Abstract
Cysteine desulfurase (NFS1) is highly expressed in a variety of tumors, which is closely related to ferroptosis of tumor cells and affects prognosis. The relationship between NFS1 and the development of gastric cancer (GC) remains unknown. Here we showed that NFS1 expression was significantly higher in GC tissues compared to adjacent normal tissues. Patients with high expression of NFS1 in GC tissues had a lower overall survival rate than those with low expression. NFS1 was highly expressed in cultured GC cells compared to normal gastric cells. Knockdown of NFS1 expression reduced the viability, migration and invasion of GC cells. In cultured GC cells, NFS1 deficiency promoted ferroptosis. Mechanistically, NFS1 inhibited ferroptosis by upregulating the signal transduction and activator of transcription 3 (STAT3) signaling pathway in cultured GC cells. NFS1 knockdown using siRNA inhibited the STAT3 pathway, reduced the expression of glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11), and elevated intracellular levels of reactive oxygen species (ROS), ferrous ion (Fe2+), and malondialdehyde (MDA) in cultured GC cells. A specific STAT3 activator significantly reversed the inhibitory effect of NFS1 deficiency on ferroptosis in cultured GC cells. These in vitro results were further confirmed by experiments in vivo using a mouse xenograft tumor model. Collectively, these results indicate that NFS1 is overexpressed in human GC tissues and correlated with prognosis. NFS1 inhibits ferroptosis by activating the STAT3 pathway in GC cells. These results suggest that NFS1 may be a potential prognostic biomarker and therapeutic target to treat GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the latest global cancer statistics, gastric cancer (GC) has the fifth highest incidence among all malignancies and caused 768,000 deaths in 2020, ranking fourth among cancer-related deaths (Sung et al. 2021). The 5-year overall survival rate of patients with early GC can reach 90%. Due to the non-specificity of early symptoms, most patients are diagnosed with advanced GC and miss the best opportunity for surgical treatment. This results in the 5-year survival rate of patients with advanced GC being lower than 30% (Cutsem et al. 2016). Distant metastasis and postoperative recurrence are the main reasons for poor prognosis (Katai et al. 2018). In order to improve the therapeutic effect of GC patients, it is urgent to find new biomarkers for early diagnosis and effective therapeutic targets. For patients with advanced GC, chemotherapy resistance is the main obstacle affecting the efficacy of the current standard chemotherapy regimen based on 5-FU (Wong et al. 2023). Therefore, it is of great significance to find new and effective treatment methods to overcome chemotherapy resistance for improving the curative effect of advanced GC patients.
Ferroptosis is a novel form of programmed cell death characterized by excessive accumulation of lipid peroxides and iron. This process differs significantly from other forms of cell death such as apoptosis, autophagy, and necrosis in terms of morphology, genetics, metabolism, and molecular biology (Dixon et al. 2012). Erastin, a ferroptosis inducer, inhibits the cellular uptake of cystine and leads to glutathione depletion by inhibiting cystine/glutamate antiporters, ultimately leading to excessive accumulation of lipid reactive oxygen species and resulting in cell death (Dixon et al. 2014). Recent studies have demonstrated that many types of tumor cells can be killed by inducing ferroptosis, including breast cancer (Chen et al. 2022a, b), pancreatic carcinoma (Müller et al. 2023), and GC (Xu et al. 2023). More importantly, tumor cells that have developed resistance to conventional therapy are susceptible to ferroptosis (Moujalled et al. 2022). Thus, targeting ferroptosis may be an effective treatment strategy for reducing acquired resistance to conventional chemotherapy.
Cysteine desulphurase (NFS1) is a rate-limiting enzyme in the biogenesis of iron-sulfur clusters (Sies and Jones 2020). As an important cofactor of Fe-S proteins, Fe-sulfur clusters are involved in many cellular processes such as lipid biosynthesis, iron homeostasis and energy metabolism (Stehling et al. 2014). Defects in the synthetic biogenesis of iron-sulfur clusters can lead to metabolic diseases and the occurrence and development of human malignant tumors (Alvarez et al. 2022). Combined with inhibition of cysteine transport, NFS1 deficiency can lead to insufficient storage of iron-sulfur clusters and induce iron starvation leading to ferroptosis (Alvarez et al. 2022). Previous studies have shown that NFS1 is highly expressed in a variety of tumors, including lung cancer (Alvarez et al. 2022), breast cancer (Chafe et al. 2021) and colorectal cancer (Lin et al. 2022), which is associated with ferroptosis in tumor cells and affects the prognosis. However, no studies have reported causal relationship between NFS1 expression and the occurrence and development of GC.
As a key oncogene with dual functions of transcriptional activation and signal transduction, signal transducer and activator of transcription 3 (STAT3) has been confirmed to be a key negative regulator of ferroptosis in GC cells (Ouyang et al. 2022). Inhibition of STAT3 can trigger ferroptosis and re-sensitize resistant cancer cells to chemotherapy (Ouyang et al. 2022). Therefore, this study focused on whether NFS1 affects ferroptosis in GC cells through the STAT3 signaling pathway. The expression of NFS1 in GC tissues and its relationship with ferroptosis in GC are worth studying. To test this hypothesis, we evaluated the expression of NFS1 in human GC tissues and its association with prognosis. We further investigated the effects of NFS1 silencing on cell viability, tumor growth, and ferroptosis in cultured human GC cells. Finally, we evaluated the STAT3 signaling pathway after NFS1 silencing in human GC cells.
Materials and methods
Antibodies
The antibodies used in this study for Western blot or immunohistochemistry are as follows: NFS1 (Bioss, AD10560018, 1:1000 dilution for Western blot; 1:200 dilution for immunohistochemistry); GPX4 (Bioss, AB10101745, 1:2000 dilution for Western blot); P-STAT3 (Abcam, GR3380060-4, 1:1000 dilution for Western blot); STAT3 (Santa Cruz, K2917, 1:500 dilution for Western blot); SLC7A11 (Bioss, AH05032796, 1:1000 dilution for Western blot); GAPDH (Zsbio, 200040908, 1:2000 dilution for Western blot).
Patient specimens
Paraffin specimens of cancerous and adjacent paracancerous normal tissues were collected from 93 patients with GC who underwent radical gastrectomy in Hefei Hospital affiliated to Anhui Medical University from January 2014 to December 2016, and the clinical data of these patients were also collected. In addition, 7 fresh GC tissues and their corresponding paracancerous normal tissues were collected and preserved at -80°C for mRNA and protein extraction. All specimens were taken from patients who had not received any antitumour treatment before surgery and were extracted with the patient’s written informed consent. This study was reviewed and approved by the Ethics Committee of Hefei Hospital affiliated to Anhui Medical University (2023-KEYAN-076).
Cell culture
The human GC cell lines MGC803, SGC7901, MKN-7, and SNU-1 and the normal gastric mucosa epithelial cell line GES-1 were purchased from iCell Bioscience Inc (Shanghai, China). All cells were cultured in an incubator with 5% CO2 and at 37 °C. All media were supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Beyotime, Shanghai, China). MGC803, SGC7901, MKN-7, and SNU-1 cells were maintained in RPMI-1640 medium (Cytiva, Shanghai, China).
Plasmid and siRNA transfection
Cells were cultured in a 6-well plate and transfected with siRNA when the cell confluence reached 60%∼70%. Lipofectamine 3000 was first mixed with serum-free Opti-MEM, while NFS1 siRNA or scramble control was mixed with plasmids into nuclear adjuvant P3000 and Opti-MEM at room temperature for 5 min. These two mixtures were then mixed for 30 min at room temperature and then added into a well with serum-free medium for 6 h. Finally, the complete medium was replaced, and the cells were then cultured for 24 h before other treatments.
Wound-healing assay
Cells were cultured on a 6-well plate for 48 h. After the culture media were removed, the cells were scratched with a pipette tip. After washing with PBS 2 times, 1 mL DMEM was added. Analysis was performed at 24 h post scratch.
Transwell invasion assay
Transwell assays were performed using a Transwell membrane coated with a matrix gel (BD Biosciences). MGC803 and SGC7901 cells were harvested 48 h after transfection, re-suspended in 200 µL of serum-free DMEM and cultured in the upper chamber. In the bottom chamber, 600 µL DMEM containing 10% FBS was added. After incubation for 24 h, the cells passing through the membrane were fixed in 4% formaldehyde and stained with 0.1% crystal violet. Five visual fields were randomly selected under the microscope and counted.
Cell viability assay
Cell viability was determined by the CCK8 method. MGC803 and SGC7901 cells in the logarithmic growth stage were collected and cultured in a 96-well plate (3000 cells/well) with a total volume of 100 µl medium per well. The cells were transfected after adherence and incubated in an incubator for 24 h. The medium was discarded, and a mixture of 90 µl complete medium and 10 µl CCK8 solution was added and then cultured in the incubator for 1 h. The absorbance value of each well was measured using an enzyme label (wavelength 450 nm), and the cell proliferation curve was finally drawn.
Cell live/death assay
Cell live/death was assessed using the CytoFLEX Flow cytometer assay kit. In brief, 1 × 106 MGC803 and SGC7901 cells were cultured in a 6-well plate. At 48 h after transfection, cells were collected, washed and suspended in binding buffer. The cells were then incubated with 5 µL Annexin V-FITC and 10 µL propyl iodide in the dark at room temperature for 20 min. Cell live/death was assessed within 1 h.
Intracellular ROS, MDA, and Fe2+ determination
ROS detection kit was used to detect intracellular ROS levels by flow cytometry and fluorescence microscopy. In short, the cells of each group were collected and added with a concentration of 10umol/L Dihydroethidium, incubated at 37℃ for 20 min, and the cells were collected for fluorescence intensity detection. The MDA detection kit (TBA microplate method) was used to prepare samples and standard curves according to the manufacturer’s instructions, and optical density (OD) values were measured at 532 nm. Intracellular Fe2+ levels were assessed using an iron colorimetric assay kit.
Transmission electron microscopy
Cells were collected and centrifuged at 800 rpm, and the supernatant was discarded. Cell pellets were fixed with 2.5% glutaraldehyde. After gradient dehydration with ethanol, the cells were permeabilized, embedded, and sliced. The sections were stained with uranium dioxyacetate and lead citrate and observed under a transmission electron microscope.
Quantitative real-time PCR (RT‒qPCR)
Total RNA extracted from cultured cells or tumor tissues using the Trizol (Invitrogen) protocol was reverse-transcribed into cDNA. Using this cDNA as a template, the expression of NFS1 in tumor tissues or cultured cells was detected by RT‒qPCR. Using GAPDH as an internal parameter, the 2−ΔΔCT method was used for relative quantitative analysis. The target gene was amplified by PCR, and the primers are shown in Table S1.
Western blot
Human tissue and cell samples were homogenized using the RIPA buffer and then incubated on the ice for 1 h. Cell proteins were collected by centrifugation at 4 °C, and protein concentrations were measured by a BCA kit. After denaturation at 95°C for 5 min, the proteins were separated through SDS‒PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Skim milk was used for blocking for 2 h. The membrane was washed with TBST and incubated overnight at 4 ℃ with primary antibody. After washing with TBST 3 times, the secondary antibody was incubated for 1 h, and the images were obtained by ECL.
Immunohistochemistry
The specimen tissues were sequentially fixed with formalin, embedded in paraffin and sliced into 5-micron sections. Samples were then deparaffinized, hydrated, and blocked with 3% BSA solution for 1 h at room temperature. Antiphosphorylated NFS1 primary antibody (concentration: 1:100) was added and incubated at 37 °C for 1 h. Secondary antibody was added (concentration was 1:400) and incubated at 37 °C for 20 min. Color developing agent was added dropwise. Finally, gradient ethanol was used successively for dehydration, and neutral gum drops were used for sealing and drying. Immunostaining scores were calculated according to the following formula: staining intensity (negative: 0; weak positive: 1 point; moderate positive: 2 points; strong positive: 3 points) × positive area rate (0%: 0 points; 1% ∼ 25%: 1 point; 26% ∼ 50%: 2 points; 51% ∼ 75%; 3 points; 76% ∼ 100%: 4 points).
Tumor xenograft assay
Animal experiments were examined and approved by the Ethics Committee of Anhui Medical University (20,180,365). Four-week old male BALB/c-nu mice were randomly divided into 3 groups with 5 mice per group. A total of 1 × 106 control cells or NFS1 knockdown MGC803 cells were injected subcutaneously into the right posterior ventral quadrant of mice. Vital signs of mice were observed and recorded every day. The tumors were removed for after 5 weeks of inoculation, and the size and quality of tumor was measured. The volume calculation formula was as follows: 0.5× length × width 2. Some tissues of each tumor were detected by Western blot and RT‒qPCR.
Statistical analysis
SPSS 23.0 (IBM Corporation, USA) was used for statistical analysis in this study. The results of the in vitro experiments were needed to repeat three times, and Student’s t test was used to compare the differences in quantitative data between the two groups. The correlation between NFS1 expression and clinicopathologic parameters in patients with GC was analysed by the chi-square test. Survival analysis was performed by log-rank test and Kaplan‒Meier analysis. A P value lower than 0.05 was considered statistically significant. GraphPad Prism 8 software was used to draw charts.
Results
NFS1 is highly expressed in human GC tissues and is associated with a poor prognosis
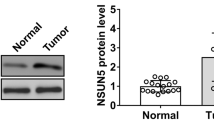
First, the expression of NFS1 was analysed by using the GC data form The Cancer Genome Atlas (TCGA). Compared with normal tissues, NFS1 was significantly higher in GC tissues (Fig. 1A). To validate NFS1 mRNA expression, GC tissues and corresponding paracancerous normal tissues were collected from 7 tumors. The expression level of NFS1 mRNA was detected by qRT‒PCR, and the results showed that the level of NFS1 mRNA in 7 GC tissues was significantly higher than that in adjacent normal tissues (Fig. 1B). Additionally, protein levels of samples from 7 patients’ tissues were also detected by Western blot. Similarly, NFS1 protein levels in GC tissues were significantly higher than those in their adjacent normal tissues (Fig. 1C and D).
NFS1 is highly expressed in gastric cancer tissues and is associated with a poor prognosis. The expression of NFS1 mRNA was determined based on the TCGA databases **P < 0.01; (A). NFS1 mRNA expression was analyzed by RT-qPCR in GC and adjacent normal tissues; **P < 0.01 (B). NFS1 expression was analyzed by Western blot in GC and adjacent normal tissues; **P < 0.01 (C, D). Representative NFS1 IHC staining images in GC tissue and paracancerous tissue (E). The statistical analysis of NFS1 expression in GC and adjacent normal tissues according to the IHC scoring method; n = 93 (F). Kaplan–Meier survival curve of GC patients with low and high NFS1 expression in our study (G), and the KM plotter database (H). The mRNA expression (I) and protein level (J) of NFS1 in the GES-1 and GC cell lines; **P < 0.01
Finally, 93 paraffin specimens of GC tissues and corresponding adjacent normal tissues were collected for immunohistochemistry. Representative images of NFS1 expression are shown in Fig. 1E. The positive expression rate of NFS1 protein was significantly higher in GC tissues (73.1%, 68/93) than in adjacent normal tissues (30.1%, 28/93) (Fig. 1F). Table 1 shows the relationship between NFS1 expression and clinicopathological parameters in patients with GC. The results revealed that increased NFS1 expression was significantly correlated with lymph node metastasis (P = 0.014) and TNM stage (P = 0.012). Survival analysis was performed to determine the relationship between NFS1 and prognosis in patients with GC. Patients with high expression of NFS1 had significantly shorter overall survival (OS) than those with low expression of NFS1 (log-rank P = 0.006, Fig. 1G). Similar results were confirmed by a large cohort in the KM plotter database (https://kmplot.com/analysis/) (log-rank P < 0.001, Fig. 1H). Cox regression analysis based on 93 GC tissue was performed. Both univariate and multivariate Cox regression analysis showed that NFS1 expression was significantly correlated with OS in patients with GC (Table 2). These results suggest that NFS1 is a valuable biomarker for predicting OS for GC. NFS1 mRNA expression was higher in GC cell lines (MGC803, SGC7901, MKN-7 and SNU-1) than in a nonmalignant gastric cell line (GES-1; Fig. 1I). MGC803 and SGC7901 cells were selected for follow-up studies due to their highest levels of NFS1 protein expression (Fig. 1J).
NFS1 knockdown inhibits the malignant phenotype of GC cells in vitro
In in vitro experiments, three siRNAs were transfected to knock out NFS1. In MGC803 cells, the expression of NFS1 was most significantly down-regulated after transfection of NFS1 siRNA-2. Thus, siRNA-2 of NFS1 was selected for subsequent experiments (Fig. 2A). The levels of NFS1 mRNA and protein were decreased in MGC803 and SGC7901 cells transfected with siRNA-NFS1 (Fig. 2B and C). The viability of tumor cells was detected by CCK-8. The results showed that the viability of MGC803 and SGC7901 cells was decreased significantly after transfection with siRNA-NFS1 (Fig. 2D). After knockdown of NFS1, a significant increase in the proportion of early apoptotic cells, as detected by Annexin V/PI staining via flow cytometry, was observed in MGC803 and SGC7901 cells (Fig. 2E). As shown in Fig. 2F and G, the migration and invasion ability of MGC803 and SGC7901 cells transfected with siRNA-NFS1, evaluated by Wound healing and Transwell assay, was decreased at 0 h and 24 h after transfection compared to the scramble group. Therefore, knocking out NFS1 inhibits the viability, invasion and migration of GC cells.
NFS1 knockdown inhibits GC cells malignant phenotypes in vitro. (A) RT–qPCR was used to detect the expression level of NFS1 mRNA in MGC803 cells after transfection. The expression levels of NFS1 mRNA (B) and protein (C) were detected in MGC803 and SGC7901 cells lines after transfection with siRNA-NFS1. The proliferation ability of tumor cells was detected by CCK-8 after transfection with siRNA-NFS1 (D). Flow cytometry and Annexin V/PI staining (left ) were performed in MGC803 and SGC7901 cells after knockdown of NFS1. Statistical results (right) showed the differences between groups (E). Representative images (left ) of the scratches (F) and transwell (G) experiments of MGC803 and SGC7901 cells after NFS1 knockdown (Scale Bar = 20 μm). Statistical results (right) showed the differences between groups. **P < 0.01
Knockdown of NFS1 induces ferroptosis in GC cells
NFS1 expression is associated with ferroptosis in various tumors, including lung cancer, breast cancer, and colon cancer (Alvarez et al. 2022; Chafe et al. 2021; Lin et al. 2022). However, the relationship between NFS1 and ferroptosis in GC has not been reported. Here, we first knocked down NFS1 in MGC803 and SGC7901 cells and evaluated mitochondrial morphology by transmission electron microscopy. As shown in Fig. 3A, a large number of mitochondria and mitochondrial ridge atrophy were found in cells transfected with NSF1 siRNA. Apoptosis inhibitor (Z-VAD-FMK), ferroptosis inhibitor (Ferristatin-1) and necroptosis inhibitor (Nec-1) were used to identify the death pattern of MGC803 after NFS1 knockdown. Fer-1 exhibited the strongest protective effect on MGC803 cells after NFS1 knockdown, indicating that NFS1 knockdown mainly induced ferroptosis of GC cells (Fig. 3B). To further evaluate the relationship between NFS1 and ferroptosis in GC cells, key markers of ferroptosis such as ROS, MDA, and Fe2 + and two major regulators of ferroptosis, glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11) were examined in MGC803 and SGC7901 cells in our study. In MGC803 and SGC7901 cells transfected with NFS1 siRNA, protein expressions of GPX4 (Fig. 3C) and SLC7A11 (Fig. 3D) were significantly down-regulated. In contrast, ROS (Fig. 3E), Fe2+ (Fig. 3F), and MDA (Fig. 3G) levels in MGC803 and SGC7901 cells were significantly increased when cells were transfected with NSF1 siRNA. These results demonstrate that NFS1 knockdown promotes ferroptosis in GC cells.
NFS1 knockdowgenes co-expressed with NFS1 induces ferroptosis in GC. (A) TEM was performed to assess the ferroptotic mitochondrial phenotype of MGC803 and SGC7901 cells after transfecting with siRNA-NFS1 or scramble for 48 h. Scale bars: 5 μm and 500 nm. (B) Cell viability assay in MGC803 cells after knockdown NFS1treated in the presence or absence of the indicated inhibitors. Western blotting was used to detect the protein expression of GPX4 (C) and SLC7A11 (D) after knockdown of NFS1 in GC cells. The level of ROS (E), Fe2+(F), and MDA (G) content were determined by the appropriate kits. **P < 0.01
NFS1 knockdown inhibits the malignant phenotype of GC cells through the STAT3 pathway
The STAT3 pathway is closely related to the occurrence and development of tumors (Mengie Ayele et al. 2022). STAT3 is abnormally overexpressed in GC and promotes the progression of GC (Judd et al. 2006). Here, we evaluated whether NFS1 affects the development of GC by regulating STAT3. Western blot results showed that phosphorylation levels of STAT3 (p-STAT3) in MGC803 and SGC7901 cells were significantly down-regulated in MGC803 and SGC7901 cells transfected with NFS1 siRNA (Fig. 4A). To investigate whether knockdown NFS1 can inhibit GC cells progression through STAT3, we performed salvage experiments on MGC803 and SGC7901 cells. The results showed that incubation with the STAT3 agonist colcolvelin significantly restored the decreased viability of MGC803 and SGC7901 cells induced by NFS1 siRNA transfection (Fig. 4B). The same result was shown in the experiment of detecting the proportion of early apoptosis of MGC803 and SGC7901 (Fig. 4C). Colivelin incubation also significantly reversed the inhibitory effect of siNFS1 on GC cell migration (Fig. 4D) and invasion (Fig. 4E). These results demonstrate that NFS1 deficiency inhibits the malignant phenotype of GC cells by downregulating the STAT3 signaling pathway.
siRNANFS1 inhibits the malignant phenotype of GC cells through STAT3 pathway. (A) STAT3 and p-STAT3 in MGC803 and SGC7901 cells were detected by western blot after transfecting with siRNA-NFS1. Statistical results (right) showed the differences between groups. **P < 0.01. (B) Cell viability of MGC803 and SGC7901 cells was determined by CCK-8 assay. (C) Cell live/death of MGC803 and SGC7901 cells was detected by flow cytometry. Annexin V/PI staining (left) and statistical results (right) showed the differences between groups. **P < 0.01. Wound healing (D) and transwell assay (E) were used to analyze MGC803 and SGC7901 cells migration and invasion. Representative images (left ) and statistical results (right) showed the differences between groups. **P < 0.01. The si-N + c indicates the si-NFS1 + colivelin group
siRNANFS1 promotes ferroptosis by inhibiting STAT3 pathway in cultured GC cells
STAT3 is a key negative regulator of ferroptosis in GC cells, and inhibition of STAT3 function can trigger ferroptosis and re-sensitize resistant cancer cells to chemotherapy (Ouyang et al. 2022). In our above experiments, it was confirmed that down-regulation of NFS1 can induce ferroptosis in GC cells. Therefore, we attempted to investigate whether siRNA-NFS1 affects ferroptosis in GC cells through the STAT3 pathway. Western blot analysis showed that after knocking out NFS1, the expression of NFS1, pSTAT3, GPX4, and SCL7A11 in MGC803 and SGC7901 cells was significantly down-regulated. Incubation of colivelin in siRNA-NFS1 transfected MGC803 and SGC7901 cells, the results showed that the declining trend of p-STAT3/STAT3, GPX4, and SCL7A11 was reversed (Fig. 5A and B). Compared with those in the siRNA-NFS1 group, the levels of ROS (Fig. 5C), Fe2+ (Fig. 5D), and MDA (Fig. 5E) in MGC803 cells in the siRNA-NFS1 + colcolvelin group were significantly decreased. The detection results of ROS, Fe2+ and MDA showed the same changes in rescue experiment of SGC7901 cells (Fig. 5C, D and E). These results suggest that NFS1 regulates ferroptosis in GC cells through the STAT3 pathway.
siRNANFS1 promotes ferroptosis by inhibiting STAT3 pathway activation in GC cells. Western blot showed that after adding colcolvelin, the down-regulation trend of NFS1, pSTAT3, GPX4, and SCL7A11 in siRNA-NFS1 MGC803 (A) and SGC7901 (B) cells was significantly reversed. Compared with the siRNA-NFS1 group, the levels of ROS (C), Fe2+ (D) and MDA (E) in MGC803 and SGC7901 cells in the siRNA-NFS1 + colcolvelin group were significantly decreased. **P < 0.01. The si-N + c indicates the si-NFS1 + colivelin group
Silencing of NFS1 inhibits tumor growth in vivo
A mouse xenograft tumor model was established to investigate the role of NFS1 in the progression of GC in vivo. Fifteen nude mice were randomly divided into 3 groups with 5 mice each group and were injected subcutaneously with MGC803, siRNA-NFS1-NC MGC803 and siRNA-NFS1 MGC803, respectively. Tumor resection was performed to obtain relevant data after the experiment finished. Figure 6A shows the comparison of tumor morphology among the three groups. SiRNA-NFS1 inhibition significantly attenuated tumor size in mice compared with siRNA-NFS1-NC. Volume and weight were also significantly reduce in siRNA-NFS1 group (Fig. 6B and C). Levels of NFS1 mRNA and protein in subcutaneous xenografts were significantly down-regulated in the siRNA-NFS1 MGC803 group (Fig. 6D). This suggests that silencing of NFS1 could inhibit the growth of GC cells.
Knockdown of NFS1 inhibited gastric tumor growth in vivo. (A) The tumor morphology of MGC803 subcutaneous xenografts among the three groups. Tumor volumes (B) and weights (C) in siRNA-NFS1-MGC803 group were significant smaller than those of the other two groups. **P < 0.01. (D) The mRNA expression and protein level of NFS1 in MGC803 subcutaneous xenograft were significantly down-regulated in siRNA-NFS1 MGC803 group. **P < 0.01
Discussion
The main risk factors for GC include Helicobacter pylori infection, improper diet, unclean diet and smoking (Yang et al. 2021). Although the incidence of GC has decreased, the prognosis of patients with GC is still poor, and there are still 780,000 deaths due to GC every year (Bray et al. 2018). The emergence of chemoresistance is a major obstacle to standard chemotherapy with first-line fluorouracil in patients with advanced GC who cannot be cured by surgery (Wang et al. 2018). The rapid progress of targeted therapy and immunotherapy in recent decades has provided a new means for the management of GC. However, there are still many shortcomings in these therapeutic strategies, and the overall therapeutic effect is disappointing (Wang et al. 2018; Kim et al. 2018). As a new form of cell death, ferroptosis has been found to affect the biological behavior of GC, such as proliferation, metastasis, invasion and drug resistance. Inducing the ferroptosis of GC cells has become a new research direction and may become a new treatment for GC (Ni et al. 2021).
As an essential enzyme in eukaryotes, NFS1 influences tumor progression by playing a key role in Fe-S cluster metabolism and Fe-S cluster-dependent pathways (Alvarez et al. 2022; Sviderskiy et al. 2020). Simultaneous inhibition of NFS1 and cysteine transport can activate the iron-starvation response in vitro, which in turn activates ferroptosis (Alvarez et al. 2022). Studies have shown that NFS1 is overexpressed in a variety of human cancers and influences ferroptosis in tumor cells (Alvarez et al. 2022; Chafe et al. 2021; Lin et al. 2022; Asghari Alashti et al. 2022; Pang et al. 2021). However, to date, no studies have elucidated the expression of NFS1 in GC and its potential function in the progression of GC. In this study, we explored the relationship between NFS1 and stomach cancer. The results showed that NFS1 was highly expressed in GC tissues, and high expression of NFS1 was associated with lymph node metastasis and late TNM stage. The overall survival time of patients with high expression of NFS1 was shorter, which was an independent risk factor for the prognosis of patients with GC. The results of in vitro and in vivo cell experiments showed that knocking out NFS1 inhibited the growth of GC cells and tumors and promoted ferroptosis by inhibiting the STAT3 pathway. Therefore, NFS1 may be a potential prognostic predictor of GC and a new therapeutic target.
Ferroptosis has been verified to play an important role as a potentiator for chemotherapy and immunotherapy (Wang et al. 2019; Liang et al. 2019). Recent studies have confirmed that it is possible to kill many types of tumor cells, including stomach cancer, by inducing ferroptosis. NFS1 has been shown to be highly expressed in lung adenocarcinoma tissues and to protect cancer cells from ferroptosis (Alvarez et al. 2022). Chafe SC et al. showed that NFS1 was highly expressed in triple-negative breast cancer tissues, and inhibition of NFS1 expression could enhance tumor ferroptosis and significantly inhibit tumor growth (Chafe et al. 2021). Lin et al. analysed the correlation between the expression level of NFS1 and clinical indicators in tumor tissues of colorectal cancer patients and found that patients with high expression of NFS1 had a low response rate for oxaliplatin and poor prognosis. Knockout of NFS1 can enhance the oxidative stress mediated by platinum-based chemotherapeutics and induce multiple types of cell death in colorectal cancer cells at the same time, including apoptosis, ferroptosis, pyroptosis and necroptosis (Lin et al. 2022). In addition, it has been confirmed that NFS1 is highly expressed in liver cancer (Gao et al. 2019), prostate cancer (Lv et al. 2021) and glioma (Yun et al. 2022) and is associated with the ferroptosis of tumor cells. Our study demonstrates for the first time that NFS1 is highly expressed in GC and protects GC cells from ferroptosis. Targeting NFS1 to induce ferroptosis to enhance anticancer activity may be a promising therapeutic strategy for GC.
STAT3, as an oxidative response transcription factor, has been confirmed to be closely related to stress-related ferroptosis (Linher-Melville and Singh 2017). Over-activated STAT3 is closely related to cell proliferation, metastasis, angiogenesis and immunosuppression and plays a key role in the tumorigenesis of most human cancers, including GC (Mora et al. 2002; Burke et al. 2001; Song and Grandis 2000; Chen et al. 2022a, b). Studies have confirmed that STAT3 regulates ferroptosis in various tumor cells in different forms. The results of Li et al. demonstrated that IL-6 induced ferroptosis resistance via the JAK2/STAT3 pathway plays a key role in the carcinogenesis of head and neck carcinoma (Li et al. 2022). A recent study confirmed that Thiostrepton regulates ferroptosis in pancreatic cancer cells by STAT3/GPX4 signaling (Zhang et al. 2022). The STAT3/P53/SLC7A11 axis has been reported to regulate ferroptosis in osteosarcoma cells (Luo et al. 2021). Another study reported that dimethyl fumarate induces diffuse large B-cell lymphoma cell ferroptosis via the NF-κB/STAT3 axis (Schmitt et al. 2021). For GC, a recent report indicated that STAT3, as a key negative regulator of ferroptosis, regulates GC cell ferroptosis by directly regulating GPX4, FTH1 and SLC7A11 (Ouyang et al. 2022). The study by Lin et al. demonstrated that inhibiting NFS1 expression can significantly increase ROS levels (Lin et al. 2022), and numerous studies have confirmed that ROS can influence the STAT3 pathway (Zhou et al. 2024; Zhao et al. 2022; Lu et al. 2023). STAT3 has been identified by Shumin et al. as a key negative regulator of ferroptosis in gastric cancer cells (Ouyang et al. 2022). Therefore, we hypothesize that NFS1 may affect ferroptosis by regulating the STAT3 pathway via ROS. In the present study, activation of STAT3 by colvelin partially reversed the inhibitory effect of NFS1 knockout on cell viability, invasion and migration and inhibited ferroptosis in GC cells. In summary, our study suggests that NFS1 may mediate GC ferroptosis through the STAT3 pathway.
The limitations of this study should be explained. First, ferroptosis is only one of many complex pathological mechanisms affecting cancer cell growth, and other possible mechanisms involved should be evaluated. Second, in addition to regulating the STAT3 pathway, NFS1 may also affect a variety of pathways regulating extracellular ferroptosis. Finally, a larger number of clinical samples are needed to further verify the reliability of NFS1 as a prognostic indicator for gastric cancer.
Conclusion
Our study shows that NFS1 is highly expressed in GC tissues and cells. High expression of NFS1 is closely associated with lymph node metastasis, late stage, and poor survival outcome. The NFS1 level can be used as a biomarker to predict the prognosis of GC. NFS1 can improve the viability, invasion and migration of GC cells and promote tumor growth. Moreover, NFS1 knockdown induced ferroptosis in GC cells by regulating the STAT3 pathway. Our results suggest that NFS1 is a potential therapeutic target for GC.
Data availability
No datasets were generated or analysed during the current study.
References
Alvarez SW, Sviderskiy VO, Terzi EM et al (2022) NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis [published correction appears in Nature. 609(7929):E12]. Nature. 2017;551(7682):639–643
Asghari Alashti F, Goliaei B, Minuchehr Z (2022) Analyzing large scale gene expression data in colorectal cancer reveals important clues; CLCA1 and SELENBP1 downregulated in CRC not in normal and not in adenoma. Am J Cancer Res 12(1):371–380
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA Cancer J Clin 68(6):394–424
Burke WM, Jin X, Lin HJ et al (2001) Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene 20(55):7925–7934
Chafe SC, Vizeacoumar FS, Venkateswaran G et al (2021) Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci Adv 7(35):eabj0364
Chen C, Xie B, Li Z et al (2022a) Fascin enhances the vulnerability of breast cancer to erastin-induced ferroptosis. Cell Death Dis 13(2):150
Chen L, Chen D, Li J et al (2022b) Ciclopirox drives growth arrest and autophagic cell death through STAT3 in gastric cancer cells. Cell Death Dis 13(11):1007
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Dixon SJ, Patel DN, Welsch M et al (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3:e02523
Gao Q, Zhu H, Dong L et al (2019) Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma [published correction appears in Cell. 179(5):1240]. Cell. 2019;179(2):561–577.e22
Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS (2006) STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology 131(4):1073–1085
Katai H, Ishikawa T, Akazawa K et al (2018) Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer 21(1):144–154
Kim ST, Cristescu R, Bass AJ et al (2018) Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 24(9):1449–1458
Li M, Jin S, Zhang Z, Ma H, Yang X (2022) Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer Lett 527:28–40
Liang C, Zhang X, Yang M, Dong X (2019) Recent progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater 31(51):e1904197
Lin JF, Hu PS, Wang YY et al (2022) Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal Transduct Target Ther 7(1):54
Linher-Melville K, Singh G (2017) The complex roles of STAT3 and STAT5 in maintaining redox balance: lessons from STAT-mediated xCT expression in cancer cells. Mol Cell Endocrinol 451:40–52
Lu J, Su Z, Li W et al (2023) ASCT2-mediated glutamine uptake of epithelial cells facilitates CCL5-induced T cell infiltration via ROS-STAT3 pathway in oral lichen planus. Int Immunopharmacol 119:110216
Luo Y, Gao X, Zou L, Lei M, Feng J, Hu Z (2021) Bavachin Induces Ferroptosis through the STAT3/P53/SLC7A11 Axis in Osteosarcoma cells. Oxid Med Cell Longev 2021:1783485
Lv Z, Wang J, Wang X et al (2021) Identifying a ferroptosis-related gene signature for Predicting biochemical recurrence of prostate Cancer. Front Cell Dev Biol 9:666025
Mengie Ayele T, Tilahun Muche Z, Behaile Teklemariam A, Bogale Kassie A, Chekol Abebe E (2022) Role of JAK2/STAT3 signaling pathway in the Tumorigenesis, Chemotherapy Resistance, and treatment of solid tumors: a systemic review. J Inflamm Res 15:1349–1364
Mora LB, Buettner R, Seigne J et al (2002) Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 62(22):6659–6666
Moujalled D, Southon AG, Saleh E et al (2022) BH3 mimetic drugs cooperate with Temozolomide, JQ1 and inducers of ferroptosis in killing glioblastoma multiforme cells. Cell Death Differ 29(7):1335–1348
Müller F, Lim JKM, Bebber CM et al (2023) Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ 30(2):442–456
Ni H, Qin H, Sun C et al (2021) MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res Ther 12(1):325
Ouyang S, Li H, Lou L et al (2022) Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol 52:102317
Pang Y, Tan G, Yang X et al (2021) Iron-sulphur cluster biogenesis factor LYRM4 is a novel prognostic biomarker associated with immune infiltrates in hepatocellular carcinoma. Cancer Cell Int 21(1):463
Schmitt A, Xu W, Bucher P et al (2021) Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 138(10):871–884
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21(7):363–383
Song JI, Grandis JR (2000) STAT signaling in head and neck cancer. Oncogene 19(21):2489–2495
Stehling O, Wilbrecht C, Lill R (2014) Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie 100:61–77
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Sviderskiy VO, Blumenberg L, Gorodetsky E et al (2020) Hyperactive CDK2 activity in basal-like breast Cancer imposes a genome Integrity Liability that can be exploited by targeting DNA polymerase ε. Mol Cell 80(4):682–698e7
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388(10060):2654–2664
Wang T, Fahrmann JF, Lee H et al (2018) JAK/STAT3-Regulated fatty acid β-Oxidation is critical for breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab 27(6):1357
Wang W, Green M, Choi JE et al (2019) CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569(7755):270–274
Wong TL, Loh JJ, Lu S et al (2023) ADAR1-mediated RNA editing of SCD1 drives drug resistance and self-renewal in gastric cancer. Nat Commun 14(1):2861
Xu X, Li Y, Wu Y et al (2023) Increased ATF2 expression predicts poor prognosis and inhibits sorafenib-induced ferroptosis in gastric cancer. Redox Biol 59:102564
Yang L, Kartsonaki C, Yao P et al (2021) The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health 6(12):e888–e896
Yun D, Wang X, Wang W et al (2022) A Novel Prognostic signature based on glioma essential ferroptosis-related genes predicts clinical outcomes and indicates treatment in Glioma. Front Oncol 12:897702
Zhang W, Gong M, Zhang W et al (2022) Thiostrepton induces ferroptosis in pancreatic cancer cells through STAT3/GPX4 signalling. Cell Death Dis 13(7):630
Zhao Z, Wang Y, Gong Y et al (2022) Celastrol elicits antitumor effects by inhibiting the STAT3 pathway through ROS accumulation in non-small cell lung cancer. J Transl Med 20(1):525
Zhou H, Li J, He Y, Xia X, Liu J, Xiong H (2024) SLC25A17 inhibits autophagy to promote triple-negative breast cancer tumorigenesis by ROS-mediated JAK2/STAT3 signaling pathway. Cancer Cell Int 24(1):85
Acknowledgements
Not applicable.
Funding
This study was funded by natural science foundation of Anhui province (grant no. 2008085MH1294).
Author information
Authors and Affiliations
Contributions
Y.J. and Z.W. designed the study. Y.J., L.L., W.L., K.L., and Y.W. performed the majority of the experiments. Y.J. and Y.W. analyzed the data. Y.J., L.L., W.L., K.L., and Y.W. provided assistance for the experiments and data analysis. Y.J., L.L. and W.L. wrote the draft of the manuscript. Z.W. revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All specimens were taken from patients who had not received any antitumour treatment before surgery and were extracted with the patient’s written informed consent. This study was reviewed and approved by the Ethics Committee of Hefei Hospital affiliated to Anhui Medical University (2023-KEYAN-076).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Li, L., Li, W. et al. NFS1 inhibits ferroptosis in gastric cancer by regulating the STAT3 pathway. J Bioenerg Biomembr (2024). https://doi.org/10.1007/s10863-024-10038-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10863-024-10038-7