Abstract
Interventions have modest impact on reducing excessive gestational weight gain (GWG) in pregnant women with overweight/obesity. This two-arm feasibility randomized control trial tested delivery of and compliance with an intervention using adapted dosages to regulate GWG, and examined pre-post change in GWG and secondary outcomes (physical activity: PA, energy intake: EI, theories of planned behavior/self-regulation constructs) compared to a usual care group. Pregnant women with overweight/obesity (N = 31) were randomized to a usual care control group or usual care + intervention group from 8 to 2 weeks gestation and completed the intervention through 36 weeks gestation. Intervention women received weekly evidence-based education/counseling (e.g., GWG, PA, EI) delivered by a registered dietitian in a 60-min face-to-face session. GWG was monitored weekly; women within weight goals continued with education while women exceeding goals received more intensive dosages (e.g., additional hands-on EI/PA sessions). All participants used mHealth tools to complete daily measures of weight (Wi-Fi scale) and PA (activity monitor), weekly evaluation of diet quality (MyFitnessPal app), and weekly/monthly online surveys of motivational determinants/self-regulation. Daily EI was estimated with a validated back-calculation method as a function of maternal weight, PA, and resting metabolic rate. Sixty-five percent of eligible women were randomized; study completion was 87%; 10% partially completed the study and drop-out was 3%. Compliance with using the mHealth tools for intensive data collection ranged from 77 to 97%; intervention women attended > 90% education/counseling sessions, and 68–93% dosage step-up sessions. The intervention group (6.9 kg) had 21% lower GWG than controls (8.8 kg) although this difference was not significant. Exploratory analyses also showed the intervention group had significantly lower EI kcals at post-intervention than controls. A theoretical, adaptive intervention with varied dosages to regulate GWG is feasible to deliver to pregnant women with overweight/obesity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rates of obesity in United States adults ages 20–59 years are higher among women (38–41%) than men (34–38%; Hales et al., 2017). One explanation for this disparity is that women have a greater susceptibility for weight gain, weight loss/regain, and long-term weight retention as they transition to adulthood and childbearing years (Meldrum et al., 2017). Gestational weight gain (GWG), weight gain that is needed for a healthy pregnancy, is a critical factor that increases women’s obesity risk. The Institute of Medicine (IOM; Rasmussen & Yaktine, 2009) recommends weight gain ranges for women with underweight (body mass index [BMI] < 18.5: 12.5–18 kg), normal weight (BMI 18.5–24.9: 11.5–16 kg), overweight (BMI 25.0–29.9: 7–11.5 kg), and obesity (BMI > 30.0: 5.0–9.0 kg). Pregnant women with overweight and obesity are at increased risk for excessive GWG, and experience unique challenges with weight regulation, which in turn, elevates risks for adverse outcomes (e.g., preeclampsia, gestational diabetes), postpartum weight retention, and long-term obesity (Butte et al., 2003; Gunderson & Abrams, 1999; Rasmussen & Yaktine, 2009). There is a critical need for interventions that effectively regulate GWG.
Efforts to prevent excessive GWG as reported in randomized control trials (e.g., Farpour-Lambert et al., 2018; Vincze et al., 2019) have had modest effects on GWG in women with normal pre-pregnancy BMI and little success among pregnant women with overweight/obesity (e.g., Herring et al., 2016; Peaceman et al., 2018; Phelan et al., 2011; Vesco et al., 2014). There is also emerging evidence from several qualitative and prospective cohort studies (e.g., Chang et al., 2015; Lindsay et al., 2015; Nagourney et al., 2019; Symons Downs et al., 2014) that pregnant women with overweight/obesity often over-estimate the amount of weight they should gain, under-estimate energy intake, have low motivation for eating healthy and engaging in physical activity, and have difficulties self-regulating energy intake and expenditure. Further exacerbating the problem is that GWG and fetal growth are not “one size fits all” processes that can be tested in a traditional ubiquitous intervention approach. Thus, an intervention that tailors the dosage based on a woman’s responsiveness (i.e., GWG within IOM ranges) as pregnancy progresses may be a beneficial strategy to regulate GWG. For example, Unick et al. (2017) suggested that an adaptive “stepped care” approach may be useful for weight regulation because it can provide more intervention to individuals who have poor initial behavior change success (e.g., “non-responders”). James et al. (2018) also suggested that personalizing the intervention, especially in the early weeks of treatment, may promote long-term weight regulation. An adaptive approach may also help with retention of pregnant women with overweight/obesity, a commonly cited barrier in GWG randomized control trial interventions (e.g., Phelan et al., 2011; Vesco et al., 2014) as it can provide the right type and amount of support at the ideal time that it is necessary to promote behavior change (Nahum-Shani et al., 2018). That is, increasing the intensity of the intervention gradually over time (based on each woman’s need for more assistance to effectively regulate her GWG) can reduce participant burden and fatigue that often lead to attrition.
To this end, we developed a theoretically driven, behavioral intervention that uses intensive data (e.g., daily weight, physical activity, energy intake) to adapt the dosage to the unique needs of pregnant women with overweight/obesity in an effort to regulate GWG (Dong et al., 2012, 2013, 2014; Guo et al., 2016). The intervention was designed with the Multiphase Optimization Strategy (Collins, 2018), is based on principles of control systems engineering (Hekler et al., 2018; Rivera et al., 2018) and adaptive interventions (Almirall et al., 2014), and produced a dynamical, mathematical model of energy balance (Dong et al., 2012, 2013, 2014; Guo et al., 2016, 2018, 2020; Pauley et al., 2018; Symons Downs et al., 2018; Thomas et al., 2012). This model includes the Theories of Planned Behavior (Ajzen, 1991) and Self-Regulation (Carver & Scheier, 1998) constructs targeting physical activity and energy intake/healthy eating, which in turn, are predicted to influence GWG. Our prior research describes the intervention and measurement protocols (Symons Downs et al., 2018), development of the intervention dosages (Pauley et al., 2018), evidence-base of the content (Diabetes Prevention Program, 2002; Dong et al., 2014; Symons Downs et al., 2009, 2013, 2017a, 2019), and our energy balance model (Dong et al., 2012, 2013, 2014; Guo et al., 2016, 2018, 2020). To our knowledge, no prior studies have used principles of systems science and adaptive interventions to regulate GWG in pregnant women with overweight/obesity. A first step toward understanding the utility of this intervention is to conduct a feasibility trial (Collins, 2018). The aims of this study were to: (a) characterize participant compliance (i.e., adherence to the measurement protocol and session attendance) with the intensive longitudinal data collection protocol (needed to dynamically model GWG) and intervention implementation; (b) describe frequency of exposure to the adaptive intervention dosages; and (c) determine pre-post change in GWG (primary outcome) and explore secondary outcomes (physical activity, energy intake, motivational determinants, self-regulation) between the intervention and control groups. We hypothesized that participant compliance would be acceptable, the adaptive intervention would be feasible to implement, and intervention women would have lower GWG than controls. Because this was a feasibility trial, evaluation of secondary outcomes is under-powered; thus, these analyses are considered to be exploratory.
Methods
Conceptual framework
The intervention was based on a conceptual framework that expanded a mathematical, dynamical model of energy balance (Thomas et al., 2012). Specifically, it included two Theory of Planned Behavior (TPB; Ajzen, 1991) models to inform physical activity (PA) and energy intake (EI) behaviors and two self-regulation modules depicting how success expectancies during the intervention influence a woman’s motivation to achieve a goal to be active and eat healthy (Carver & Scheier, 1998). It also included an intervention delivery module (i.e., goal-setting, self-monitoring, active learning) to understand its influence on the TPB motivational determinants to improve PA and EI, and in turn, regulate GWG (see Fig. 1; Dong et al., 2012, 2013, 2014; Guo et al., 2016, 2018, 2020). Principles of control systems engineering were used to inform how our intervention impacted GWG and predict when to augment intervention dosages (Rivera et al., 2007). This trial was registered at clinicaltrials.gov NCT03945266.
TPB theory of planned behavior; I1…In: intervention components; i: exogenous variables that serve as inputs for behavioral models; yi: system outputs; ξ1: behavioral belief × evaluation of outcome; ξ2: normative belief × motivation to comply; ξ3: control belief × power of control belief; I1: healthy eating education; I2: healthy eating weekly plan; I3: healthy eating active learning; I4: goal setting; I5: physical activity education; I6: physical activity weekly plan; I7: physical activity session; I9: daily weight scale; I10: dietary record; I11: PA monitor output
Design
This study was a two-arm, feasibility randomized control trial with pregnant women with overweight/obesity were randomized 1:1 to usual care control group or usual care + adaptive intervention group. Approval from the Institutional Review Board of a northeast university was obtained for all research activities (STUDY00003752); all women gave verbal and written informed consent to participate. The primary outcome was GWG. According to Julios (2005), a sample size of N = 12 per group is adequate to assess feasibility. Considering an expected 20% drop-out based on our pilot work (Symons Downs et al., 2009, 2017a, 2019), a sample size of 30 participants (15 per group) provided 80% power to detect a standardized effect size for GWG of 1.2 standard deviations using a two-sided test with significance level of 0.05. Because we were not powered to detect effects in secondary outcomes, the reported results for secondary outcomes are considered to be exploratory (Thabane et al., 2010).
Participants
Women were eligible to participate in this study if they were ages 18–40 years and had: (1) pre-pregnancy overweight/obesity (BMI range 25–45 kg/m2; > 40 kg/m2 with physician consultation), (2) at enrollment, singleton pregnancy > 8 and up to 12 weeks gestation, (3) physician consent to participate, and 4) were English-speaking and residing in or near Central Pennsylvania. Exclusion criteria were: (1) multiple gestation, (2) diabetes at study entry, (3) not having pre-pregnancy overweight/obesity, (4) severe allergies or dietary restrictions, (5) contraindications to prenatal PA (American College of Obstetrics and Gynecologists [ACOG], 2015) and, (6) not residing in area for duration of study.
Procedures
Pregnant women with overweight/obesity were recruited for enrollment between 8 and 12 weeks gestation using on-site clinic, community-based, and Web-based strategies (Symons Downs et al., 2018). Clinic nurses referred women at their first prenatal appointment to a project staff member who was onsite to give them study information. Women who saw a study flyer called a toll-free number and spoke with a project staff member about the study. The project staff member obtained verbal assent to ask questions and screened each woman (onsite at the clinic or by phone) for eligibility based on the inclusion criteria. If eligible and interested, the woman was scheduled for her pre-intervention assessment (between 8 and 12 weeks gestation) at the university’s Clinical Research Center (CRC) where a study clinician assessed height, weight, blood pressure, and conducted a physical exam to identify health symptoms that may preclude study participation (ACOG, 2015). All women completed self-report measures of PA, EI, motivational determinants (attitude, subjective norm, perceived behavioral control, intention), self-regulation and demographics/health history using secure, web-based data software (Research Electronic Data Capture; REDCap; Harris et al., 2009). Women were given instructions on how to use the mobile health (mHealth) devices (i.e., Wi-Fi scale, activity monitor, MyFitnessPal smartphone app) to measure weight, PA, and EI. MyFitnessPal was used to collect information on diet quality for dietary counseling over the course of the intervention; because of the inaccuracies associated with self-reported dietary intake data (McClung et al., 2018), daily EI was estimated with a validated back-calculation method (described below). All participants were compensated for pre- and post-intervention assessments ($50 each), and using the mHealth tools and completing online surveys over the course of the study ($20 every 4 weeks). Intervention women were also compensated $20 for their time with attending 90% + of intervention sessions (Zweben et al., 2009). Study staff used strategies (e.g., text/phone call reminders, detailed calendars with appointments) to encourage compliance with the data collection and intervention protocols.
Randomization
Randomization to intervention (n = 15) or control (n = 16) groups used 1:1 allocation; participants were entered consecutively. After the participant’s informed consent was signed and the pre-intervention assessment period was complete, a staff member requested randomization by a unique participant identification number and informed the woman of her study assignment. Women were randomized to the study groups at the pre-intervention assessment and completed study procedures from ~ 8 to 36 weeks gestation.
Usual care
All women received the usual prenatal care, which included prenatal education and regular check-up appointments throughout pregnancy with their healthcare provider. No feedback was provided on their GWG, PA, or EI behaviors.
Intervention
Women randomized to the intervention group received the usual prenatal care + the following: (a) education on GWG (e.g., guidelines, plotting weight gain against IOM guidelines), PA (e.g., guidelines, safety), EI/healthy eating (e.g., calorie goals in pregnancy, diet quality, energy density, water intake), and importance of related factors (e.g., strategies to improve mood/sleep); (b) goal-setting/action plans for setting and achieving GWG/PA/EI goals; (c) self-monitoring of GWG (daily use of Wi-Fi weight scale), PA (daily use of wrist-worn activity monitor), and EI/healthy eating (MyFitnessPal phone app; using 3 days/week: 2 week days, 1 weekend day) with mHealth devices; and (d) content on growth of the fetus over the pregnancy (e.g., facts about when the baby is growing eye brows or sucking his/her thumb) and how the woman’s GWG/PA/EI related to infant birth weight and sleep/feeding preferences). Content was delivered to all intervention women in weekly 45–60 min, one-on-one, onsite sessions with a registered dietitian over the study with a maximum of 24 weekly modules (depending on enrollment start) as the “baseline” intervention. Sessions covered the education content and customized GWG, PA, and calorie/healthy eating goals. Visual diagrams of each woman’s weight, PA, and EI were plotted from real-time data collection from mHealth tools and a back-calculation equation to estimate EI (Guo et al., 2016, 2018, 2020). The registered dietitian reviewed each woman’s prior week diet quality from the MyFitnessPal app and PA from the activity monitor data and made customized recommendations for the following week related to women’s goals (e.g., increase fruits/vegetables, reduce sweets; increase PA by 10 min/day). A didactic interaction between the dietitian and participant occurred that allowed for a personalized discussion of content, feedback on behavioral progress, and strategies/plans to overcome barriers in the upcoming week. The dietitian also offered motivational encouragement toward goal progress.
Each woman’s GWG was monitored weekly and compared to her IOM goal ranges and predicted estimates. Every 3–4 weeks, we used a decision rule (Rivera et al., 2018) to either: (a) maintain the current dosage if she was meeting (within) her IOM goal range or (b) adapt the dosage (“step-up” intensity) if a woman was exceeding her IOM goal range (i.e., weekly GWG: overweight 0.23–0.33 kg/week; obese 0.17–0.27 kg/week). We designed up to five possible adapted “step-up” dosages with PA/EI/self-regulation activities that were based on prior evidence (Diabetes Prevention Program (DPP), 2002; Symons Downs et al., 2019; see Table 1) and packaged in a way that considered a balance between the integration of additional (more intensive) intervention support to help a woman regulate her GWG with participant burden and cost (Collins, 2018; Rivera et al., 2018). For example, because onsite interactive PA/EI strategies require more participant time and intervention resources, they were built into the adaptive design so that only women needing more intensive intervention to regulate GWG got them. More specifically, step-up 1 included hands-on PA (45-min onsite activity session led by a fitness instructor that promoted moderate to vigorous PA based on prenatal guidelines with 5-min warm-up, 30-min aerobic activity [choice of treadmill, cycle ergometer, or low impact aerobics routine], 5-min resistance exercises [e.g., hand weights, lunges, etc.] and 5-min cool-down; ACOG, 2015), customized workout booklet with trimester-specific, at-home workouts (e.g., aerobics, swimming, resistance activities), addition of step goal (minimum of 10,000 steps/day) to their personalized PA goals, 30-min onsite healthy eating demonstration session led by a registered dietitian with meal preparation/cooking and customized recipe booklet based on a woman’s food preferences, and implementation intentions specifying where, when, and how to connect PA/EI goals to successful outcomes (Gollwitzer & Sheeran, 2006; Symons Downs & Singer, 2004). Step-up 2–5 added more PA/EI sessions and self-regulation content. Step-ups were cumulative so that a woman could have received the maximum intervention dosage of baseline + step-up 1 + step-up 2 + step-up 3 + step-up 4 + step-up 5.
Measures
All participants completed the following measures at the pre- and post-intervention assessments. In addition, assessments were obtained over the course of the study for weight (daily), PA (daily), energy intake (daily), and TPB/self-regulation constructs (e.g., weekly/ monthly). Weight was measured using the Aria Wi-Fi weight scale (Fitbit Inc., 2019, San Francisco, CA), a valid and reliable tool to estimate weight in the general population (Hood et al., 2019). Women weighted themselves the first thing in the morning when they woke up wearing minimal/no clothing; they were able to see their weights each day. The scale transmitted weights automatically to secure participant online accounts; data were accessed and stored in REDCap. Each woman’s GWG was monitored weekly and compared to her IOM goal ranges for pre-pregnancy BMI status (IOM; Rasmussen & Yaktine, 2009). Predicted estimates and decision rules were based on the extent to which each woman’s GWG was below/within or above her IOM goal range; decisions to adapt the intervention were made in 3–4 week cycles similar to a women’s prenatal care visit schedule. GWG was calculated as weight at post-intervention—weight at pre-intervention.
PA was assessed using the Jawbone UP3 (San Francisco, CA) wrist-worn activity monitor that is a valid and acceptable tool for measuring PA (Evenson et al., 2015; Ferguson et al., 2015). Our pilot work (Symons Downs et al., 2016) showed the Jawbone accurately estimated activity kcal within 76 cal/day of the “gold standard” Actigraph monitor (ActiGraph, LLC, 2019). Women were asked to wear the monitor 24 h/day for the study duration. Activity expenditure kcal was extracted via secure participant online accounts and stored in REDCap.
EI was estimated with a back-calculation method (Guo et al., 2016, 2018, 2020; Symons Downs et al., 2018) as a function of maternal weight (W), PA, and resting metabolic rate (RMR) as:
The variables are as follows: k = 1, 2, …, N corresponding to day 1-day N. T represents sampling time which in this case was T = 1 day. Maternal W was measured by Aria Wi-Fi scale in kilograms; Jawbone activity monitor was used to assess PA in kcals. Daily RMR was estimated as a function of maternal W using a validated empirical equation that was proposed (Thomas, 2009) and fit using quadratic regression data (Butte et al., 2003, 2004): RMR(k) = 0.1976 W(k)2 − 13.424 W(k) + 1457.6. The validated estimated equation was used to estimate RMR given that daily objective assessments of RMR were not feasible. The use of this validated estimated equation is supported by our previous work (Leonard et al., 2019) showing that the back-calculation of EI when using the estimated RMR equation vs. an objective RMR assessment (i.e., mobile metabolism device) was equivalent in a sample of pregnant women with overweight/obesity.
TPB and self-regulation Self-reported measures were collected weekly, biweekly, and monthly based on our pilot work showing that some constructs (e.g., subjective norm, perceived behavioral control, intention) had greater within-person variability than others (e.g., attitude, self-regulation); this provided sufficient data to inform our energy balance model and reduced participant burden. PA TPB Constructs. The TPB scales used for this study were developed specifically for pregnant women (Blanchard et al., 2009; Hausenblas & Symons Downs, 2004; Symons Downs & Hausenblas, 2003, 2007) from guidelines of Ajzen (1991). Attitude was assessed monthly with seven differential pairs (e.g., 1 = useless to 7 = useful) describing how women felt about engaging in PA for at least 30 min per day on most, if not all, days of the week. Subjective Norm was assessed weekly with three Likert scale items (1 = strongly disagree to 7 = strongly agree) measuring perceived support from important others to engage in PA for 30 min/day on most days of the week. Perceived Behavioral Control was assessed weekly with three items (e.g., 1 = extremely difficult/very little control/strongly disagree to 7 = extremely easy/complete control/strongly agree). Intention to engage in PA for 30 min/day on most days of the week was assessed weekly with six items (e.g., 1 = strongly disagree to 7 = strongly agree). Internal consistency reliability for the PA TPB items ranged from alpha = 0.73–0.97 at pre- and post-intervention. EI TPB Constructs were developed from prior research (Symons Downs et al., 2014) including Blanchard et al. (2009) and Murnaghan et al. (2010) and using the same Likert scales as used for the PA items. Attitude was assessed monthly with 14 differential pairs (e.g., 1 = useless to 7 = useful): 7 assessing attitude about eating healthy foods each day in the next week and 7 measuring attitude about limiting unhealthy foods (e.g., sugary beverages, snacks). Subjective Norm was assessed weekly with six items (e.g., three items measuring women’s perceptions of the extent to which significant others provided support to eat healthy; three items assessing perceived pressure from others to limit unhealthy foods). Perceived Behavioral Control was assessed weekly with six items (e.g., ease/difficulty in eating healthy and limiting unhealthy foods). Intention was assessed weekly using 12 items (e.g., 1 = strongly disagree/definitely not/not at all to 7 = strongly agree/definitely/very much). Internal consistency reliability for the EI TPB items ranged from alpha = 0.62–0.94 and limit unhealthy food items ranged from alpha = 0.67–0.96 at pre- and post-intervention. PA/EI Self-Regulation was assessed biweekly with 16 items; 8 items assessed prospective (i.e., in the next week) and eight items assessed retrospective (i.e., in the past week) self-regulation (Carver & Scheier, 1998; Sniehotta et al., 2005; Umstattd et al., 2009). Items were divided into seven subscales: self-monitoring, goal-setting, action planning, coping planning, scheduling, cuing, and affective reaction. Internal consistency reliability ranged from 0.82 to 0.97 for PA items and 0.74 to 0.93 for the healthy eating items.
Analyses
Descriptive statistics (means, standard deviations, and/or frequencies and percentages) were used to report participant characteristics, intervention dosage assignment, and participant compliance. An interpolation method to replace missingness in weight measurements followed by filtering to smooth the interpolated weight was performed before applying the energy balance models (Guo et al., 2016, 2020). Contrasts were constructed from linear mixed models, which account for repeated measures per subject (e.g., pre and post weight measurements), to assess the change within and between the intervention and control groups with respect to the study measurement variables (e.g., GWG). Baseline BMI status (overweight vs. obese) was included as a covariate because baseline BMI is a key component of the GWG guidelines (IOM; Rasmussen & Yaktine, 2009). The denominator degrees of freedom for the linear mixed models were determined using the method of Kenward and Roger (1997). Results from the mixed models are reported as model-adjusted means and 95% confidence intervals (CIs). Model-adjusted means take into account covariates as well as unbalanced data arising from factors such as study design, unequal number of observations per group, or missing data (e.g., daily missing weight, PA, EI; weekly/monthly survey data). With respect to our data, the model-adjusted means take into account the one covariate (baseline BMI status) and the sample size imbalance due to using all available data, as appropriate, in cases where a subject had a pre-measurement but is missing a post-measurement or vice-versa. Visual inspection of residual diagnostics for the mixed models did not reveal any obvious deviations from parametric modeling assumptions. All hypothesis tests were two-sided and all analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Two women (1 intervention, 1 control) were diagnosed with gestational diabetes during the study and remained in the study and the final sample for analyses.
Results
Participant characteristics
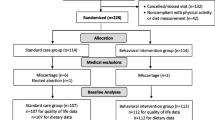
Flow of participants through the study is presented in Fig. 2. As shown, 149 women were screened for eligibility and N = 31 were randomized to the intervention (n = 15) or usual care control group (n = 16). The remaining 118 were excluded due to: not meeting inclusion criteria (n = 51), declined to participate (n = 16), lost to follow-up (n = 34) and eligible but not randomized (n = 17; could not commit to the study, n = 9; did not want to travel to campus, n = 1; decided to move n = 1; non-responsive to scheduling n = 6); randomization of eligible women was 65% (n = 31/48; 21% of all women contacted/screened for eligibility). Retention was high, 27 of 31 (87%) completed the study (e.g., study assessments and post-assessment week). Of the non-completers, n = 3 had miscarriages (1 intervention, 2 controls; we successfully replaced 1 of these women in the control group for n = 16 to meet recruitment goals) prior to starting the intervention protocol and n = 1 intervention woman withdrew (3% drop-out rate). Women participated in the study on average for 26.6 weeks from the pre- to post-intervention assessment weeks. Women were on average 29.6 years old (SD = 4.1 years, range 20–37 years); see Table 2. Mean gestational age at study start was 10.2 weeks (SD = 1.7; range 8–12 weeks) and 36.6 weeks at study end (i.e., post-intervention assessment; SD = 1.0, range 33–38 weeks). Mean weight at study start was 89.9 kg (SD = 20.2; range 59.5–131.9 kg). Mean pre-pregnancy weight was 88.9 kg (SD = 21.0, range 60.8–133.4 kg). Mean pre-pregnancy BMI was 32.6 kg/m2 (SD = 7.2, range 25.0–48.9 kg/m2). Most women were Caucasian (97%), married (84%), employed full time (87%), had at least a college degree (90%), had a family income of ≥ $40,000 (71%), and nulliparous (71%).
Intervention dosage assignment
Among women randomized to the intervention (n = 13), 1 woman (7.6%) received only the baseline intervention dosage with 100% in-person sessions; 3 women (23.1%) received baseline + step-up 1 with 100% in-person sessions; 6 women (46.2%) received baseline + step-up 1 + step-up 2 with 84.4% in person and 15.6% remote sessions; and 3 women (23.1%) received baseline + step-up 1 + step-up 2 + step-up 3 with 42.3% in-person and 57.7% remote sessions; no women exceeded step-up 3. Augmented dosages occurred between gestational weeks 21–31; M gestational age = 21.8 weeks (SD = 4.9) weeks for step-up 1, 27.8 (SD = 3.4) weeks for step-up 2, and 31.5 (SD = 3.2) weeks for step-up 3.
Participant compliance
All women (100%) attended the onsite pre-intervention session and 97% attended the post-intervention session. Both intervention and control women weighed themselves daily using the Wi-Fi scale on 79% of the days of the study (mean [M] = 139.0 days, SD = 50.1), and wore the wrist-worn activity monitor daily on 77% of the study days (M = 128.0 days, SD = 51.1). They also completed EI records (3 days/week: 2 week days, 1 weekend day) using the smartphone app on 80% of the study days (M = 55.9 days, SD = 25.7), and completed 91% (M = 21, SD = 7.7) of the weekly surveys and 97% (M = 5.0, SD = 1.4) of their monthly surveys. Women in the intervention group attended 90.4% of the education/counseling sessions (M = 22; Range = 22–27, mode = 24 weeks depending on gestational age at enrollment); 86.7% in person delivery, 13.0% remote delivery. Self-regulation content was integrated into these sessions. Overall compliance rates for dosage augmentations were 81.3%, 92.8%, and 67.5% for step-up 1, 2, and 3, respectively (see Table 3).
Study measurement variables
Model-adjusted pre-post intervention means, mean change estimates, and 95% confidence intervals for the study variables by intervention and control groups are presented in Table 4. GWG. As expected in pregnancy, weight significantly increased from pre- to post-intervention for both the intervention and control groups. The mean change in weight for the intervention group (6.9 kg; 95% CI: 3.8, 10.0) did not significantly differ from the mean change in weight for the control group (8.8 kg; 95% CI: 5.1, 12.3). PA. The change in Jawbone PA kcals from pre- to post-intervention was not significantly different between or within intervention (34.9 kcals; 95% CI: − 245.6, 315.4) and control groups (− 78.9 kcals; 95% CI: − 273.5, 115.7). EI. As expected based on public health guidance to support fetal growth during pregnancy, EI kcals significantly increased from pre- to post-intervention for both the intervention and control groups. The mean change in EI kcals for the intervention group (410.2 kcals; 95% CI: 32.4, 787.9) was significantly less compared to the control group (1134.8 kcals; 95% CI: 697.3, 1572.3); p = 0.02.
TPB constructs
The change in PA attitude, subjective norm, perceived behavioral control, and intention from pre- to post-intervention were not significantly different between or within the intervention and control groups. There were no significant differences between the two groups with respect to change in attitude, subjective norm, perceived behavioral control, and intention for EI and limiting unhealthy foods. However, there were significant pre- to post-intervention decreases within the control group for EI attitude (M change = − 2.5; 95% CI: − 4.8, − 0.2), subjective norm (M change = − 1.4; 95% CI: − 2.7, − 0.1), and intention (M change = − 3.2; 95% CI: − 5.8, − 0.7).
Self-regulation
Change in PA prospective self-regulation and EI prospective and retrospective self-regulation were not significantly different between the intervention and control groups. PA retrospective self-regulation significantly increased in the intervention group (M change = 5.6; 95% CI: 1.2, 10.1) and when compared to the control group (M change = 0.3; 95% CI: − 4.0, 4.7), the group difference was marginal, p = 0.09. Within the control group, there was a significant decrease in PA prospective self-regulation (M change = − 4.9; 95% CI: − 8.4, − 1.4).
Discussion
The goals of this study were to characterize participant compliance with the intensive longitudinal data collection protocol and intervention implementation, describe frequency of exposure of the adaptive intervention dosages, and examine intervention and control group differences in the primary outcome of GWG. We also explored between and within group differences on secondary outcomes of PA, EI, motivational determinants, and self-regulation. Overall, compliance with the intensive data collection and intervention protocols and longitudinal study retention were good. The intervention group had 21% lower GWG than controls, and exploratory analyses also showed the women in the intervention group had significantly lower EI kcals at post-intervention than women in the control group. These findings, described in more detail below, suggest that an adaptive intervention that varies dosages to regulate GWG is feasible to deliver to pregnant women with overweight/obesity.
First, participant recruitment, compliance, and retention in this study were particularly good. There was a 65% recruitment rate of eligible women, which is comparable to recruitment rates of past prenatal behavioral interventions (e.g., 32–69%; Carpenter et al., 2016; Coleman-Phox et al., 2013). and reasons for non-eligibility were mainly due to not meeting the inclusion criteria. Common barriers (e.g., no time, not wanting to travel) were less of an issue for this sample than not meeting the inclusion criteria or loss of follow-up which are common issues across studies and not specific to this population of pregnant women with overweight/obesity. Compliance with the intensive longitudinal data collection protocol ranged across measures. All of the women completed the onsite pre-intervention assessment, all but one woman completed the onsite post-assessment, and most completed the daily/weekly/biweekly measures at home. More specifically, all participants completed daily weights (Aria Wi-Fi scale) and wore the Jawbone activity monitor on 79% and 77% of the study days, respectively. They also completed dietary intake records (MyFitnessPal app) on at least three days/week during 80% of the study weeks, and completed online surveys of their motivational determinants/self-regulation during 91% of the study weeks and 97% of the study months. The overall study retention rate was high with 87% completing the study and only 3% drop-out (only one woman withdrew from the study). Attendance at baseline education/counseling sessions (> 90%) and dosage augmentations step-up 1 (81%) and step-up 1 + 2 (93%) were very good. These compliance findings are in support of past research showing that pregnant women report high use of internet and smartphone technology and high willingness to participate in interventions using mHealth tools (Urrutia et al., 2015) which are easily implemented within clinic settings. Further, consistent with Collins (2018), we anticipated good compliance because we conducted the preparation phase of the Multiphase Optimization Strategy which included pilot studies (e.g., Pauley et al., 2018; Symons Downs et al., 2017b, c) that provided critical information about pregnant women with overweight/obesity preferences for and barriers to study participation, using mHealth tools, and receiving the intervention dosages; these pilot studies informed the strategies used to promote study compliance and the design of the intensive data collection and intervention protocols used in this study. These compliance findings illustrate the benefits of conducting formative work with a target population prior to conducting an intervention. While compliance for step-up 3 was lowest (68%), it should be noted that two of the three women in this dosage assignment were the least compliant in the study which brought the overall compliance rate down. In sum, these findings paired with past research (Urrutia et al., 2015) show the potential for scalability of the intervention, particularly within clinic settings given the incorporation of mHealth tools, and that it is possible to engage and retain pregnant women with overweight/obesity in an intensive, adaptive intervention over the course of pregnancy to regulate GWG. However, future researchers may want to examine the potential utility of repackaging multiple mHealth tools into one platform (e.g., a suite of apps) to explore additional strategies to increase scalability. It is also important to note that while the women in this study had overweight/obesity and resided in rural communities around [location blinded for review], they were also mostly Caucasian, educated, and employed which may have influenced their ability and interest in participating in this study. Future research is needed to replicate these study findings with more diverse samples of women to confirm our compliance findings.
Second, we were able to successfully deliver the adaptive dosages. Among women in the intervention group, one received only the baseline dosage and the remaining women (92.4%) received at least one dosage augmentation; 10/13 (77%) had GWG within the IOM (2009) guidelines. The most frequently delivered dosage was baseline + step-up 1 + step-up 2 (46.2%). No women were randomized beyond dosage step-up 3, mostly because women reached the end of the study (post-intervention assessment at 36-weeks gestation) before they were eligible to receive the next step-up. This also indicates, however, that we were not able to test content in step-up 4 and 5 (e.g., partial meal replacements, motivational messaging delivered by phone/text). Given recent evidence that partial meal replacements may be useful for regulating GWG (Phelan et al., 2018), and our pilot work indicating that pregnant women with overweight/obesity were willing to receive meal replacements (Pauley et al., 2018), we will explore the repackaging of this content within the other dosages (e.g., step-up 1–3). We had originally proposed up to seven dosages in our pilot work but the participants found dosages 6 and 7 to be too intensive so they were removed from the design (Pauley et al., 2018). Thus, while we identified the “maximum intensity” of intervention to deliver, a more practical number of step-ups is likely less (e.g., 3–4) given timeline constraints of delivering the full intervention prior to childbirth. Further, repackaging of these fewer step-ups may move some content (e.g., personalized recipe and workout booklets) to the baseline dosage. Also, it is encouraging that women attended 90% or more of the intervention sessions. These findings provide initial evidence that pregnant women with overweight/obesity are willing to receive more intensive intervention if needed to manage their weight over pregnancy. However, it is also important to acknowledge that many of the women in this study worked either full-time for the university or at a job in close proximity to campus which may have made it easier for them to attend onsite sessions. In addition, some women preferred to receive their additional sessions via remote delivery. In an effort to improve intervention scalability and reach more diverse women in the future, all of the intervention content will be modified for remote synchronous/asynchronous delivery.
Moreover, consistent with our hypothesis, women in the intervention group had 21% lower GWG at post-intervention compared to women in the control group, and as noted above, the majority of women (77%) in the intervention had GWG within the IOM (2009) guidelines. Given the challenges with regulating GWG among pregnant women with overweight/obesity, these findings are promising and suggest that the intervention had some impact on regulating GWG among the intervention group which is relevant for testing the intervention in a future larger randomized trial. Further, when GWG was explored by subgroups, it is interesting that intervention women with overweight had almost 55% less GWG compared to control group women with overweight whereas the group difference in GWG for women with obesity was minimal. Future research is needed to better understand if this intervention is more beneficial for women with overweight compared to women with obesity.
In addition, exploratory analyses of the secondary outcomes showed the intervention group had significantly lower EI kcals at post-intervention than women in the control group. Interestingly, the difference was almost three times as high in the controls than in the intervention group which implies that our intervention education, dietary counseling, and healthy eating sessions (e.g., cooking demonstrations, understanding energy density) were able to slow the increase in EI among women in the intervention group. Although there was not a significant group difference for PA kcals, exploration of the means showed intervention women slightly increased in PA kcals over the study period whereas controls decreased. Further modifications may be needed to the intervention to better promote PA such as including asynchronous video workouts that women can do at home rather than just following the customized workout booklet. Also consistent with past research (Symons Downs et al., 2017a), there was a trend toward significance for an increase in PA intention and retrospective self-regulation in the intervention vs. control group. These findings suggest the intervention may influence key determinants of PA such as action planning (e.g., when/where to be active) and setting reminders for PA (e.g., laying out sneakers, phone notifications); both of which may be better utilized to increase PA behavior. There were no significant group differences for the remaining TPB constructs for PA, EI, or EI limiting unhealthy foods, PA prospective regulation, and EI retrospective and prospective regulation. However, there was a significant within group increase in PA retrospective self-regulation for intervention women and a trend toward significance for an increase in EI retrospective regulation for both the intervention and control groups over the study period. These findings suggest that women in the intervention may have used PA and EI self-regulatory practices over the course of the intervention more than women in the control group. Further, there were observed pre- to post-intervention decreases in EI prospective self-regulation for both groups and these changes tended toward significance. It is possible that women may have initially over-estimated their EI self-regulation (Millar, 2017) and became aware of this over-estimation in relation to their actual EI behaviors via tracking with the MyFitnessPal app and made subsequent adjustments. However, future research with a fully-powered trial is needed to confirm any assumptions on the secondary outcomes.
This novel feasibility study had several strengths. To our knowledge, this is the first study to test an intervention that adapts intervention intensity over the course of pregnancy to regulate GWG. We confirmed that our adaptive intervention is feasible to deliver, pregnant women with overweight/ obesity had high compliance with the intensive data collection/intervention protocols, and had an impact on GWG and some secondary outcomes. Despite the novelty of this study and its strengths, there were some limitations. We were not adequately powered to detect significant effects on the secondary outcomes. Also, despite the focus on a high-risk population of pregnant women with overweight/obesity and the sample demographics matching those of most women residing in rural communities across Central Pennsylvania, our sample was largely homogenous (mostly Caucasian, middle income, educated) which may have led to higher motivation to comply with the study procedures. Future research is needed to test this intervention in a more diverse sample of pregnant women with overweight/obesity. Lastly, modifications to the intervention (e.g., remote synchronous and asynchronous delivery, repackaging mHealth tools) are warranted to increase scalability and reach women residing in communities not in proximity to a university setting.
Conclusion
These preliminary findings suggest that a theoretically-driven, adaptive behavioral intervention that varies intervention dosages to regulate GWG is feasible to deliver and may impact GWG and some secondary outcomes (e.g., EI kcal; PA intention and self-regulation). The next step is to conduct a fully-powered randomized control trial to confirm the Healthy Mom Zone intervention can effectively and efficiently regulate GWG and impact secondary outcomes among a more diverse sample of pregnant women with overweight/obesity.
References
ActiGraph, LLC. Retrieved November 17, 2019, from https://www.actigraphcorp.com/
Ajzen, I. (1991). The theory of planned behavior. Organizational Behavior and Human Decision, 50, 179–211
Almirall, D., Nahum-Shani, I., Sherwood, N. E., & Murphy, S. A. (2014). Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Translational Behavioral Medicine, 4, 260–274
American College of Obstetrics and Gynecologists (ACOG). (2015). ACOG committee opinion No. 650: Physical activity and exercise during pregnancy and the postpartum period. Obstetrics and Gynecology, 126, e135–e142
Blanchard, C. M., Fisher, J., Sparling, P. B., Shanks, T. H., Nehl, E., Rhodes, R. E., Courneya, K. S., & Baker, F. (2009). Understanding adherence to 5 servings of fruits and vegetables per day: A theory of planned behavior perspective. Journal of Nutrition Education and Behavior, 41, 3–10
Butte, N. F., Ellis, K. J., Wong, W. W., Hopkinson, J. M., & Smith, E. O. (2003). Composition of gestational weight gain impacts maternal fat retention and infant birth weight. American Journal of Obstetrics and Gynecology, 189, 1423–1432
Butte, N. F., Wong, W. W., Treuth, M. S., Ellis, K. J., & O’Brian Smith, E. (2004). Energy requirements during pregnancy based on total energy expenditure and energy deposition. American Journal of Clinical Nutrition, 79, 1078–1087
Carpenter, R. E., Emery, S. J., Rassi, D., Uzun, O., & Lewis, M. J. (2016). Recruitment of pregnant women to an exercise-intervention study. Randomized Controlled Trial, 36, 200–207
Carver, C., & Scheier, M. F. (1998). On the self-regulation of behavior. Cambridge University Press.
Chang, M., Nitzke, S., Buist, D., Cain, D., Horning, S., & Eghtedary, K. (2015). I am pregnant and want to do better but I can’t: Focus groups with low income overweight and obese pregnant women. Maternal and Child Health Journal, 19, 1060–1070
Coleman-Phox, K., Laraia, B., Alder, N., Vieten, C., Thomas, M., & Epel, E. (2013). Recruitment and retention of pregnant women for a behavioral intervention: Lessons from the maternal adiposity, metabolism, and stress (MAMAS) study. Preventing Chronic Disease, 10, e31
Collins, L. M. (2018). Optimization of behavioral, biobehavioral, and biomedical interventions: The multiphase optimization strategy (MOST). Springer.
Diabetes Prevention Program (DPP) Research Group. (2002). The diabetes prevention program (DPP) description of lifestyle intervention. Diabetes Care, 25, 2165–2171
Dong, Y., Deshpande, S., Rivera, D. E., Downs, D. S., & Savage, J. S. (2014). Hybrid model predictive control for sequential decision policies in adaptive behavioral interventions. In Proceedings of the American control conference (pp. 4198–4203).
Dong, Y., Rivera, D. E., Downs, D. S., Savage, J. S., Thomas, D. M., & Collins, L. M. (2013). Hybrid model predictive control for optimizing gestational weight gain behavioral interventions. In Proceedings of the American control conference (pp. 1970–1975).
Dong, Y., Rivera, D. E., Thomas, D. M., Navarro-Barrientos, J. E., Symons Downs, D., Savage, J. S., & Collins, L. M. (2012). A dynamical systems model for improving gestational weight gain behavioral interventions. In Proceedings of the American Control Conference (pp. 4059–4064).
Evenson, K. R., Goto, M. M., & Furberg, R. D. (2015). Systematic review of the validity and reliability of consumer-wearable activity trackers. International Journal of Behavioral Nutrition and Physical Activity, 12, 159
Farpour-Lambert, N. J., Ells, L. J., Martinez de Tejada, B., & Scott, C. (2018). Obesity and weight gain in pregnancy and postpartum: An evidence review of lifestyle interventions to inform maternal and child health policies. Frontiers in Endocrinology, 9, 546. https://doi.org/10.3389/fendo.2018.00546
Ferguson, T., Rowlands, A. V., Olds, T., & Maher, C. (2015). The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: A cross-sectional study. International Journal of Behavioral Nutrition and Physical Activity, 12, 42
Fitbit Inc. Retrieved November 17, 2019, from https://fitbit.com/aria
Gollwitzer, P. M., & Sheeran, P. (2006). Implementation intentions and goal achievement: A meta-analysis of effects and processes. Advances in Experimental Psychology, 38, 69–119
Gunderson, E. P., & Abrams, B. (1999). Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiologic Reviews, 21, 261–275
Guo, P., Rivera, D. E., Pauley, A. M., Leonard, K. S., Savage, J. S., & Symons Downs, D. (2018). A “Model-on-Demand” methodology for energy intake estimation to improve gestational weight control interventions. In Proceedings of the IFAC World Congress (Vol. 51, pp. 144–149).
Guo, P., Rivera, D. E., Savage, J. S., Hohman, E. E., Pauley, A. M., Leonard, K. S., & Symons Downs, D. (2020). System identification approaches for energy intake estimation: Enhancing interventions for managing gestational weight gain. IEEE Transactions on Control Systems Technology, 28, 63–78. https://doi.org/10.1109/TCST.2018.2871871
Guo, P., Rivera, D. E., Symons Downs, D., & Savage, J. S. (2016). Semi-physical identification and state estimation of energy intake for interventions to manage gestational weight gain. In Proceedings of the American control conference (pp. 1271–1276). https://doi.org/10.1109/ACC.2016.7525092
Hales, C. M., Carroll, M. D., Fryar, C. D., & Ogden, C. L. (2017). Prevalence of obesity among adults and youth: U.S., 2015–2016. National Center for Health Statistics Data Brief, 288, 1–8
Hausenblas, H. A., & Symons Downs, D. (2004). Prospective examination of the Theory of Planned Behavior applied to physical activity behavior during women’s first trimester of pregnancy. Journal of Reproductive and Infant Psychology, 22, 199–210
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. G. (2009). Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381
Hekler, E. B., Rivera, D. E., Martin, C. A., Phatak, S. S., Freigoun, M. T., Korinek, E., Klasnja, P., Adams, M. A., & Buman, M. P. (2018). Tutorial for using control systems engineering to optimize adaptive mobile health interventions. Journal of Medical Internet Research, 20, e214. https://doi.org/10.2196/jmir.8622
Herring, S. J., Cruice, J. F., Bennett, G. G., Rose, M. Z., Davey, A., & Foster, G. D. (2016). Preventing excessive gestational weight gain among African American women: A randomized clinical trial. Obesity (Silver Spring), 24, 30–36
Hood, K. M., Marr, C., Kirk-Sorrow, J., Farmer, J., IV., Lee, M., & Kern, M. (2019). Validity and reliability of a Wi-Fi smart scale to estimated body composition. Health and Technology, 9, 839–856
James, B. L., Roe, L. S., Loken, E., & Rolls, B. J. (2018). Early predictors of weight loss in a 1-year behavioural weight-loss programme. Obesity Science and Practice, 4, 20–28
Julios, S. A. (2005). Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics, 4, 287–291
Kenward, M. G., & Roger, J. H. (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics, 53, 983–997
Leonard, K. S., Guo, P., Pauley, A. M., Hohman, E. E., McNitt, K. M., Rivera, D. E., Savage, J. S., & Symons Downs D. (2019). Accuracy of back-calculated energy intake: Comparing estimated vs. measured resting metabolic rate. In Poster presentation at The Obesity Society, Obesity Week Annual Meeting, 3-7 November 2019, Las Vegas, Nevada.
Lindsay, K. L., Heneghan, C., McNulty, B., Brennan, L., & McAuliffe, F. M. (2015). Lifestyle and dietary habits of an obese pregnant cohort. Maternal and Child Health Journal, 19, 25–32
Meldrum, D. R., Morris, M. A., & Gambone, J. C. (2017). Obesity pandemic: causes, consequences, and solutions: But do we have the will? Fertility and Sterility, 107, 833–839
McClung, H. L., Ptomey, L. T., Shook, R. P., Aggarwal, A., Gorczyca, A. M., Sazonov, E. S., Becofsky, K., Weiss, R., & Das, S. K. (2018). Dietary intake and physical activity assessment: Current tools, techniques, and technologies for use in adult populations. American Journal of Preventive Medicine, 55, e93-104
Millar, B. M. (2017). Clocking self-regulation: Why time of day matters for health psychology. Health Psychology Review, 11, 345–357
Murnaghan, D. A., Blanchard, C. M., Rodgers, W. M., LaRosa, J. N., MacQuarrie, C. R., MacLellan, D. L., & Gray, B. J. (2010). Predictors of physical activity, healthy eating and being smoke-free in teens: A theory of planned behaviour approach. Psychology and Health, 25, 925–941
Nagourney, E. M., Goodman, D., Lam, Y., Hurley, K. M., Henderson, J., & Surkan, P. J. (2019). Obese women’s perceptions of weight gain during pregnancy: A theory-based analysis. Public Health Nutrition, 22, 2228–2236. https://doi.org/10.1017/S1368980019000703
Nahum-Shani, I., Smith, S. N., Spring, B. J., Collins, L. M., Witkiewitz, K., Tewari, A., & Murphy, S. A. (2018). Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52, 446–462
Pauley, A. M., Hohman, E., Savage, J. S., Rivera, D. E., Guo, P., Leonard, K. S., & Symons Downs, D. (2018). Gestational weight gain intervention impacts determinants of healthy eating and exercise in overweight/obese pregnant women. Journal of Obesity, 6469170.
Peaceman, A. M., Clifton, R. G., Phelan, S., Gallagher, D., Evans, M., Redman, L. M., Knowler, W. C., Joshipura, K., Haire-Joshu, D., Yanovski, S. Z., Couch, K. A., Drews, K. L., Franks, P. W., Klein, S., Martin, C. K., Pi-Sunyer, X., Thom, E. A., Van Horn, L., Wing, R. R., …, LIFE-Moms Research Group. (2018). Lifestyle interventions limit gestational weight gain in women with overweight or obesity: LIFE-Moms prospective meta-analysis. Obesity, 26, 1396–1404.
Phelan, S., Phipps, M. G., Abrams, B., Darroch, F., Schaffner, A., & Wing, R. R. (2011). Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The fit for delivery study. The American Journal of Clinical Nutrition, 93, 772–779
Phelan, S., Wing, R. R., Brannen, A., McHugh, A., Hagobian, T. A., Schaffner, A., Jelalian, E., Hart, C. N., Scholl, T. O., Munoz-Christian, K., Yin, E., Phipps, M. G., Keadle, S., & Abrams, B. (2018). Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. American Journal of Clinical Nutrition, 107, 183–194
Rasmussen, K. M., & Yaktine, A. L. (Eds.). (2009). Weight gain during pregnancy: Reexamining the guidelines. National Academies Press (US).
Rivera, D. E., Heckler, E., Savage, J. S., & Symons Downs, D. (2018). Intensively adaptive interventions using control systems engineering: Two illustrative examples. In L. M. Collins & K. Kugler (Eds.), Optimization of Behavioral, biobehavioral, and biomedical interventions (pp. 121–173). Cham, Switzerland: Springer.
Rivera, D. E., Pew, M. D., & Collins, L. M. (2007). Using engineering control principles to inform the design of adaptive interventions: A conceptual introduction. Drug and Alcohol Dependence, 88, S31–S40
Sniehotta, F. F., Schwarzer, R., Scholz, U., & Schüz, B. (2005). Action planning and coping planning for long-term lifestyle change: Theory and assessment. European Journal of Social Psychology, 35, 565–576
Symons Downs, D., & Hausenblas, H. A. (2003). Exercising for two: Examining pregnant women’s second trimester physical activity intention and behavior using the framework of the theory of planned behavior. Women’s Health Issues, 13, 222–228
Symons Downs, D., & Hausenblas, H. A. (2007). Pregnant women’s third trimester physical activity behaviors, body mass index, and pregnancy outcomes. Psychology and Health, 22, 545–549
Symons Downs, D., & Singer, R. N. (2004). Goal setting and implementation intentions: Preliminary support for increasing exercise behavior. Journal of Human Movement Studies, 45, 419–432
Symons Downs, D., DiNallo, J. M., Birch, L. L., Paul, I. M., & Ulbrecht, J. S. (2017a). Randomized face-to-face vs. home exercise interventions in pregnant women with gestational diabetes. Psychology of Sport and Exercise, 30, 73–81.
Symons Downs, D., Feinberg, M., Hillemeier, M. H., Weisman, C. S., Chase, G. A., Chuang, C. H., Parrott, R., & Francis, L. A. (2009). Design of the Central Pennsylvania Women’s Health Study (CePAWHS) strong healthy women intervention: Improving preconceptional health. Maternal and Child Health Journal, 13, 18–28.
Symons Downs, D., Nigg, C. R., Hausenblas, H. A., & Rauff, E. L. (2013). Understanding of why people change physical activity behavior. In C. R. Nigg (Ed.), American college of sports medicine behavioral aspects of exercise.
Symons Downs, D., Savage, J. S., & Rauff, E. L. (2014). Falling short of guidelines? Nutrition and weight gain knowledge in pregnancy. Journal of Women’s Health, 3, 1000184. https://doi.org/10.4172/2167-0420.1000184
Symons Downs, D., Savage, J. S., Rivera, D. E., Pauley, A. M., Hess, L., & Guo, P. (2016). Design and preliminary findings of an individually-tailored “just-in-time” adaptive intervention to manage prenatal weight gain. Annals of Behavioral Medicine, 50, S162.
Symons Downs, D., Savage, J. S., Rivera, D. E., Leonard, K. S., Pauley, A. M., Hohman, E. E., & Guo, P. (2017b). Using mHealth and eHealth methods to promote weight control among women. Annals of Behavioral Medicine, 51(S1), 1587–1588.
Symons Downs, D., Savage, J. S., Rivera, D. E., Leonard, K. S., Pauley, A. M., Hohman, E. E., & Guo, P. (2017c). Influence of a feasibility study on the design of an individually-tailored, adaptive intervention to manage weight in overweight and obese pregnant women. Annals of Behavioral Medicine, 51(S1), 1451–1452.
Symons Downs, D., Savage, J. S., Rivera, D. E., Smyth, J. M., Rolls, B. J., Hohman, E. E., McNitt, K. M., Kunselman, A. R., Stetter, C., Pauley, A. M., Leonard, K. S., & Guo, P. (2018). Individually tailored, adaptive intervention to manage gestational weight gain: Protocol for a randomized controlled trial in women with overweight and obesity. JMIR Research Protocols, 7, e150. https://doi.org/10.2196/resprot.9220
Symons Downs, D., Smyth, J. M., Heron, K. E., Feinberg, M. E., Hillemeier, M., & Materia, F. T. (2019). Beliefs about using smartphones for health behavior change: An elicitation study with overweight and obese rural women. Journal of Technology in Behavioral Science, 4, 33–41.
Thabane, L., Ma, J., Chu, R., Cheng, J., Ismaila, A., Rios, L. P., Robson, R., Thabane, M., Giangregorio, L., & Goldsmith, C. H. (2010). A tutorial on pilot studies: The what, why and how. BMC Medical Research Methodology, 10, 1. https://doi.org/10.1186/1471-2288-10-1
Thomas, D. M. (2009). A dynamical fetal-maternal model of gestational weight gain. Personal Communication.
Thomas, D. M., Navarro-Barrientos, J. E., Rivera, D. E., Heymsfield, S. B., Bredlau, C., Redman, L. M., Martin, C. K., Lederman, S. A., Collins, L. M., & Butte, N. F. (2012). Dynamic energy-balance model predicting gestational weight gain. The American Journal of Clinical Nutrition, 95, 115. https://doi.org/10.3945/ajcn.111.024307
Umstattd, M. R., Motl, R., Wilcox, S., Saunders, R., & Watford, M. (2009). Measuring physical activity self-regulation strategies in older adults. Journal of Physical Activity and Health, 6, S105–S112
Unick, J. L., Pellegrini, C. A., Demos, K. E., & Dorfman, L. (2017). Initial weight loss response as an indicator for providing early rescue efforts to improve long-term treatment outcomes. Current Diabetes Report, 17, 69. https://doi.org/10.1007/s11892-017-0904-1
Urrutia, R. P., Berger, A. A., Ivins, A. A., Beckham, A. J., Thorp, J. M., Jr., & Nicholson, W. K. (2015). Internet use and access among pregnant women via computer and mobile phone: Implications for delivery of perinatal care. JMIR mHealth and uHealth, 3, e25
Vesco, K. K., Karanja, N., King, J. C., Gillman, M. W., Leo, M. C., Perrin, N., McEvoy, C. T., Eckhardt, C. L., Smith, K. S., & Stevens, V. J. (2014). Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity (Silver Spring), 22, 1989–1996
Vincze, L., Rollo, M., Hutchesson, M., Hauck, Y., MacDonald-Wicks, L., Wood, L., Callister, R., & Collins, C. (2019). Interventions including a nutrition component aimed at managing gestational weight gain or postpartum weight retention: A systematic review and meta-analysis. JBI Database of Systematic Reviews and Implementation Reports, 17, 297–364
Zwebe, A., Fucito, K. M., & O’Malley, S. S. (2009). Effective strategies for maintaining research participation in clinical trials. Drug Information Journal, 43, 459–467
Acknowledgements
We would like to acknowledge the assistance of the Healthy Mom Zone team who assisted with participant recruitment and data collection for this study. The project described was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health through grant 1 R01 HL119245-01 and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000127 and TR002014. The content is solely responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
The project described was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health through grant 1 R01 HL119245-01 and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000127 and TR002014.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr. DSD, Dr. JSS, Dr. DER, Dr. AMP, Dr. KSL, Dr. EH, Dr. PG, CS, and AK. The first draft of the manuscript was written by Dr. DSD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with principles of the Declaration of Helsinki. Approval was granted by the Pennsylvania State Institutional Review Board (Date: 12/1/2015, Study# 00003752).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Downs, D.S., Savage, J.S., Rivera, D.E. et al. Adaptive, behavioral intervention impact on weight gain, physical activity, energy intake, and motivational determinants: results of a feasibility trial in pregnant women with overweight/obesity. J Behav Med 44, 605–621 (2021). https://doi.org/10.1007/s10865-021-00227-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-021-00227-9