has complete control over the heart rate, and the tissue is made to beat artificially quickly or slowly in the absence of autonomic hormones that regulate heart rate in the intact animal. For example, adrenaline and other sympathetic hormones are necessary to produce high heart rates in the body, but to construct the so-called dynamic restitution curve, electrical pacing is used. Because adrenaline and its analogs are known to shorten QT interval, the QT intervals measured at high heart rate produced by adrenaline in an intact animal should be shorter than QT intervals measured at the same heart rate produced by pacing with no adrenaline present. In other words, by refraining from pacing the tissue, we were able to observe QT intervals at the heart rate dictated by the isoprenaline concentration. We advocate giving nonpaced restitution the new and specific name of endogenous restitution to correspond to what physicians call QT dynamicity in humans.
2 Methods
2.1 Surgical procedure
A well-characterized (with respect to histology, electrophysiology, and cardiac function) model of heart failure induced by chronic left ventricular infarction in the rabbit was used in this study [25–28]. Male New Zealand White rabbits, 3–4 kg in weight (n = 26), were premedicated with a fentanyl–fluanisone combination (Hypnorm, Janssen) 0.3 ml/kg intramuscularly. They were intubated and connected to a respirator delivering oxygen and halothane. Using aseptic technique, the pericardium was opened and usually two but up to four ties (4/0 Ethibond) were placed around the left anterior descending coronary arteries to achieve a blanching of approximately 40% of the ventricular surface (n = 15). Quinidine was given intravenously just prior to each ligation and allowed time to wear off before the pericardial sac was closed. A pediatric defibrillator was used with the paddles in direct contact with the heart when necessary. In the remaining 11 rabbits, sham surgery was conducted in which the pericardium was opened, then sutured closed without coronary ligation.
During the same surgery, bipolar permanent pediatric pacemaker leads (Pacepath 820, Pacesetter Systems) were placed in the right ventricle for future recording of intracardiac electrocardiogram (ECG) following the technique of Manley et al. [29]. Briefly, a pacemaker lead was introduced into the right internal jugular vein via a neck incision after excising the tines. The tip of the lead was positioned in the apex of the right ventricle using X-ray visualization. After confirming the presence of a suitable endocardial electrocardiogram, the pacemaker lead was secured to the jugular vein by sutures. The distal end of the pacemaker lead was tunneled subcutaneously to be exteriorized via a mid-dorsal incision. At the end of the surgical procedures, the rabbit was put in an upper body jacket with zippered pockets for storing and protecting the external parts of the pacemaker leads from damage by the rabbit. Because the pacemaker lead had a ring to tip distance similar to the length of the rabbit ventricle, the recording approximated a unipolar recording of ventricular activation. All surgical, chronic maintenance, and data collection procedures conformed to the standards for animal care in the UK, specifically the Animals (Scientific Procedures) Act of 1986.
2.2 Assessment of cardiac function
Ultrasound was conducted on rabbits in the seventh week after surgery under sedation with Hypnorm. Standard echocardiographic measurements of cardiac dimensions were made. Left ventricular ejection fraction was computed from online planimetry measurements of the left ventricular cavity in the short axis view. Although the sonographer was not informed of the surgical status of the animals, the hypokinetic motion of ventricular walls differentiated ligated rabbits from sham rabbits.
2.3 Electrocardiographic recordings
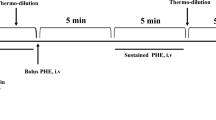
Intracardiac ECG recordings were made in conscious, unsedated animals on two separate occasions, namely, in the seventh and eighth weeks after surgery. The animals were placed in a wooden box slightly larger than the animal with a hole in the front and sides allowing the animal to breathe and see outside. The external pacemaker lead was removed from the jacket and threaded through a hole in the side of the box and connected to an ECG amplifier and data acquisition system (MP100 Biopac Systems, Inc.). ECGs were recorded at a rate of 1,000 Hz with the room lights dimmed and with a minimum of ambient noise. Filtering was set to pass 0.15 to 35 Hz signals. Figure 1 shows the ECG samples obtained from a sham and a ligated animal at baseline. After recording a baseline intracardiac ECG, a Venflon intravenous cannula (22 gauge) was inserted into one marginal ear vein and secured by adhesive tape. When the rabbit’s heart rate returned to baseline, 1.0 μmol/L isoprenaline was given intravenously as a bolus in volumes ranging from 0.25 to 2.0 ml to achieve a target heart rate of 300 bpm. Bolus injections were given when heart rates returned to the baseline heart rate noted before the first injection, and remained stable for at least 3 min. Isoprenaline stock solutions were preserved by addition of ascorbic acid and titrated to achieve a pH of 4.
2.4 Data analysis
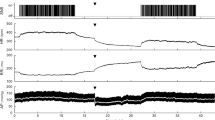
Offline analysis of ECG files was conducted using a program written in the Python programming language. R peaks and T peaks were found automatically and overlaid ten beats at a time to allow manual correction of misclassified peaks. RR interval was measured as the time from R peak to R peak of the QRS waveform. QT interval was approximated by the time from R peak to T peak (QTp). Ectopic and post-ectopic beats obvious on visual inspection were excluded from plots and further data analysis. Figure 2 shows examples of temporal RR and QTp changes produced by four doses of isoprenaline from a sham and a ligated animal.
For each interval of isoprenaline-induced heart rate increase and return to baseline, we plotted QTp against the preceding TQ interval for all of the beats in the interval. Figure 3 shows examples of QTp restitution from a sham and a ligated rabbit below time plots of TQ and QTp. Because hysteresis was seen in the majority of restitution plots, we decided to divide the restitution plot into four segments which we named biphasic, positive, negative, and recovery. Slopes of the four segments were calculated from their endpoints as the difference in QTp divided by the difference in TQ (in milliseconds per millisecond), making use of the fact that the slope formed by two endpoints of an arc gives the average slope of all the tangent slopes of the arc. The figure legends for Fig. 3 describe the criteria used to define the five points used to mark the start and end of the four segments. We also calculated time spent in each of the four segments and divided RR difference and QTp difference by these times to obtain rates of change of RR and of QTp (in milliseconds per second). For each rabbit, we averaged the slope and rate values over all of the isoprenaline challenges without regard to volume of isoprenaline given, for several reasons. One was poor reproducibility of dose effects between the seventh and eighth weeks in the same animal. Another was the difference between animals in heart rate response to identical dosages, resulting in higher volumes of isoprenaline being required to reach the target heart rate of 300 in some animals than others. We used unpaired t tests for comparisons of sham and ligated rabbits. Statistical significance was inferred at p < 0.05. Mean values are reported with standard deviation values.
Examples of isoprenaline-induced QTp, TQ changes (top), and restitution trajectories (bottom) from a sham surgery (left) and a coronary ligation surgery (right) rabbit. Overlaid are the five points used to define the four segments of restitution plot hysteresis. Point 1 maximum RR before RR decrease due to isoprenaline, Point 2 maximum QTp, Point 3 minimum TQ, Point 4 minimum QTp, Point 5 whichever of RR or QTp that recovered first to point 1. The biphasic segment was point 1 to point 2. The positive segment was point 2 to point 3. The negative segment was point 3 to point 4. The recovery segment was point 4 to point 5
3 Results
3.1 Study population
None of the 11 sham surgery rabbits and five of the 15 ligation surgery rabbits died peri-operatively. Three of the perioperative deaths were caused by defibrillator malfunction. Electrocardiograms were not obtainable in one sham surgery and in two ligation surgery rabbits because of destruction of the exterior portion of the pacemaker lead by the rabbit. The T wave was not clearly identifiable in two sham and two surgery rabbits. One sham surgery rabbit was further excluded from analysis because the drops in RR value with isoprenaline challenge were >180 ms (Fig. 4). RR and QTp intervals were measured in the remaining seven sham and six ligation surgery rabbits. Although each rabbit was challenged with isoprenaline at least six times (0.25–2.0 ml in two consecutive weeks), restitution slope as defined by our model of four segments was not analyzable approximately half of the time due to a variety of reasons. They were: lack of heart rate increase, stepped (nonmonotonic) increases in heart rate, lack of QTp decrease, loss of measurable T wave peak at fast heart rate due to reduction in T wave amplitude and convergence with P wave, and in one rabbit, the appearance of T wave alternans at fast heart rate. Restitution hysteresis that did not exhibit a negative slope region (coincidence of TQ minimum and QTp minimum) was also excluded from analysis. The range of number of isoprenaline challenges averaged per rabbit was 2 to 5.
Reduction in RR value (RR drop = baseline RR − minimum RR) achieved after intravenous infusions of 1 μmol/L isoprenaline plotted against volume of infusion for eight sham (32 isoprenaline challenges) and six ligated (23 isoprenaline challenges) rabbits. The two points representing RR drops >180 were from the same rabbit. This rabbit was excluded from further analysis because inclusion (exclusion) produced a statistically significant (insignificant) difference in RR drop between ligated and sham rabbits with a p value of 0.025 (p = 0.075)
Baseline and experimental characteristics of the rabbits are given in Table 1. To summarize, left ventricular ejection fraction was significantly greater in the sham than in the ligated rabbits. Baseline heart rate had a tendency to be lower in the sham than in the ligated rabbits (p = 0.07). Baseline QTp had a tendency to be shorter in the sham than in the ligated rabbits (p = 0.1). These three results were as expected. Sham and ligated rabbits did not differ in volumes of isoprenaline given (p = 0.6) nor in minimum RR achieved (p = 0.4). Figure 4 shows the drop in RR from baseline to minimum plotted against isoprenaline dose (sham n = 32, ligation n = 23). The tendency for isoprenaline-induced RR drop to be greater for sham rabbits can be seen in this figure.
3.2 Restitution slopes and rates of change
Table 2 gives the QTp/TQ restitution slopes and the rates of change of RR and QTp intervals for the four hysteresis segments. Figure 5 shows box plots of these three quotients for the biphasic, positive, and negative segments. We describe the segments in turn.
Box plots of restitution slopes and rates of RR and QTp interval change per unit time in seconds for six ligation surgery and seven sham surgery rabbits. Within each panel, from left to right are the distribution of values for the biphasic hump segment, positive slope segment, and negative slope segment of QTp hysteresis. The box edges represent the 25th and 75th percentile, the horizontal line within the box represents the median, the whiskers represent tenth and 90th percentile, and the discrete points represent all of the values outside the tenth to 90th percentile. See Table 2 for the mean and standard deviation values of these parameters
3.2.1 Biphasic hump
For the initial segment where QTp increased although RR had begun to decrease, there was no statistically significant difference between sham and ligated rabbits in restitution slope, rate of RR drop, or rate of QTp increase.
3.2.2 Positive slope
For the classically defined restitution segment where both RR and QTp decreased, restitution slope was significantly greater for ligated rabbits, with an average slope value of 1.27 ± 0.66 for ligated and 0.35 ± 0.14 for sham rabbits (p = 0.0040). The rate of RR decrease was similar (p = 0.5), but QTp drop rate was significantly faster (− 3.0 ms/s) in ligated rabbits than in sham rabbits (− 1.9 ms/s, p = 0.040).
3.2.3 Negative slope
For the restitution segment where QTp continued to decrease although RR had begun to recover, restitution slope was significantly greater for ligated rabbits with an average slope value of −0.81 ± 0.52 for ligated vs −0.35 ± 0.14 for sham rabbits (p = 0.04). The rate of RR increase was similar (p = 0.8), but QTp drop rate was significantly faster (−2.6 ms/s) in ligated rabbits than in sham rabbits (−0.95 ms/s, p = 0.02). A paired comparison of QTp drop rate for the ligated rabbits between the positive and negative slope segments produced a p value of 0.47 and, for the sham rabbits, 0.02. This suggested that, in ligated rabbits, QTp continued to drop at a similar rate after the TQ minimum had been reached, while in sham rabbits, the rate of QTp reduction per time tapered off significantly, once the TQ minimum had been reached.
3.2.4 Recovery slope
For the restitution segment where both RR and QTp increased in tandem, no significant differences were seen in any of the parameters calculated. The restitution slope averages were 0.61 for ligated and 0.25 for sham rabbits.
Time spent in each of the four segments was statistically similar for sham and ligated rabbits, as was change in RR, suggesting that the difference in magnitude or rate of RR response to isoprenaline could not account for the difference in restitution slope and rate of QTp change between sham and ligated rabbits.
4 Discussion
As stated in Section 1, the goal of the present study was to see if a restitution slope >1 could be observed during beta-adrenergic stimulation in a rabbit model of ischemia-induced congestive heart failure. We found that isoprenaline infusion indeed produced an average restitution slope in the positive segment >1 (1.27) in heart failure rabbits and a slope <1 (0.35) in sham-operated rabbits. In addition, we observed a clear region of negative slope in many of the isoprenaline challenges that led to hysteresis in the restitution trajectory. The slope of the negative segment was also steeper in heart failure rabbits. The positive and negative restitution slopes were steeper in the heart failure rabbits despite the tendency for failing rabbits to start with a faster baseline heart rate, leading them to undergo smaller increases of heart rate to the 300-bpm target heart rate. Because failing and sham surgery rabbits displayed similar rates of heart rate change over those hysteresis segments and because the two groups received similar doses of isoprenaline, we were able to exclude those factors as causes for the difference in restitution slopes as well. In contrast, rate of change of QTp (QTp change per unit time) was higher in the heart failure rabbits for the positive and negative slope segments and also for the biphasic segment, albeit with a p value of 0.07, suggesting that greater magnitude of QTp dynamics itself was a core feature of failing hearts, contributing to the steeper restitution.
4.1 Endogenous restitution
The term restitution has of late, come to be used to describe any relationship between heart rate and some measure of repolarization, such as action potential duration, refractory period, or electrocardiographic QT interval. Heart rate is usually quantified by its reciprocal, the beat to beat interval or diastolic interval,Footnote 3 which has units of time. Beyond the choice of particular independent and dependent variables, there are also different categories of restitution, depending on the stimulation protocol used to vary the heart rate. Originally, restitution referred solely to the relationship between a single beat change in heart rate and repolarization. Such restitution curves were obtained by electrically pacing cardiac tissue at a constant heart rate, followed by one perturbatory stimulus at a different heart rate, recording the repolarization measure of the perturbed beat, then repeating the constant rate–single perturbed rate sequence with varied timing of the perturbatory stimulus [30–32]. Such a restitution curve is not unique. The position of the restitution curve depends on the constant heart rate used before the perturbatory stimulus, a phenomenon attributed to cardiac memory, the name of which suggests that the tissue has a memory of the underlying heart rate [32]. To prevent confusion caused by the recent expansion in the meaning of the term restitution, we have suggested that this type of classical restitution be renamed “standard restitution” [24]. Ventricular premature contractions occurring spontaneously in otherwise stable heart rate conditions in man can produce a standard restitution function, so long as the coupling interval of the premature beat varies widely enough to provide a spread of points.
A second category of restitution refers to the relationship between constant heart rate and repolarization. This type of restitution is obtained by pacing cardiac tissue at a constant heart rate until the repolarization measure stabilizes, then repeating this for other heart rates [33]. The plot of this relationship was originally called the steady state action potential duration curve [32]. In practice, investigators do not wait for the repolarization measure to stabilize completely, but deliver a fixed number of pacing stimuli (usually eight to 20), then move on to the next heart rate. Koller et al. called the heart rate–repolarization relationship obtained in this manner, dynamic restitution [9], and thereby expanded the meaning of the term restitution. The closest analog of dynamic restitution in man would be a plot of QT intervals compiled from different stable heart rates, such as found over the day. The equation used to derive Bazett’s formula, QT = 0.44 × square root of RR, could be regarded as the dynamic restitution curve for a population [34].
In the current study, we studied restitution under unpaced conditions. Currently, there is no special name for restitution that exists under unpaced conditions, although it has been studied by many, both in vivo and in vitro. We suggest calling this type of restitution endogenous restitution because it represents the behavior of the QT interval when it is allowed to change in lock step with spontaneous changes of heart rate. In the intact animal, heart rate varies frequently throughout the day, after a change in posture, during exercise or emotional duress, or after startle by a large sound. In the intact animal, changes in heart rate are mediated by the autonomic (sympathetic and parasympathetic) nervous system, which affects the QT interval independently of heart rate. Endogenous restitution, therefore, describes the heart rate–repolarization relationship that exists within us at any moment in time. Plots of action potential duration against preceding cycle length during experimentally induced fibrillation should also be called endogenous restitution, since pacing is turned off after fibrillation is initiated.
The relationship between endogenous restitution and the two types of classical restitution described above is complex. As mentioned in Section 1, standard and dynamic restitution are static measures. They represent a collection of steady state points at which cardiac memory is fixed. If one plots both standard and dynamic restitution curves on one graph [32, 33, 36], the spaces between the curves represent what is dynamically possible, everything that is not steady state, where cardiac memory is in flux. If, to simplify the argument, we assume that repolarization is not affected by autonomic input, then endogenous restitution represents a trajectory through this space during transitions between the various steady states mapped out on that graph. Some studies have analyzed this space as a succession of shifting standard restitution curves [35] or captured a “portrait” of this space by recording the behavior of trajectories formed by stepped transitions between different heart rates [36].
Our current study is unique in many ways. Heart rate changed continuously. The only study we know of in which restitution was measured during continuous heart rate change is by Lux and Ershler, who demonstrated the effects of applying a linear ramp pacing protocol to repolarization in the dog [37]. Isoprenaline added a third dimension to the restitution space, and isoprenaline concentration also changed continuously. Dynamically, we used a transient stimulus to perturb the heart and followed its trajectory back to steady state, instead of studying the transition from one steady state to a different one.
4.2 Negative slope in restitution
Hysteresis in restitution is a well-known phenomenon [38]. Its presence complicates the definition and measurement of restitution slope, and several different methods have been developed for circumnavigating this problem [39–42]. We believe that our current report marks the first time the existence of negative restitution has been reported in vivo for any category of restitution. Negative restitution simply represents continued QT decrease as heart rate begins its return to slower rate, i.e., it is nothing but QT response lagging behind that of RR, of the sinus node recovering more quickly than the ventricular myocardium. Figure 6 shows a cobweb map of the dynamics of negative restitution slope. Under adrenergic stimulation, cycle length is shortening with each beat and restitution curves are being shifted down with each beat as well. Starting at point (TQn, QTn), TQn + 1 is determined by RR[n] − QTn (horizontal line). QTn + 1 is determined by restitution curve n + 1 (vertical line). Iteration of this analysis shows how negative restitution can be obtained. If the restitution curve shift is greater than the cycle length reduction, a negative slope results. Negative slope has been reported in in vitro pacing studies in which pacing was switched from a slower rate to a faster rate [35, 36, 43] and is a special case of this cobweb example, in which cycle length reduction is zero. The slope was approximately − 1 in all three studies because fixed cycle length dictated that gradual reductions in action potential duration due to cardiac memory led to equivalent increases in diastolic interval [36]. In our opinion, demonstration of the existence of negative restitution is important because it guarantees the existence of a point of infinite magnitude slope between the positive and negative segments. The heartbeats themselves jump between discrete points on this curve, but the curve itself is continuous, and the fact that the slope passes through infinity implies that the ratio of difference in QT to difference in diastolic interval between successive beats can be arbitrarily large. Although a slope >1 implies instability and arrhythmia susceptibility in standard and dynamic restitution curves, the theoretical implications of having a large slope in endogenous restitution curves are entirely unknown at this time and are a subject for future research.
Cobweb map showing the dynamics of negative restitution slope. Under adrenergic stimulation, cycle length is shortening with each beat and restitution curves are being shifted down with each beat as well. Starting at point (TQn, QTn), TQn + 1 is determined by RR[n] − QTn (horizontal line). QTn + 1 is determined by restitution curve n + 1 (vertical line). Iteration of this analysis shows how negative restitution can be obtained. If the restitution curve shift is greater than the cycle length reduction, a negative slope results. The presence of negative restitution slope in paced studies [35, 36, 43] is a special case of this example in which cycle length reduction is zero
4.3 Effects of adrenergic stimulation on repolarization
Isoprenaline has been used frequently over the years to mimic sympathetic activity. Because computerized measurement techniques did not exist, early studies focused largely on measurements of discrete time points after isoprenaline infusion. Nevertheless, hysteresis was noted (RR decrease before QT) [44], as was dose-dependent reduction in action potential duration in fixed atrial pacing [45] and presence of biphasic change in action potential duration (i.e., an increase preceding the decrease) [46]. Sympathetic nerve stimulation was shown to shorten refractory period in epicardial and endocardial tissue by similar amounts [47] and produce biphasic change in monophasic action potential duration despite fixed atrial rate [48]. More recent studies indicate emerging interest in the restitution slope. Taggart et al. [49] reported a value of 1.05 for the standard restitution slope using programmed stimulation in man and demonstrated that it increased with isoprenaline infusion. Ng et al. [50] reported that sympathetic nerve stimulation led to reduced refractory period, increased restitution slope, and reduced ventricular fibrillation threshold. They further found a significant association between restitution slope and ventricular fibrillation threshold. With regard to ionic mechanism, isoprenaline’s action on repolarization has been shown to depend on the slowly activating component of the delayed rectifier potassium current (IKs). Isoprenaline enhances IKs in a dose-dependent fashion, negatively shifts IKs activation voltage dependence, and accelerates IKs activation [51]. Consistent with this result, shortening of repolarization by isoprenaline is prevented by IKs block in vitro and in vivo [52].
4.4 Limitations
We were forced to choose the T peak as our measure of repolarization rather than the time from R peak to end of the T wave because, at fast heart rates, the T wave end became impossible to distinguish from the start of the following negative P wave. Even using the T peak, we were unable to analyze data at high heart rates in some isoprenaline challenges. A study in man reporting that the slope of daytime Q–T peak vs RR did not predict sudden death after myocardial infarction in contrast to Q–T end [12] would make Q–T end preferable for study. However, from a restitution hypothesis viewpoint, there is no reason to believe that QT dynamicity calculated using T end is superior to T peak in predicting arrhythmia vulnerability, since both reflect action potential duration in some sections of the heart according to recent studies, e.g., in one in vivo study in swine utilizing recordings from multiple endocardial and epicardial sites, it was demonstrated that the peak and end of the T wave coincided, respectively, with the times of earliest and latest end of repolarization over the whole heart [53]. In another study, the T peak and T end were reported to correspond to end of repolarization in epicardium and M cells, respectively [54]. In a previous study using New Zealand White rabbits, the same breed used in the current study, the stimulus to T peak time recorded by the intracardiac pacemaker lead and action potential duration measured from transmembrane recordings in six ventricular septa were related by the regression equation, stimulus_to_Tpeak = 0.9 × APD[100%] + 25 (ms), r = 0.96, over an APD range of 200–450 ms [29]. Finally, work by Nearing and Verrier [55] suggests that T wave changes such as T wave alternans are minimal in the terminal portion and that the greatest amplitude differences reside in the segment preceding the peak (see Fig. 1 of the reference). For these various reasons, we feel there is justification in using Q–T peak. Furthermore, our results may have applications to studies of repolarization dynamics in rodents whose high heart rates make T wave end hard to identify.
A second limitation of our study was that many isoprenaline challenges were excluded from the final analysis for the six cases enumerated in Section 3. We discuss three of them here in more detail. (1) We do not know why stepped increases in heart rate occurred. Perhaps the animals were startled by the pharmacologically induced sudden increase in heart rate and contributed their own sympathetic surge. In these cases, there were multiple measurable restitution slopes, but it was unclear how to treat them statistically. (2) In cases of lack of QTp decrease, we considered treating them as zero-valued restitution slope, but then the question arose of whether it made sense to give values of 0 to all four slopes when they were ill-defined in the case of lack of QTp change, e.g., recovery slope defined as recovery of both RR and QTp would have to be redefined as recovery of RR only. On the other hand, there is precedence in analyzing only measurable responses in human data. Spontaneous baroreflex sensitivity is calculated as the slope between blood pressure and heart rate only for time segments over the 24-h record in which both are increasing. Baroreflex sensitivity measured in this way correlates closely with measurements using pharmacological challenges [56] and suggests one way of dealing with the unpredictability of cardiac responses displayed in vivo. (3) Loss of measurable T wave peak at fast heart rate was the most frustrating because there was a clear response in heart rate and QTp and a technique for quantifying repolarization other than intracardiac ECG might have allowed us to follow their values to their minimum. Exclusions due to this reason happened at higher doses of isoprenaline and might have been mitigated by increasing the number of challenges using lower doses, if we had had real-time QTp measurement capabilities.
A third limitation of our study was that we were unable to prove a direct correlation between arrhythmias and steeper restitution slope by demonstrating arrhythmias in the rabbits with steeper slope. We did not have telemetric ECG recordings that would have enabled us to compare arrhythmia frequency between rabbits with steeper and shallower slopes.
5 Conclusion
Rate of change of QTp per unit time and QTp restitution slope during beta-adrenergic stimulation are both greater in heart failure rabbits than in control rabbits. The fact that the positive QTp restitution slope was >1 in the heart failure rabbits is compatible with the increased propensity for arrhythmic death in patients with heart failure, according to the restitution hypothesis.
Notes
Ventricular fibrillation is an irregular rhythm of the heart which is a major cause of sudden death. Ventricular tachycardia is a fast periodic rhythm that frequently leads to ventricular fibrillation.
Adrenaline is one of the naturally occurring hormones in the body that causes heart rate to increase by stimulating receptors in the heart, one of which is the beta-adrenergic receptor. Ischaemia means lack of blood flow, such as from clogged arteries. Ischaemia stresses or injures tissue.
To apply nonlinear dynamical theory, restitution slope must be calculated using the diastolic interval as the independent variable, and not beat to beat interval. That said, in steady state pacing experiments, the choice of independent variable does not affect the slope value because the restitution curve is merely shifted horizontally by a constant.
Abbreviations
- ECG:
-
electrocardiogram
- RR:
-
time from R peak to R peak on electrocardiogram
References
Maison-Blanche, P., Coumel, P.: Changes in repolarization dynamicity and the assessment of the arrhythmic risk. PACE 20, 2614–2624 (1997)
Moleiro, F., Misticchio, F., Castellanos, A., Myerburg, R.J.: Paradoxical behavior of the QT interval during exercise and recovery and its relationship with cardiac memory. Clin. Cardiol. 22, 413–416 (1999)
Fauchier, L., Babuty, D., Poret, P., Autret, M.L., Cosnay, P., Fauchier, J.P.: Effect of verapamil on QT interval dynamicity. Am. J. Cardiol. 83, 807–808 (1999)
Weiss, J.N., Garfinkel, A., Karagueuzian, H.S., Qu, Z., Chen, P.-S.: Chaos and the transition to ventricular fibrillation: a new approach to antiarrhythmic drug evaluation. Circulation 99, 2819–2826 (1999)
Guevara, M.R., Ward, G., Shrier, A., Glass, L.: Electrical alternans and period-doubling bifurcations. IEEE Comp. Cardiol. 562, 167–170 (1984)
Chialvo, D.R., Gilmour, R.F., Jalife, J.: Low dimensional chaos in cardiac tissues. Nature 343, 653–657 (1990)
Karma, A.: Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 4, 461–472 (1994)
Watanabe, M., Otani, N.F., Gilmour, R.F. Jr.: Biphasic restitution of action potential duration and complex dynamics in ventricular myocardium. Circ. Res. 76, 915–921 (1995)
Koller, M.L., Riccio, M.L., Gilmour, R.F. Jr.: Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am. J. Physiol. 275, H1635–1642 (1998)
Riccio, M.L., Koller, M.L., Gilmour, R.F., Jr.: Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ. Res. 84, 955–963 (1999)
Garfinkel, A., Kim, Y.-H., Voroshilovsky, O., Qu, Z., Kil, J.R., Lee, M.-H., Karagueuzian, H.S., Weiss, J.N., Chen, P.-S.: Preventing ventricular fibrillation by flattening cardiac restitution. Proc. Natl. Acad. Sci. U. S. A. 97, 6061–6066 (2000)
Chevalier, P., Burri, H., Adeleine, P., Kirkorian, G., Lopez, M., Leizorovicz, A., André-Fouët, X., Chapon, P., Rubel, P., Touboul, P.: QT dynamicity and sudden death after myocardial infarction: results of a long-term follow-up study. J. Cardiovasc. Electrophysiol. 14, 227–233 (2003)
Milliez, P., Leenhardt, A., Maison-Blanche, P., Vicaut, E., Badilini, F., Siliste, C., Benchetrit, C., Coumel, P.: Usefulness of ventricular repolarization dynamicity in predicting arrhythmic deaths in patients with ischemic cardiomyopathy (from the European Myocardial Infarct Amiodarone Trial). Am. J. Cardiol. 95, 821–826 (2005)
Merri, M., Moss, A.J., Benhorin, J., Locati, E., Alberti, M., Badilini, F.: Relation between ventricular repolarization duration and cardiac cycle length during 24-hour holter recordings. Circulation 85, 1816–1821 (1992)
Tavernier, R., Jordaens, L., Haerynck, F., Derycke, E., Clement, D.L.: Changes in the QT interval and its adaptation to rate, assessed with continuous electrocardiographic recordings in patients with ventricular fibrillation, as compared to normal individuals without arrhythmias. Eur. Heart J. 18, 994–999 (1997)
Smetana, P., Pueyo, E., Hnatkova, K., Batchvarov, V., Laguna, P., Malik, M.: Individual patterns of dynamic QT/RR relationship in survivors of acute myocardial infarction and their relationship to antiarrhythmic efficacy of amiodarone. J. Cardiovasc. Electrophysiol. 15, 1147–1154 (2004)
Marchlinski, F.E., Cain, M.E., Falcone, R.A., Corky, R.F., Spear, J.F., Josephson, M.E.: Effects of infarction, procainamide, coupling interval and cycle length on refractoriness of extrastimuli. Am. J. Physiol. 248, H606–613 (1985)
Kieran, E.B., Coote, J.H., Ng, G.A.: Is electrical restitution the key determinant in ventricular fibrillation initiation? Electrophysiological studies on the effects of autonomic stimulation on isolated rabbit hearts. Circulation 104, II–48 (2001)
Taggart, P., Sutton, P., Simon, R., Eliot, D., Gill, J.: Effect of beta-adrenergic stimulation on the action potential restitution curve in humans. Circulation 104, II–48 (2001)
Lown, B., Verrier, R.L.: Neural activity and ventricular fibrillation. N. Engl. J. Med. 294, 1165–1170 (1976)
Zipes, D.P., Miyazaki, T.: The autonomic nervous system and the heart. Basis for understanding interactions and effects on arrhythmia development. In: Zipes, D.P., Jalife, J. (eds.) Cardiac Electrophysiology: From Cell to Bedside, p. 312. Saunders, Philadelphia (1990)
Kent, K.M., Smith, E.R., Redwood, D.R., Epstein, S.E.: Electrical stability of acutely ischemic myocardium. Influences of heart rate and vagal stimulation. Circulation 47, 291–298 (1973)
Vanoli, E., De Ferrari, G.M., Stramba-Badiale, M., Hull, S.S. Jr., Foreman, R.D., Schwartz, P.J.: Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 68, 1471–1478 (1991)
Watanabe, M.A.: Standard restitution curves during action potential duration alternans. Heart Rhythm 3, 720–721 (2006)
Pye, M.P., Black, M., Cobbe, S.M.: Comparison of in vivo and in vitro haemodynamic function in experimental heart failure: use of echocardiography. Cardiovasc. Res. 31(6), 873–881 (1996)
Pye, M.P., Cobbe, S.M.: Arrhythmogenesis in experimental models of heart failure: the role of increased load. Cardiovasc. Res. 32(2), 248–257 (1996)
Ng, G.A., Cobbe, S.M., Smith, G.L.: Non-uniform prolongation of intracellular Ca 21 transients recorded from the epicardial surface of isolated hearts from rabbits with heart failure. Cardiovasc. Res. 37, 489–502 (1998)
McIntosh, M.A., Cobbe, S.M., Smith, G.L.: Heterogeneous changes in action potential and intracellular Ca2+ in left ventricular myocyte sub-types from rabbits with heart failure. Cardiovasc. Res. 45(2), 397–409 (2000)
Manley, B.S., Chong, E.M.F., Walton, C., Economides, A.P., Cobbe, S.M.: An animal model for the chronic study of ventricular repolarisation and refractory period. Cardiovasc. Res. 23, 16–20 (1989)
Mendez, C., Gruhzit, C.C., Moe, G.K.: Influence of cycle length upon refractory period of auricles, ventricles, and A–V node in the dog. Am. J. Physiol. 184, 287–295 (1956)
Boyett, M.R., Jewell, B.R.: A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J. Physiol. 285, 359–380 (1978)
Elharrar, V., Surawicz, B.: Cycle length effect on restitution of action potential duration in dog cardiac fibers. Am. J. Physiol. 244, H782–H792 (1983)
Hoffman, B.F., Suckling, E.E.: Effect of heart rate on cardiac membrane potentials and the unipolar electrogram. Am. J. Physiol. 179, 123–130 (1954)
Bazett, H.C.: An analysis of the time relationship of electrocardiograms. Heart 7, 353–370 (1920)
Watanabe, M.A., Koller, M.L.: A mathematical analysis of the dynamics of cardiac memory and accommodation. Theory and experiment. Am. J. Physiol. 282, H1534–H1547 (2002)
Kalb, S.S., Dobrovolny, H.M., Tolkacheva, E.G., Idriss, S.F., Krassowska, W., Gauthier, D.J.: The restitution portrait: a new method for investigating rate-dependent restitution. J. Cardiovasc. Electrophysiol. 15, 698–709 (2004)
Lux, R.L., Ershler, P.R.: Cycle length sequence dependent repolarization dynamics. J. Electrocardiol. 36 Suppl, 205–208 (2003)
Hall, G.M., Bahar, S., Gauthier, D.J.: Prevalence of rate-dependent behaviors in cardiac muscle. Phys. Rev. Lett. 82, 2995–2998 (1999)
Neilson, J.M.: Dynamic QT interval analysis. In: Osterhues, H.H., Hombach, V., Moss, A.J. (eds.) Advances in Non-invasive Electrocardiographic Monitoring Techniques. Kluwer Academic, Dordrecht (2000)
Lang, C.C.E., Flapan, A.C., Neilson, J.M.M.: The impact of QT lag compensation on dynamic assessment of ventricular repolarization: reproducibility and the impact of lead selection. PACE 24, 366–373 (2001)
Pueyo, E., Smetana, P., Laguna, P., Malik, M.: Estimation of the QT/RR hysteresis lag. J. Electrocardiol. 36, 187–190 (2003)
Fossa, A.A.: The impact of varying autonomic states on the dynamic beat-to-beat QT–RR and QT–TQ interval relationships. Br. J. Pharmacol. 154, 1508–1515 (2008)
Vick, R.L.: Action potential duration in canine Purkinje tissue: effects of preceding excitation. J. Electrocardiol. 4(2),105–115 (1971)
Biberman, L., Sarma, R.N., Surawicz, B.: T-wave abnormalities during hyperventilation and isoproterenol infusion. Am. Heart J. 81, 166–174 (1971)
Giotti, A., Ledda, F., Mannaioni, P.F.: Effects of noradrenaline and isoprenaline, in combination with α- and β-receptor blocking substances, on the action potential of cardiac Purkinje fibres. J. Physiol. 229, 99–113 (1973)
Murayama, M., Mashima, S., Shimomura, K., Takayanagi, K., Tseng, Y.Z., Murao, S.: An experimental model of giant negative T wave associated with QT prolongation produced by combined effect of calcium and isoproterenol. Jpn. Heart J. 22, 257–265 (1981)
Martins, J.B., Zipes, D.P.: Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ. Res. 46, 100–110 (1980)
Murayama, M., Harumi, K., Mashima, S., Shimomura, K., Murao, S.: Prolongation of ventricular action potential due to sympathetic stimulation. Jpn. Heart J. 18, 259–265 (1977)
Taggart, P., Sutton, P., Chalabi, Z., Boyett, M.R., Simon, R., Elliott, D., Gill, J.S.: Effect of adrenergic stimulation on action potential duration restitution in humans. Circulation 107, 285–289 (2003)
Ng, G.A., Brack, K.E., Patel, V.H., Coote, J.H.: Autonomic modulation of electrical restitution, alternans, and ventricular fibrillation initiation in the isolated heart. Cardiovasc. Res. 73, 750–760 (2007)
Han, W., Wang, Z., Nattel, S.: Slow delayed rectifier current and repolarization in canine cardiac Purkinje cells. Am. J. Physiol. 280, H1075–H1080 (2001)
Volders, P.G., Stengl, M., van Opstal, J.M., Gerlach, U., Spätjens, R.L., Beekman, J.D., Sipido, K.R., Vos, M.A.: Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation 107, 2753–2760 (2003)
Xia, Y., Liang, Y., Kongstad, O., Liao, Q., Holm, M., Olsson, B., Yuan, S.: In vivo validation of the coincidence of the peak and end of the T wave with full repolarisation of the epicardium and endocardium in swine. Heart Rhythm 2, 162–169 (2005)
Shimizu, W.: Effects of sympathetic stimulation on various repolarization indices in the congenital long QT syndrome. Ann. Noninvasive Electrocardiol. 7, 332–342 (2002)
Nearing, B.D., Verrier, R.L.: Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J. Appl. Physiol. 92, 541–549 (2002)
Pitzalis, M.V., Mastropasqua, F., Passantino, A., Massari, F., Ligurgo, L., Forleo, C., Balducci, C., Lombardi, F., Rizzon, P.: Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation 97(14), 1362–1367 (1998)
Acknowledgements
The experimental portion of this research project was conducted at Glasgow Royal Infirmary, with the financial support of British Heart Foundation Project Grant PG/02/155 and Stuart M. Cobbe. The analytical portion of this research project was conducted at St. Louis University School of Medicine. TK received salary support from Sankyo Co, Ltd., Shizuoka, Japan. The authors thank Robert F. Gilmour Jr. and Paul Belk for expert advice concerning the manuscript.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author contributions
All authors contributed to the study: TK and NN in the analysis and interpretation of data, MNH and MD in the conception and design of the study and in conducting the experiments, SMC in the conception and design of the study, and MAW in all of the above and in writing the computer program for data analysis. All authors also read the manuscript critically and approved the final version, with the exception of MNH, who passed away before the study was completed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimotsuki, T., Niwa, N., Hicks, M.N. et al. Isoprenaline increases the slopes of restitution trajectory in the conscious rabbit with ischemic heart failure. J Biol Phys 36, 299–315 (2010). https://doi.org/10.1007/s10867-009-9185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-009-9185-5