Abstract
Theoretically driven smartphone-delivered behavioral interventions that target mechanisms underlying eating behavior are lacking. In this study, we administered a 28-day self-paced smartphone-delivered intervention rooted in an operant conditioning theoretical framework that targets craving-related eating using mindful eating practices. At pre-intervention and 1-month post-intervention, we assessed food cravings among adult overweight or obese women (N = 104; M age = 46.2 ± 14.1 years; M BMI = 31.5 ± 4.5) using ecological momentary assessment via text message (SMS), self-reported eating behavior (e.g., trait food craving), and in-person weight. Seventy-eight participants (75.0%) completed the intervention within 7 months (‘all completers’), and of these, 64 completed the intervention within 3 months (‘timely completers’). Participants experienced significant reductions in craving-related eating (40.21% reduction; p < .001) and self-reported overeating behavior (trait food craving, p < .001; other measures ps < .01). Reductions in trait food craving were significantly correlated with weight loss for timely completers (r = .30, p = .020), this pattern of results was also evident in all completers (r = .22, p = .065). Taken together, results suggest that smartphone-delivered mindful eating training targeting craving-related eating may (1) target behavior that impacts a relative metabolic pathway, and (2) represent a low-burden and highly disseminable method to reduce problematic overeating among overweight individuals.
ClinicalTrials.gov registration: NCT02694731.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 60% of the risk for chronic illnesses such as diabetes and cardiovascular diseases in U.S. adults is mediated by health-related behavior, such as physical activity, dietary intake, and substance use (Bauer et al., 2014). To date, health behavior interventions targeting changes in diet and exercise have yielded weight-loss results that tend to fade over time (Dombrowski et al., 2014). Current behavioral interventions may meet with short-lived results because of the logistical burdens of engaging with such interventions. Alternatively, the interventions themselves may not impact the underlying mechanisms that drive eating behavior.
One way to address logistical burdens of engagement with lifestyle interventions is by delivering interventions via mobile phones. A fast-growing majority of US adults owns a smartphone (64% in 2015; Smith, 2015). More recent statistics indicate that 72% of US adults access news on their mobile phones (Mitchell et al., 2016). Mobile intervention delivery yields several advantages over traditional in-person methods. For example, mobile intervention delivery enables individuals to carry intervention tools with them wherever they go, thus allowing individuals to access behavioral support tools at the times and locations they need them the most (Klasnja & Pratt, 2012). Such accessibility is likely to increase adherence and engagement, which are associated with longer-lasting behavioral change and resultant health-related benefits. For example, in one 6-month three-group trial targeting weight loss, participant retention and weight loss were greater in the group receiving the intervention delivered via smartphone app (93% retained, −4.6 kg lost) than in groups receiving the intervention via a website (55% retained, −1.3 kg lost) or a paper-based group (53% retained; −2.9 kg lost; Carter et al., 2013). Additionally, mobile intervention delivery overcomes barriers to treatment engagement such as scheduling, travel, and time commitments. In sum, mobile intervention platforms may address burdensome or inaccessible elements of traditional lifestyle interventions. However, not all mobile interventions have yielded optimal results (Free et al., 2013).
Another reason why traditional diet and exercise programs may not achieve their intended weight-loss goals is that they may not effectively address the behavioral mechanisms that underpin obesity for many individuals. Craving-related eating is a commonly reported reason for difficulties with adhering to healthy eating plans (Massey & Hill, 2012; Meule et al., 2011; Potenza & Grilo, 2014), and relative to normal-weight people, overweight and obese people report more frequency and more intense cravings for highly palatable food (Chao et al., 2014; Mason & Epel, 2015). Indeed, the modern food environment is replete with cues to eat and easy access to highly palatable foods, which biases eating behavior away from innate, internal cues towards artificial, external cues. This externally cued “non-homeostatic eating,” also referred to as reward-related eating, contrasts with “homeostatic eating,” or eating cued by the innate physiological hunger system that detects caloric needs (Lowe & Levine, 2005).
Reward-related eating may stem from operant conditioning, which is one of the most basic learning processes known to man given the importance of caloric intake needed for survival. Behaviors that net positive effects are learned via positive and negative reinforcement (the core tenants of operant conditioning) and are reinforced by their consequences (rewards). In the context of eating, operant learning was adaptive when food sources were scarce, however, it is arguably maladaptive in an environment replete with caloric options. For example, repeatedly eating hyperpalatable food can contribute to conditioning individuals to expect pleasurable responses both (1) upon consuming a such food, which can trigger continued eating for pleasure, and (2) when observing stimuli they come to associate with the food, which can trigger eating (Volkow et al., 2008). Such stimuli can activate learned associations that trigger automatic eating—for reward or relief—in the absence of hunger (Dallman, 2010). This behavior is in line with theories of “emotional eating” (eating in response to triggers tied to our emotions rather than to feelings of physical hunger) as well as disinhibited eating (overeating in the presence of palatable foods or other stimuli such as stress), whereas adaptive stress responses would trigger a loss of appetite (Adam & Epel, 2007; Greeno & Wing, 1994; van Strien & Ouwens, 2003).
Habitual eating of highly palatable foods contributes to the reinforcement of craving for these habitually eaten foods. Such craving-related eating is a critical barrier to dietary adherence and resultant weight change, and reductions in craving-related eating have been associated with greater weight loss (Smithson & Hill, 2017). Yet, to date, traditional diet and exercise programs focus on dietary monitoring and physical activity, rather than the habitual behaviors developed and driven by reinforcement that underpins craving-related eating (Skinner, 1963).
Recent work in smoking cessation interventions has found craving to be a critical link of both positive and negative reinforcement (Brewer et al., 2013; Elwafi et al., 2013). Just as food cravings drive eating behavior, cigarette cravings drive smoking. Mindfulness training directly targets the experience of craving itself: For example, mindfulness training not only doubles quit rates for smoking, but also decouples the experiences of cigarette craving from smoking (Brewer et al., 2011; Elwafi et al., 2013). In contrast, cognitive interventions for addictions “treat around” craving by employing substitute or avoidance strategies.
Mindfulness can be defined as the awareness that arises when paying attention in the present moment, on purpose and nonjudgmentally (Kabat-Zinn & Hanh, 2009). In other words, when someone is “being mindful,” the attitudinal quality of not judging and allowing their experience to unfold with curiosity rather than trying to control it helps them not be pushed or pulled by positive and negative affective states.
Researchers have begun to use mindfulness principles to develop interventions targeting obesity by interrupting habits that perpetuate craving-related eating (Dalen et al., 2010; Forman et al., 2013; Godfrey et al., 2015; Katterman et al., 2014; O’Reilly et al., 2014). Such interventions target craving experiences by increasing awareness and acceptance of one’s emotional experience to reduce reactivity (e.g., eating in response to an emotion; Forman & Butryn, 2015). Indeed, increases in mindful eating have been associated with reductions in eating of sweet foods and desserts, as well as reductions in fasting blood glucose (Mason et al., 2016b). One intervention model that brings together mindfulness with principles of operant conditioning uses three core steps to address patterns of behaviors enacted without awareness. The first step focuses on increasing awareness of one’s habitual behavior, as it is essential that one becomes aware of the triggers (e.g., emotional experiences, places, people, situations) and behavioral responses (e.g., eating cookies) that comprise the habit. The second step is clearly seeing the outcomes that follow from enacting a given habit by forming testable hypotheses for individuals to consider (e.g., “Do I truly feel better after eating cookies?”). This step promotes the recalibration of the experience of reward, which is a critical component of operant conditioning: future behavior is driven by perceived reward value (Skinner, 1963). The third step is learning to exist with, rather than distract oneself from, the experience of craving. Cultivating a curiosity about one’s in-the-moment experience and the skills to co-exist with the discomfort that accompanies experiences of craving promote one’s ability to observe the craving until it has subsided. All three steps rely on core principles of mindfulness: developing a non-judgmental awareness of one’s experience.
Traditional behavioral weight-loss interventions generally focus on increasing awareness of one’s eating behavior through dietary self-monitoring (Baker & Kirschenbaum, 1993; Wadden, 1993) and goal setting, which fit with the first step of cultivating awareness. Dietary self-monitoring can be effective in promoting weight loss when practiced consistently. One review of 15 studies reported that individuals who consistently completed dietary self-monitoring logs lost the most weight, and the more thorough the logs, the greater the weight loss (Burke et al., 2011). However, daily tracking and logging of everything one eats is time-consuming and difficult to sustain over time. Dietary self-monitoring may also fall short in that it does not emphasize clarity around the outcomes that follow craving-related eating (step 2) or the skills to coexist with, rather than act on, food cravings (step 3). Similarly, goal setting is a common component of interventions targeting behavior change (e.g., Pearson, 2012), and identifying and increasing awareness of one’s goals fits with increasing one’s clarity of how behavior serves (or does not serve) such goals. From an operant conditioning standpoint, clearly seeing the consequences of one’s eating behavior (i.e., how this impacts goals) is critical for changing the perceived reward value of the behavior, and thus for driving behavior change. Finally, cultivating the ability to exist with cravings (rather than to habitually react by eating) may better address problematic craving-related eating that contributes to weight gain. This may also help individuals move from prescriptive and externally motivated behavior (e.g., staying under a calorie limit for the day), to internally focused behavior (e.g., noticing what it feels like to stop eating when one is full).
Study overview
As indicated for early intervention testing by the Obesity-Related Behavioral Intervention Trials (ORBIT) framework (Czajkowski et al., 2015), we administered a theory-driven, self-paced, smartphone-delivered intervention focusing on craving-related eating in a proof-of-concept, single-arm, unmasked trial (Phase IIA). This intervention included self-paced lessons delivered over a minimum of 28 days, with one new lesson becoming available each day. We defined intervention completion within a 3-month period (approximately one lesson every 2–3 days) as ‘timely completion.’ We followed participants’ progress for up to 7 months due to a limited funding source and timeframe (12-months total) for this study. We reported on feasibility (retention and engagement), change in behavioral targets (craving-related eating), and associations between changes in behavioral targets and a clinical outcome (weight). We hypothesized that (1) recruitment would be feasible and that most participants would complete the intervention, (2) craving-related eating as indexed by ecological momentary assessment (EMA) and self-reported eating behavior would be significantly reduced following intervention completion, and (3) reductions in our intervention target (craving-related eating) would be associated with improvement in a clinical outcome (weight). We also explored user engagement metrics and report those alongside recruitment and feasibility results. This trial was registered at ClinicalTrials.gov (NCT02694731).
Methods
Study design and sample size
This was a single-arm clinical trial that assessed participants at pre- and post-intervention. The target sample size of 105 was based on the effect size found in a study of an intensive 6-month dietary intervention that reduced frequency of craving-related eating from 64.4% of the time at pre-intervention to 26.4% of the time at post-intervention (a 59% reduction; Gilhooly et al., 2007). Given that the present intervention is substantially less intensive than that administered by Gilhooly and colleagues, we predicted a 30% reduction in the frequency of craving-related eating (from 64 to 44%, having arrived at 44% by solving for XX% in the following equation: [(64.4% − XX%)/64.4%] = 30%) and used a two-sided paired t test, as was done in previous investigations of changes in food craving over time (Gilhooly et al., 2007). Attrition rates commonly observed in smartphone-delivered intervention studies approximate ~25% (Brindal et al., 2013; Carter et al., 2013; Thomas & Wing, 2013), so we sought to recruit 105 participants to allow for an analytic sample of 70 participants. This would provide 88% power to detect a two-sided statistically significant reduction in craving-related eating at the .05 level while adjusting for attrition (Lachin, 1981).
Participant recruitment
We recruited participants using social media outlets such as Facebook and Google ads, as well as campus-wide email digests at the University of California, San Francisco (UCSF). We sent targeted physical letters to UCSF patients who were potentially eligible and who had consented to be contacted by research studies via a secure university participant matching service. All participants were directed to a web-based screening survey (www.qualtrics.com) to verify eligibility (see Participants). Eligible participants identified dates when they could receive text message assessments and accessed an online scheduling service (www.youcanbook.me) to book a date and time for their pre-intervention visit. During the first text message assessment period, participants were disqualified from the study if they did not (1) show adequate engagement by responding to at least 7 of the 9 messages, and (2) report at least 3 instances of craving-related eating (see Measures for detailed text message assessment protocol).
Participants
Eligible participants were women aged at least 18 years, were overweight or obese (as defined by having a body mass index (BMI) of 25 or greater), did not have diabetes (per self-report), had a smartphone operating iOS or Android, experienced food cravings most days of the week, responded to at least 7 of 9 messages at pre-intervention assessment, and reported at least 3 instances of craving-related eating in these messages. Participants were required to attend an in-person baseline assessment in the San Francisco Bay Area. Enrollment in the study was rolling; participants began the intervention after completing all baseline study components.
Intervention
We administered a 28-day mindful eating intervention in the form of a mobile application that functions on both iOS and Android platforms. Intervention components center on (1) the scientific underpinnings of how food cravings arise and are reinforced, (2) research on the behavioral conditioning processes by which responses to food cravings become habitual, and (3) research showing how mindfulness directly targets craving to change behavior. The content for this intervention was developed based upon a combination of clinical experience with individuals with binge eating disorder and previously developed in-person and app-based mindfulness training protocols for addictions (Brewer et al., 2009, 2011; Garrison et al., 2015). Content was successively iterated with several groups of pilot testers (both clinical and non-clinical populations) using individual and focus group-based feedback before being finalized for the current trial. Course materials teach users to attend to three aspects of eating: Why, what, and how: Why they eat, including environmental and emotional triggers unrelated to homeostatic hunger; What types of food are most likely to lead to and reinforce cravings; and How to eat with awareness and mindful attention to physiological cues. The intervention lessons (modules) and app features are designed to help individuals efficiently incorporate the didactic content and mindfulness tools and practices into their normal daily activities and eating routines.
Intervention structure and components
Users access one 5- to 10-min module per day. Each module includes a video lecture with animations and straightforward guidance as to how to practice the mindfulness principles being taught (typically a meditative or mindful eating technique). Additional bonus modules are unlocked after modules 1, 2, 5, 10, and 18. Modules for future days are locked to prevent users from skipping ahead; however, users have unlimited access to review previous modules. New modules will not unlock unless the user has completed the current module and a new calendar day has started. Users can take days off from the app and resume where they left off, or delay starting a new module to practice their current skills. Thus, completing the intervention requires a minimum of 28 days of active engagement (Table 1).
In addition to the daily modules, participants can access brief review materials (e.g., videos and animations) and tools to aid in mindfully “riding out” food cravings as they occur. One such tool is the “Stress Test” wherein users complete a short mindfulness exercise that encourages them to take a moment to check in with their body to see what they are feeling at that moment and endorse specific symptoms accordingly. They then receive feedback about whether these are most consistent with stress, habit, or homeostatic hunger and are then given a suggestion to either eat mindfully (if they have not eaten in a while), or to use a mindfulness exercise to ride out a craving. A second mindfulness tool is the “Want-O-Meter” button, which guides users through a craving as they are experiencing it. A user’s responses to questions in the Want-O-Meter funnel them to additional tools, such as the RAIN exercise (Recognize, Accept, Investigate, and Note what cravings feel like as they arise and pass). The user can also choose to eat in response to the craving with awareness—rather than eat automatically or with a sense of compulsion. The app then provides a mindful eating exercise that can help users to eat a craved food in moderation. Engagement in each of these activities helps to disrupt the automatic, “mindless” indulgence of cravings and to emphasize attention to the body’s signals.
Users can set reminders that encourage them to check in with their hunger and emotional state, and to use mindfulness skills that help cultivate mindful eating habits. Users can also enter and track goals. After the 28 modules are complete, all tools and previous modules remain available indefinitely to support ongoing mindful eating and skill use.
Materials and measures
We collected anthropometric, standard self-report, and ecological momentary measures at pre-intervention and 1 month after completing the final module of the intervention (post-intervention). This allowed participants to complete the intervention at their own pace, which often included spending 1 or more days consolidating their new knowledge, practicing mindfulness skills, or using the in-app tools prior to starting the next module.
Ecological momentary assessment (EMA)
Participants received text messages at 11:30 AM, 4:30 PM, and 9:00 PM for 3 days spread over the course of 1 week (2 weekdays and 1 weekend day) 1 week before beginning the intervention (pre-intervention) and 1 month after completing the intervention (post-intervention). Participants responded to an item asking, “did you eat or drink something because of a craving in the last hour?” to which they responded yes or no. We refer to this variable as “craving-related eating” (Table 3).
Body mass index (BMI)
Participants removed their shoes and wore a paper gown with undergarments while being weighed on a Tanita BC-568 (www.tanita.com). A research assistant assessed height used a wall-mounted measure. We computed BMI as kg/m2.
Self-report questionnaires
Participants completed traditional self-report questionnaires that assess food cravings, as well as the related constructs of reward-related eating and eating to cope, enhance, conform, or socialize (i.e., non-homeostatically driven eating).
Food Craving Questionnaire–Trait–Reduced (FCQ–T–R; Meule et al., 2014)
The 15-item FCQ-T-R assesses (1) preoccupation with food, or obsessive thoughts about food and eating, (2) loss of control over eating, or difficulty regulating eating behavior when exposed to food cues, (3) positive outcome expectancy, or believing that eating is positively reinforcing, and (4) emotional craving, or the tendency to crave food when experiencing high levels of emotion. Higher FCQ-T-R scores have been associated with more frequent thinking and eating of high calorie snacks (Richard et al., 2017) and weight gain over time via increases in disinhibited eating (Meule et al., 2017). Items are answered on a 6-point scale from 1 (never) to 6 (always). The total score was computed as the sum of all items, with higher sores indicating greater trait food craving. Internal consistency in this sample was very high (baseline α = .94; 1-month post-intervention α = .95), similar to the level observed in validation studies (Meule et al., 2014) and to the highest criterion described as desirable by Bland and Altman (1997).
Reward-based Eating Drive Scale (RED; Epel et al., 2014)
The 9-item RED scale assesses reward-driven eating. Sample items include, “When I start eating, I just can’t seem to stop” (lack of control), “I don’t get full easily” (lack of satiety), and “Food is always on my mind” (preoccupation with food). Participants answered items on a scale from 1 (strongly disagree) to 5 (strongly agree). Greater RED scores have been associated with greater weight and weight gain over time (Epel et al., 2014) as well as stronger daily craving experiences (Mason et al., 2015), and reductions in RED scores have been associated with weight loss (Mason et al., 2016a, b). The total score was computed as the sum of all items, with higher scores reflecting higher reward-based eating drive. Internal consistency in this sample (baseline α = .75; 1-month post-intervention α = .84) was medium.
Palatable Eating Motives Scale (PEMS; Burgess et al., 2014)
The 19-item PEMS assesses four motives for eating tasty food (social, conformity, enhancement, and coping motives) and is modeled after the Drinking Motives Questionnaire (Cooper, 1994). Each subscale has 5 items, except the coping subscale (4 items). PEMS scores have been associated with weight gain over time (Boggiano et al., 2015a, b), binge eating (Boggiano, 2016), and ecologically assessed reasons for eating (Boggiano et al., 2015b). Items are answered on a 5-point scale (almost never/never, some of the time, half of the time, most of the time, almost always/always). The total scores for each subscale were computed as the mean of items for that subscale, with higher scores indicating greater eating of tasty food for that motive. Internal consistency in this sample (baseline α = .87; 1-month post-intervention α = .91) was high.
Intervention engagement
We quantified intervention engagement by assessing the following metrics between the first and final lesson (possible range: 28 days–150 days): percent of days using app; number of times app opened per day; and the number of minutes the app was open per day. For participants who did not complete the entire 28-day program, we quantified the above metrics between the first day of app use and the last day on which a module was completed. Internal consistency in this sample (baseline α = .87; 1-month post-intervention α = .91) was high.
Statistical analysis
To test our hypothesis that recruitment would be feasible and that most participants would complete the intervention (and to explore user engagement) we computed descriptive statistics (means) related to app use (H1). To test our hypothesis that craving-related eating, indexed both by ecological momentary assessment and self-reported eating behavior, as well as other eating-related constructs, would be significantly reduced following intervention completion, we conducted paired-samples t tests (H2). Secondarily, to examine (a) how the number of EMA assessments responded to (quantified as a count) and time to intervention completion (quantified as number of days) may have impacted change from pre- to 1-month post-intervention in EMA-assessed craving-related eating, and (b) how time to intervention completion may have impacted change from pre- to 1-month post-intervention in trait craving, we conducted logistic and linear mixed regression analyses using STATA (xtmelogit). To test our hypothesis that reductions in our target behavior (craving-related eating) would be associated with reductions in a clinical outcome (weight), we examined correlations between change in craving-related eating and weight from pre- to 1-month post-intervention (H3). We report changes in weight from pre- to 1-month post-intervention using t tests as well as linear mixed regression analysis to ascertain the impact of time to intervention completion on any observed change in weight using STATA (xtmelogit). Our a priori assessment schedule (registered in ClinicalTrials.gov) was to examine change from pre-intervention to 1-month post-intervention, so as to allow users to consolidate and practice the mindful eating skills they had learned.
We computed analyses using all participants who began the intervention, which yielded the ‘all completer’ sample, defined as participants who completed the intervention within 7 months. We also computed analyses in a ‘timely completer’ sample, defined as participants who completed the intervention within 3 months.
Results
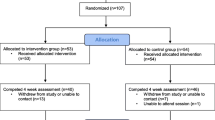
See Table 2 for baseline participant characteristics and Fig. 1 for participant recruitment and retention flow.
Hypothesis 1: Feasibility and retention
A total of 1651 individuals completed the online screener; 719 qualified to participate in the study; and 104 ultimately completed all consent procedures and participated in the study (see Fig. 1). Of all 104 participants who completed a baseline assessment and therefore were provided with the mobile intervention on their smartphones, 6 withdrew for one of the following significant life events: Death of a family member (n = 3), moving to another state (n = 1), undergoing major surgery (n = 1), and beginning a second full-time job (n = 1). The mean ± SD and the median time to intervention completion were 57.74 ± 28.13 and 48 days, respectively.
Seventy-eight (75.0%) completed the 28-day intervention between March 3, 2016, and July 31, 2016 (7 months; all completer sample). Of these 78 participants, 72 completed the in-person follow-up visit (1-month post-intervention) and 73 completed the online self-report measures at follow-up. Sixty-four participants (61.5%) completed the intervention between March 3rd, 2016, and June 1st, 2016 (3 months; timely completer sample). Of these 64 participants, 61 completed the in-person follow-up visit (1-month post-intervention) and 62 completed the online self-report measures at follow-up. There were no particular modules after which participants appeared to drop out (see Supplementary Table 1). Thus, data support our first hypothesis: We recruited our intended number of participants and the majority of participants completed the intervention.
Engagement outcomes
Participants (n = 104) completed an average of 22.9 ± 9.46 modules, and intervention completion spanned from 29 to 139 days. On average, participants accessed the app on 30.7 ± 14.29 days between their first and last module (module 28 or last completed module if they did not complete all 28 modules). On average, participants accessed the app 1.96 ± 0.99 times per day (~2 sessions), used the app for 5.99 ± 2.22 min per session, and used the app for 11.74 ± 8.89 min per day.
Hypothesis 2: Changes in craving-related eating
As shown in Table 3 (All Completers) one participant did not complete the post-intervention EMA measures of craving-related eating, and three participants did not present for an in-person follow-up assessment, and therefore did not provide weight data (one did complete the post-intervention self-report survey). As shown in Table 3, all completer analyses (n = 78 completers) and timely completer analyses (n = 64) evidenced statistically significant reductions in craving-related eating indexed by each EMA and self-report (FCQ-T-R). Participants also evidenced reductions in reward-related eating and eating for social reasons, to conform, to cope with emotions, and to enhance experiences. Logistic (a) and linear (b) mixed model analyses indicated that the patterns of change and statistical significance of reductions in (a) EMA-assessed craving-related eating (all completers: OR = 0.729, p < .001; timely completers: OR = 0.754, p < .001), and (b) self-report trait craving (all completers: b = 14.27, p < .001; timely completers: b = 12.73, p < .001) remained after accounting for missing responses at either baseline or follow-up (a), as well as the number of days to complete the intervention (a and b). The number of days to complete the intervention was not statistically significantly associated with reductions in EMA-assessed craving-related eating (all completers: r = −.06, p = .612; timely completers: r = 0.27; p = .835) or trait craving (all completers: r = −.13, p = .276; timely completers: r = .17, p = .180).
Hypothesis 3: Associations between changes in mechanistic target (craving-related eating) and clinical outcome (weight)
Greater reductions in trait food craving were associated with greater reductions in weight all completers (r = .23, p = .052) and timely completers (r = .33, p = .010). Greater reductions in EMA-assessed craving-related eating, however, were not statistically significantly associated with changes in weight in the all completer or timely completer analyses (ps > .50).
As shown in Table 3, all completer analyses did not evidence statistically significant weight loss from pre- to 1-month post-intervention. Timely completer analyses, however, indicated statistically significant weight loss, which may suggest that completing the intervention within 3 months (rather than longer) may confer greater clinical improvement. Mixed model analysis revealed that fewer days to intervention completion tended to be associated with greater weight loss among the timely completers b days to complete = −0.28, p = .082), but not across the all completers (p = .202).
Discussion
Data from this trial demonstrate that, among overweight and obese women who experience food cravings, a smartphone-delivered mindful eating intervention can effectively retain and engage participants, that it is associated with reductions in craving-related eating, and that changes in self-reported craving-related eating are associated with reductions in weight. To our knowledge, this is the first study to show that a smartphone-based mindful eating intervention can directly target and change learned mechanisms of craving-related eating and that change in these mechanisms may be associated with a clinically relevant metabolic outcome (weight). These analyses revealed that the time a user takes to complete the intervention may be an important determinant of weight-loss outcome: The association between changes in craving-related eating and weight were stronger among participants who completed the intervention within 3 months.
These analyses demonstrate that deploying mindful eating training via smartphone is feasible. Participant retention was similar to rates observed in other smartphone-delivered interventions focused on weight loss, though this intervention was less intensive than others, which renders comparison difficult. For example, our retention rate was similar to that observed in a 6-month mobile weight-loss intervention that included a smartphone application and a podcast, as well as the opportunity to interact with study counselors and other participants via social media applications (89%; Turner-McGrievy & Tate, 2011), a mixed methods intervention that included in-person group meetings and smartphone-based dietary self-monitoring (85%; Burke et al., 2012), and a mobile intervention that included text messages, apps, coaching calls, and emails that targeted weight-gain prevention (88%; Allman-Farinelli et al., 2016). Thus, understanding factors critical to retention in behavior change mobile interventions poses a challenge, as these interventions are diverse in intensity (e.g., quantity of intervention), content (e.g., type of intervention), and delivery method (e.g., combinations that may include social media, personalized feedback, in-person orientations).
A recent meta-analysis indicated that the majority of smartphone-delivered interventions targeting obesity have been tested in samples that were at least 80% White (Hutchesson et al., 2015). Our study sample was slightly more diverse (68% White), which suggests that e-health interventions may be becoming increasingly accessible (Bennett et al., 2014). Given the growing diversity of the US (Colby & Ortman, 2015) and that obesity and overweight differentially impact non-White populations (Ogden et al., 2015), it is important to examine the efficacy of smartphone-based interventions targeting weight loss in diverse populations (Bennett et al., 2014).
Participants’ significant reductions in trait craving from pre- to 1-month post-intervention suggest that the intervention is accessing a relevant behavioral target, as change in this measure has been associated with greater weight loss in more intensive trials (Gilhooly et al., 2007; Smithson & Hill, 2017), and preliminarily, in these analyses. This intervention led to significant reductions in trait-like craving, greater levels of which are associated with poorer dieting success (Meule et al., 2011) and less weight loss over time (Buscemi et al., 2017). Furthermore, this intervention led to significant reductions in eating for reward and to cope with emotions, each of which are associated with weight change over time (Boggiano et al., 2015a, b; Epel et al., 2014; Mason et al., 2016a). Thus, smartphone-delivered mindful eating training shows promise not just for short-term behavior change, but also for motivational and psychological changes that can build a basis for sustainable change.
Previous studies of in-person delivered mindfulness training targeting weight loss have yielded mixed results. One factor that may have contributed is difficulty in standardizing in-person treatment delivery. For example, one recent trial investigating a mindfulness intervention for obesity found that participants receiving mindfulness instruction achieved differential weight depending on which teacher led their group (Daubenmier et al., 2016). Smartphone-delivered interventions that deliver mindfulness training in pre-recorded modules ensure that all users receive the same content in the same format. Furthermore, they allow users to access intervention content at any moment. This may optimize acquisition and application in the real world.
A second reason why mindfulness training for obesity may have met with mixed results is that previous interventions may have focused on general, domain-neutral skills instead of specifically targeting the most meaningful drivers of eating behavior. For example, Kearney and colleagues (Kearney et al., 2012) found that receiving training in mindfulness-based stress reduction (MBSR) was not associated with reduced emotional or uncontrolled eating. Indeed, a recent review of the effects of mindfulness-based interventions on emotional eating reported that the interventions that specifically targeted emotional eating led to reductions in such eating, whereas those that promoted mindfulness practices in general (e.g., stress reduction) did not (O’Reilly et al., 2014). Formal meditation practice may contribute to the development of increased attention and awareness, which likely lead to improved ability to notice what the experience of craving feels like—but may fall short in terms of providing the needed tools to adapt one’s responses.
Moreover, formal meditation practice may often occur outside of craving experiences and may therefore be more supportive of the ability to recalibrate one’s reward learning and coexist with discomfort rather than a primary intervention for addressing craving in the moment. Interventions like the one tested here specifically support the application of mindfulness skills to the specific experiences and contexts that are most relevant for participants’ current goals (e.g., changing eating behavior so as to lose weight). For example, informal and in-the-moment mindfulness practices, such as those accessible in this smartphone-delivered intervention (e.g., RAIN), have been more directly associated than formal meditation in reducing cigarette smoking (Elwafi et al., 2013).
This study suggests that smartphone-delivered mindful eating training may specifically target craving-related eating. This is important as it supports previously hypothesized mechanistic underpinnings of how mindfulness training changes behavior, and fits with prior work on other craving-related behavior. For example, previous trials have found that mindfulness training focused on craving-related smoking can decouple the experiences of craving and smoking, thereby reducing craving-related smoking (Elwafi et al., 2013). The current study shows similar decoupling of craving and behavior, in this case, eating. This is important because (1) it is directly in line with the theoretical aspects of operant conditioning, and (2) it lends greater evidence for the core mechanistic underpinnings of how mindfulness elicits behavior change.
Further assessing smartphone-delivered mindfulness interventions focused on dismantling the effects of craving on eating behaviors will shed light on the mechanisms that are common to craving-driven behaviors, and how to best intervene upon them (e.g., Brewer et al., 2013). Taken together, self-reports (e.g., traditional self-report, in vivo assessments), biomarkers (e.g., markers of metabolic health), and brain imaging (e.g., fMRI) may clarify optimal intervention targets and their assessment in the context of the associative learning framework. These findings may help to refine and streamline treatment development such that future therapies directly target key positive and negative reinforcement in the operant conditioning pathway.
This protocol assessed craving in vivo, using ecological momentary assessment, as well as using traditional self-report measures. One systematic review found that craving is a stronger predictor of behavior (e.g., smoking) when assessed in close temporal proximity to the actual behavior, thus, EMA is likely to capture more craving experiences than an individual may recollect at a later time (Serre et al., 2015). Other work has shown that mobile-based EMA assessments of food cravings lead to more complete reporting in comparison to pencil-and-paper methods (Berkman et al., 2014). This study used smartphone-based assessments that users completed in less than 30 s, and this platform promoted more complete data collection. Thus, smartphone-based assessment, as deployed in this study, will increase the likelihood of mechanistically-based, high fidelity, and cost-effective assessments. Notably, reductions in EMA-assessed craving-related eating were not significantly correlated with changes in weight; however, this may be a measurement and statistical issue: The craving-related eating EMA item was binary, and as such we aggregated these responses into averages for each pre- and 1-month post-intervention before subtracting one from the other. This procedure likely yielded a variable with a restricted range.
Limitations and future directions
This study had several limitations that suggest future directions. The study focused on mechanistic outcomes and, given its early phase (Czajkowski et al., 2015), did not rigorously collect clinical outcomes. For example, we did not standardize the time of day at which we weighed participants; hence, weight data reported here should be interpreted with caution and future work should ensure consistent clinical measurements. Future designs should add an active control group, as these analyses are unable to account for factors such as interaction with one’s smartphone (which may, for example, increase self-monitoring behavior), goals setting, motivation, and the fact that participants were aware of their participation in a study focused on food cravings.
Future work should also collect additional measures. These might include more distal measurements of craving-related eating and weight, as this pilot trial followed participants between 2 and 6 months after intervention start. Although the study used both daily and pre-post measures of craving, which are subject to typical limitations of self-report (e.g., experimenter demand) it included only pre-post measures of weight. Future work should also include a more thorough demographic assessment which might include parental or marital status, and should include income and education levels. Future work could also add daily self-weighing that sends data to the research team directly (e.g., Chen et al., 2016) to make assessments of each craving and weight more parallel. Future studies should assess the extent to which additional modalities, such as in-app coaching or group support, facilitate increased engagement with the program. Greater intervention engagement via such modalities may lead to larger changes in clinical outcomes (i.e., weight change). Further, longer-term follow up will help to determine to degree to which this intervention may have a “sleeper effect,” akin to those observed in interventions testing metacognitive training and computer-assisted cognitive behavioral therapy (Carroll et al., 2009; Moritz et al., 2014). In this context, individuals may incorporate mindfulness as a new habit loop over time, which may contribute to gradual, but sustained, change in behavior (e.g., less craving related eating) and change in clinical outcomes (e.g., weight loss).
This trial also required that participants own a smartphone, and although that may have biased the sample, rates of smartphone ownership in the US have been accelerating in recent years (Smith, 2015). Despite this requirement, this study sample was more racially/ethnically diverse than has been reported in other mobile intervention studies (Hutchesson et al., 2015). Although providing participants with a smartphone for the purpose of a weight-loss study has been done (Wayne & Ritvo, 2014), the provision of a smartphone with internet access in and of itself can precipitate significant lifestyle changes that may contribute to, or detract from, health. For example, acquisition of a smartphone may increase use of tools to track health behavior, or might increase sedentary time spent playing computerized games. Thus, the present intervention avoided any effects of newly providing individuals with smartphones.
This study sample may have been particularly motivated to engage in behavioral change, which may have impacted outcomes. For example, though many potentially eligible participants completed the screening survey and became eligible respondents (n = 719), the majority of them (n = 493) did not complete the actual baseline survey. This may have been because individuals are increasingly frequently “surfing” the internet and simply clicking around on different links more out of curiosity than of actual motivation. Alternatively, eligible respondents wished to begin the online intervention but, upon learning that a baseline survey and in-person visit would be required before they would receive the intervention material, may not have had enough motivation to overcome this hurdle. In contrast, this may have biased the final sample of participants toward greater motivation to engage with an intervention targeting food-craving experiences. This suggests that targeting this barrier (i.e., problematic food cravings) to improved health may be important for a subset of overweight individuals. Future research should incorporate standard measures of motivation for change and/or other measures associated with adherence, such as trait-level conscientiousness (Bogg & Roberts, 2013). Future research studies using internet-based recruitment methods will also need to develop new methodologies to differentiate “curious clickers” who come to a website from those with true interest in the advertised study.
Conclusions
The present work introduces a smartphone-delivered mindful eating intervention that is supported by an operant model of eating behavior. This theory-driven intervention mechanistically targets craving-related eating, and in so doing seeks to bring about improvements in clinically relevant outcomes, such as weight. The results suggest that this intervention is feasible, engaging, and may reduce craving-related eating over time. Future work should bring this research to Phase IIB (Czajkowski et al., 2015), which would introduce a control condition and require more rigorous measurement of clinical outcomes.
References
Adam, T. C., & Epel, E. S. (2007). Stress, eating and the reward system. Physiology & Behavior, 91, 449–458.
Allman-Farinelli, M., Partridge, S. R., McGeechan, K., Balestracci, K., Hebden, L., Wong, A., et al. (2016). A mobile health lifestyle program for prevention of weight gain in young adults (TXT2BFiT): Nine-month outcomes of a randomized controlled trial. JMIR mHealth and uHealth, 4, e78.
Baker, R. C., & Kirschenbaum, D. S. (1993). Self-monitoring may be necessary for successful weight control. Behavior Therapy, 24, 377–394.
Bauer, U. E., Briss, P. A., Goodman, R. A., & Bowman, B. A. (2014). Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. The Lancet, 384, 45–52.
Bennett, G. G., Steinberg, D. M., Stoute, C., Lanpher, M., Lane, I., Askew, S., et al. (2014). Electronic health (eHealth) interventions for weight management among racial/ethnic minority adults: A systematic review. Obesity Reviews, 15, 146–158.
Berkman, E. T., Giuliani, N. R., & Pruitt, A. K. (2014). Comparison of text messaging and paper-and-pencil for ecological momentary assessment of food craving and intake. Appetite, 81, 131–137.
Bland, J. M., & Altman, D. G. (1997). Statistics notes: Cronbach’s alpha. BMJ, 314, 572.
Bogg, T., & Roberts, B. W. (2013). The case for conscientiousness: Evidence and implications for a personality trait marker of health and longevity. Annals of Behavioral Medicine, 45, 278–288.
Boggiano, M. M. (2016). Palatable eating motives scale in a college population: Distribution of scores and scores associated with greater BMI and binge-eating. Eating Behaviors, 21, 95–98.
Boggiano, M. M., Wenger, L. E., Turan, B., Tatum, M. M., Morgan, P. R., & Sylvester, M. D. (2015a). Eating tasty food to cope. Longitudinal association with BMI. Appetite, 87, 365–370.
Boggiano, M., Wenger, L. E., Turan, B., Tatum, M. M., Sylvester, M. D., Morgan, P. R., et al. (2015b). Real-time sampling of reasons for hedonic food consumption: Further validation of the palatable eating motives scale. Name: Frontiers in Psychology, 6, 1–8.
Brewer, J. A., Elwafi, H. M., & Davis, J. H. (2013). Craving to quit: Psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychology of Addictive Behaviors, 27, 366–379.
Brewer, J. A., Mallik, S., Babuscio, T. A., Nich, C., Johnson, H. E., Deleone, C. M., et al. (2011). Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence, 119, 72–80.
Brewer, J. A., Sinha, R., Chen, J. A., Michalsen, R. N., Babuscio, T. A., Nich, C., et al. (2009). Mindfulness training and stress reactivity in substance abuse: Results from a randomized, controlled stage I pilot study. Substance Abuse, 30, 306–317.
Brindal, E., Hendrie, G., Freyne, J., Coombe, M., Berkovsky, S., & Noakes, M. (2013). Design and pilot results of a mobile phone weight-loss application for women starting a meal replacement programme. Journal of Telemedicine and Telecare, 19, 166–174.
Burgess, E. E., Turan, B., Lokken, K. L., Morse, A., & Boggiano, M. M. (2014). Profiling motives behind hedonic eating: Preliminary validation of the palatable eating motives scale. Appetite, 72, 66–72.
Burke, L. E., Styn, M. A., Sereika, S. M., Conroy, M. B., Ye, L., Glanz, K., et al. (2012). Using mhealth technology to enhance self-monitoring for weight loss: A randomized trial. American Journal of Preventive Medicine, 43, 20–26.
Burke, L. E., Wang, J., & Sevick, M. A. (2011). Self-monitoring in weight loss: A systematic review of the literature. Journal of the American Dietetic Association, 111, 92–102.
Buscemi, J., Rybak, T. M., Berlin, K. S., Murphy, J. G., & Raynor, H. A. (2017). Impact of food craving and calorie intake on body mass index (BMI) changes during an 18-month behavioral weight loss trial. Journal of Behavioral Medicine, 40, 565–573.
Carroll, K. M., Ball, S. A., Martino, S., Nich, C., Babuscio, T. A., & Rounsaville, B. J. (2009). Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A 6-month follow-up of CBT4CBT. Drug and Alcohol Dependence, 100, 178–181.
Carter, M. C., Burley, V. J., Nykjaer, C., & Cade, J. E. (2013). Adherence to a smartphone application for weight loss compared to website and paper diary: Pilot randomized controlled trial. Journal of Medical Internet Research, 15, e32.
Chao, A., Grilo, C. M., White, M. A., & Sinha, R. (2014). Food cravings, food intake, and weight status in a community-based sample. Eating Behaviors, 15, 478–482.
Chen, F., Su, W., Becker, S. H., Payne, M., Sweet, C. M. C., Peters, A. L., et al. (2016). Clinical and economic impact of a digital, remotely-delivered intensive behavioral counseling program on Medicare beneficiaries at risk for diabetes and cardiovascular disease. PLoS ONE, 11, e0163627.
Colby, S. L., & Ortman, J. M. (2015). Projections of the size and composition of the US population: 2014 to 2060. US Census Bureau, 9, 1–13.
Cooper, M. L. (1994). Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment, 6, 117–128.
Czajkowski, S. M., Powell, L. H., Adler, N., Naar-King, S., Reynolds, K. D., Hunter, C. M., et al. (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 34, 971–982.
Dalen, J., Smith, B. W., Shelley, B. M., Sloan, A. L., Leahigh, L., & Begay, D. (2010). Pilot study: Mindful Eating and Living (MEAL): Weight, eating behavior, and psychological outcomes associated with a mindfulness-based intervention for people with obesity. Complementary Therapies in Medicine, 18, 260–264.
Dallman, M. F. (2010). Stress-induced obesity and the emotional nervous system. Trends in Endocrinology and Metabolism, 21, 159–165.
Daubenmier, J., Moran, P. J., Kristeller, J., Acree, M., Bacchetti, P., Kemeny, M. E., et al. (2016). Effects of a mindfulness-based weight loss intervention in adults with obesity: A randomized clinical trial. Obesity, 24, 794–804.
Dombrowski, S. U., Knittle, K., Avenell, A., Araújo-Soares, V., & Sniehotta, F. F. (2014). Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ, 348, g2646. doi:10.1136/bmj.g2646.
Elwafi, H. M., Witkiewitz, K., Mallik, S., IV, IV Thornhill, T. A., & Brewer, J. A. (2013). Mindfulness training for smoking cessation: Moderation of the relationship between craving and cigarette use. Drug and Alcohol Dependence, 130, 222–229.
Epel, E., Tomiyama, A. J., Mason, A., Laraia, B. A., Hartman, W., Ready, K., et al. (2014). The reward-based eating drive scale: A self-report index of reward-based eating. PLoS ONE, 9, e101350.
Forman, E. M., & Butryn, M. L. (2015). A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite, 84, 171–180.
Forman, E. M., Butryn, M. L., Juarascio, A. S., Bradley, L. E., Lowe, M. R., Herbert, J. D., et al. (2013). The mind your health project: A randomized controlled trial of an innovative behavioral treatment for obesity. Obesity, 21, 1119–1126.
Free, C., Phillips, G., Galli, L., Watson, L., Felix, L., Edwards, P., et al. (2013). The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med, 10, e1001362.
Garrison, K. A., Pal, P., Rojiani, R., Dallery, J., O’Malley, S. S., & Brewer, J. A. (2015). A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: A study protocol. BMC Psychiatry, 15, 83.
Gilhooly, C. H., Das, S. K., Golden, J. K., McCrory, M. A., Dallal, G. E., Saltzman, E., et al. (2007). Food cravings and energy regulation: The characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. International Journal of Obesity, 31, 1849–1858.
Godfrey, K. M., Gallo, L. C., & Afari, N. (2015). Mindfulness-based interventions for binge eating: A systematic review and meta-analysis. Journal of Behavioral Medicine, 38, 348–362.
Greeno, C. G., & Wing, R. R. (1994). Stress-induced eating. Psychological Bulletin, 115, 444.
Hutchesson, M. J., Rollo, M. E., Krukowski, R., Ells, L., Harvey, J., Morgan, P. J., et al. (2015). eHealth interventions for the prevention and treatment of overweight and obesity in adults: A systematic review with meta-analysis. Obesity Reviews, 16, 376–392.
Kabat-Zinn, J., & Hanh, T. N. (2009). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Random House LLC.
Katterman, S. N., Kleinman, B. M., Hood, M. M., Nackers, L. M., & Corsica, J. A. (2014). Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eating Behaviors, 15, 197–204.
Kearney, D. J., Milton, M. L., Malte, C. A., McDermott, K. A., Martinez, M., & Simpson, T. L. (2012). Participation in mindfulness-based stress reduction is not associated with reductions in emotional eating or uncontrolled eating. Nutrition Research, 32, 413–420.
Klasnja, P., & Pratt, W. (2012). Healthcare in the pocket: Mapping the space of mobile-phone health interventions. Journal of Biomedical Informatics, 45, 184–198.
Lachin, J. M. (1981). Introduction to sample size determination and power analysis for clinical trials. Controlled Clinical Trials, 2, 93–113.
Lowe, M. R., & Levine, A. S. (2005). Eating motives and the controversy over dieting: Eating less than needed versus less than wanted. Obesity Research, 13, 797–806.
Mason, A., & Epel, E. (2015). Craving chocolate? A review of individual differences, triggers, and assessment of food cravings. In N. Avena (Ed.), Hedonic eating. Oxford: Oxford University Press.
Mason, A., Epel, E. S., Aschbacher, K., Lustig, R. H., Acree, M., Kristeller, J., et al. (2016a). Reduced reward-driven eating accounts for the impact of a mindfulness-based diet and exercise intervention on weight loss: Data from the SHINE randomized controlled trial. Appetite, 100, 86–93.
Mason, A., Epel, E. S., Kristeller, J., Moran, P. J., Dallman, M., Lustig, R. H., et al. (2016b). Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: Data from the SHINE randomized controlled trial. Journal of Behavioral Medicine, 39, 201–213.
Mason, A., Laraia, B. A., Daubenmier, J., Hecht, F. M., Lustig, R. H., Puterman, E., et al. (2015). Putting the brakes on the “drive to eat”: Pilot effects of naltrexone and reward based eating on food cravings among obese women. Eating Behaviors, 9, 53–56.
Massey, A., & Hill, A. J. (2012). Dieting and food craving. A descriptive, quasi-prospective study. Appetite, 58, 781–785.
Meule, A., Hermann, T., & Kübler, A. (2014). A short version of the Food Cravings Questionnaire—Trait: The FCQ-T-reduced. Frontiers in Psychology, 5, 1–10.
Meule, A., Richard, A., & Platte, P. (2017). Food cravings prospectively predict decreases in perceived self-regulatory success in dieting. Eating Behaviors, 24, 34–38.
Meule, A., Westenhöfer, J., & Kübler, A. (2011). Food cravings mediate the relationship between rigid, but not flexible control of eating behavior and dieting success. Appetite, 57, 582–584.
Mitchell, A., Gottfried, J., Barthel, M., & Shearer, E. (2016). 1. Pathways to news. Retrieved from http://www.journalism.org/2016/07/07/pathways-to-news/
Moritz, S., Veckenstedt, R., Andreou, C., Bohn, F., Hottenrott, B., Leighton, L., et al. (2014). Sustained and “sleeper” effects of group metacognitive training for schizophrenia: A randomized clinical trial. JAMA Psychiatry, 71, 1103–1111.
O’Reilly, G. A., Cook, L., Spruijt-Metz, D., & Black, D. S. (2014). Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obesity Reviews, 15, 453–461.
Ogden, C. L., Carroll, M. D., Fryar, C. D., & Flegal, K. M. (2015). Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief, 219, 1–8.
Pearson, E. S. (2012). Goal setting as a health behavior change strategy in overweight and obese adults: A systematic literature review examining intervention components. Patient Education and Counseling, 87, 32–42.
Potenza, M. N., & Grilo, C. (2014). How relevant is food craving to obesity and its treatment? Eating Behavior, 5, 164.
Richard, A., Meule, A., Reichenberger, J., & Blechert, J. (2017). Food cravings in everyday life: An EMA study on snack-related thoughts, cravings, and consumption. Appetite, 113, 215–223.
Serre, F., Fatseas, M., Swendsen, J., & Auriacombe, M. (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug and Alcohol Dependence, 148, 1–20.
Skinner, B. F. (1963). Operant behavior. American Psychologist, 18, 503.
Smith, A. (2015). U.S. smartphone use in 2015. Retrieved April 3, 2015, from http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/
Smithson, E. F., & Hill, A. J. (2017). It is not how much you crave but what you do with it that counts: Behavioural responses to food craving during weight management. European Journal of Clinical Nutrition, 71, 625–630.
Thomas, J. G., & Wing, R. R. (2013). Health-E-Call, a smartphone-assisted behavioral obesity treatment: Pilot study. JMIR mHealth and uHealth, 1, e3.
Turner-McGrievy, G., & Tate, D. (2011). Tweets, Apps, and Pods: Results of the 6-month Mobile Pounds Off Digitally (Mobile POD) randomized weight-loss intervention among adults. Journal of Medical Internet Research, 13, e120.
van Strien, T., & Ouwens, M. A. (2003). Counterregulation in female obese emotional eaters: Schachter, Goldman, and Gordon’s (1968) test of psychosomatic theory revisited. Eating Behaviors, 3, 329–340.
Volkow, N. D., Wang, G.-J., Fowler, J. S., & Telang, F. (2008). Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 363, 3191–3200.
Wadden, T. A. (1993). The treatment of obesity. In A. J. Stunkard & T. A. Wadden (Eds.), Obesity: Theory and therapy (pp. 197–217). New York: Raven Press.
Wayne, N., & Ritvo, P. (2014). Smartphone-enabled health coach intervention for people with diabetes from a modest socioeconomic strata community: Single-arm longitudinal feasibility study. Journal of Medical Internet Research, 16, e149.
Acknowledgements
This study was supported by a K23 award (1K23 HL133442) from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (Ashley E. Mason); foundational funds at the UMASS Medical School (Judson A. Brewer), and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004 (Ashley E. Mason). This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
AEM, KJ, and MC completed aspects of the study, including obtaining study funding (AEM and MC), registration on clinicaltrials.gov (MC), obtaining approval of the University of California, San Francisco, Institutional Review Board (AEM and MC), participant enrollment (AEM, KJ, MC), data collection (AEM, KJ, MC), statistical analyses (AEM, MC), and manuscript preparation (AEM, KJ, MC). JB principally developed the intervention, with input on app feature content from AEM and MC, and contributed to manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
Ashley E. Mason, Kinnari Jhaveri, and Michael Cohn declare that they have no conflicts of interest. Judson A. Brewer owns stock in Claritas MindSciences, the company that produced the app.
Human and animal rights and Informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mason, A.E., Jhaveri, K., Cohn, M. et al. Testing a mobile mindful eating intervention targeting craving-related eating: feasibility and proof of concept. J Behav Med 41, 160–173 (2018). https://doi.org/10.1007/s10865-017-9884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-017-9884-5