Abstract
Previous studies have suggested that N6-methyladenosine (mA) modification of RNA affects fundamental aspects of RNA metabolism, and mA dysregulation is implicated in various human diseases, including Alzheimer’s disease (AD). This study is designed to explore the role and mechanism of methyltransferase-like 14 (METTL14) in the pathogenesis of AD. SK-N-SH cells were treated with Aβ1–42 to establish an in vitro model of AD. Cerebellin 4 (CBLN4) and METTL14 expression levels were detected by real-time quantitative polymerase chain reaction (RT-qPCR). Cell viability and apoptosis were analyzed using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay and flow cytometry assay. B-cell lymphoma-2 (Bcl-2), Bcl-2 related X protein (Bax), C-caspase-3, total-caspase-3, C/EBP homologous protein (CHOP), and glucose-related protein 78 (GRP78) protein levels were determined using Western blot. Interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) levels were analyzed using ELISA. Reactive oxygen species (ROS), malondialdehyde (MDA), and superoxide dismutase (SOD) products were examined using special assay kits. Interaction between CBLN4 and METTL14 was verified using methylated RNA immunoprecipitation (MeRIP) and dual-luciferase reporter assays. CBLN4 and METTL14 expression was decreased in Aβ1-42-treated SK-N-SH cells. Upregulation of CBLN4 relieved Aβ1-42-induced SK-N-SH cell apoptosis, inflammation, oxidative stress, and endoplasmic reticulum (ER) stress in vitro. At the molecular level, METTL14 could improve the stability and expression of CBLN4 mRNA via m6A methylation. Our findings indicated that m6A methylase METTL14-mediated upregulation of CBLN4 mRNA stability could repress Aβ1-42-triggered SK-N-SH cell injury, providing a promising therapeutic target for AD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the most prevalent dementia and neurodegenerative disease, Alzheimer’s disease (AD) occurs in the central nervous system with irreversible effects, which seriously affects activities of daily living and social functioning, imposing a costly disease burden (Scheltens et al. 2021; , Zhang et al. 2021). With longer life expectancy and demographic aging occurring, the global incidence of AD is projected to continue to rise, with an estimated 13.8 million Americans aged 65 and older living with AD by 2060 ( 2023). Pathologically, the accumulation of neuritic plaques composed of β-amyloid (Aβ) could trigger neuronal dysfunction and apoptosis by interfering with neuron-to-neuron communication at synapses (Breijyeh 2020; , Sehar 2022). Hence, targeting therapy to protect against Aβ-triggered neuronal impairment represents a valid strategy for AD treatment.
In recent years, some studies have indicated that cerebellins, a kind of secreted glycoproteins, could serve as trans-synaptic cell-adhesion molecules that are essential for synapse formation and/or function (Bourgeron and Bourgeron 2015; , Sanfilippo et al. 2021). Among them, Cerebellin 4 (CBLN4) is abundantly expressed in nearly all brain regions and involved in the form of regulating synapses by binding to presynaptic deleted in colorectal cancer (DCC) cell-contact molecules (Seigneur and Südhof 2017). Different from other cerebellins, CBLN4 only weakly binds to β neurexins (Wei et al. 2012). As a synaptic protein, CBLN4 is vital for the formation and maintenance of synaptic connections. It has been reported that CBLN4 expression is decreased in the hippocampus of a mouse model of AD (Jankowsky et al. 2001). Furthermore, the upregulation of CBLN4 relieved Aβ-triggered death in cultured hippocampal neurons (Chacón et al. 2015). However, its role and molecule mechanism in the Aβ1-42-induced in vitro model of AD remains unclear.
As the most abundant modification of internal RNA in eukaryotes, N6-methyladenosine (m6A) modification recently emerged as an important regulator of gene expression (Murakami and Jaffrey 2022; , Salaikumaran Raj and Prasad Burra Laxmi Siva 2023). In mammalian cells, m6A can be installed by methyltransferase complex (writer), composed of methyltransferase-like 14 (METTL14), METTL3, and WTAP (Liu et al. 2014). On the contrary, m6A demethylases (erasers) consisting of FTO and ALKBH5 are responsible for m6A removal (Yang et al. 2018). In addition, the regulatory function of m6A requires the recruitment of binding proteins (readers), such as YTHDF1/2/3 and IGF2BP1/2 (Deng et al. 2021). Furthermore, m6A modification has been implicated in modulating mRNA splicing, RNA stability, and translation efficiency (Meyer and Jaffrey 2014). Numerous studies have suggested that altered expression of the m6A-related proteins could participate in regulating the pathogenesis of nervous system diseases, containing AD (Zhang et al. 2022; , Zhao et al. 2021). For example, recent literature has exhibited that the overexpression of METTL3 could ameliorate AD by m6A-dependent regulation of STUB1 (Tang et al. 2023). Of note, it has been reported that METTL14 offers an RNA-binding scaffold for promoting the recognition and activation of RNA substrates and elevating the catalytic ability of METTL3 ( 2016). Apart from that, METTL14 and METTL3 levels are shown to be reduced in the Hippocampus of Tyrobp-/-mice (Lv 2022). Nevertheless, there are no reports on the potential epigenetic regulation of METTL14 in AD treatment.
Herein, a public prediction server SRAMP discovered that CBLN4 contains underlying m6A sites. Meanwhile, CBLN4 was a downstream target of METTL14 in SK-N-SH cells. Therefore, we aimed to explore the role of METTL14 and its molecular mechanism in regulating Aβ1-42-induced SK-N-SH cell injury.
Materials and methods
Cell culture
Under a 37˚C incubator with 5% CO2,human neuroblastoma cell line SK-N-SH cells (CL-0214) were cultured in a specialized medium (CM-0214, Procell, Wuhan, China). For neuronal cultures, SK-N-SH cells were differentiated with 10 µM all-trans-retinoic acid (Sigma-Aldrich, St.Louis, MO, USA) for 7 days in 2% FBS maintenance media. To construct an in vitro neuronal injury model, SK-N-SH cells were treated with 20 µM Aβ1–42 (Sigma-Aldrich, dissolved in double water) for 24 h (Meng et al. 2022).
RT-qPCR
By using the Trizol reagent (Invitrogen, Paisley Scotland, UK), SK-N-SH cell RNAs were extracted, followed by cDNA synthesis using Prime Script RT Master Mix (Takara, Tokyo, Japan). On StepOnePlus (Applied Biosystems, Carlsbad, CA, USA), an amplification reaction was performed based on TB Green Premix Ex Taq™ II kit (Takara) to determine mRNA transcript level. β-actin was used as an endogenous control. Relative expression of the target gene was calculated by the 2–ΔΔCt method. Primer sequences are presented in Table 1.
Cell viability assay
In this experiment, the viability of SK-N-SH cells with or without Aβ1–42 treatment was assessed using MTT kit (Solarbio, Beijing, China). In short, SK-N-SH cells in 96-well plates were mixed with MTT solution (20 mL, 5 mg/mL). After 4 h of incubation, the medium was removed, followed by the addition of 150 µL DMSO (Solarbio) for dissolving the formed formazan crystals. At last, a microplate reader was utilized to detect the absorbance.
Flow cytometry for cell apoptosis
After being trypsinized to form a single cell suspension, SK-N-SH cells were washed with PBS, followed by re-suspending in binding buffer. Then, cells were subjected to staining with 5 µL Annexin V-FITC and 10 µL PI solution (Beyotime, Shanghai, China) for 20 min away from light. Finally, the apoptotic rates were determined by a FACSCalibur Flow Cytometry instrument.
Western blot assay
By using RIPA lysis buffer (Keygen, Nanjing, China), total proteins of SK-N-SH cells were extracted, followed by quantification with a BCA assay kit. Then, protein samples were subjected to 10% SDS-PAGE separation and then shifted onto PVDF membranes (Invitrogen). After blocking, the membranes were probed at 4˚C with appropriately diluent primary antibodies: CBLN4 (ab237712, Abcam, Cambridge, MA, USA), B-cell lymphoma-2 (Bcl-2, ab32124, Abcam), Bcl-2 related X protein (Bax, ab32503, Abcam), C-caspase-3 (ab32042, Abcam), ER stress markers (CHOP, ab194533; GRP78, ab21685, Abcam), METTL14 (ab309096, Abcam), and β-actin (ab8227). The next day, signals were detected using ECL reagent (Solarbio) and data were processed by Image J software after secondary antibody incubation.
Enzyme-linked immunosorbent assay (ELISA)
To assess the releases of pro-inflammatory cytokines Interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), the culture medium of SK-N-SH cells was collected, followed by the measurement using human IL-1β ELISA kit (DLB50, R&D, Minneapolis, MN, USA) and human TNF-α ELISA kit (DTA00D, R&D). At last, a microplate reader was used to analyze the absorbance of these samples.
Measurement of reactive oxygen species (ROS), malondialdehyde (MDA), and superoxide dismutase (SOD)
In this research, the characteristic indicator of oxidative stress was evaluated based on ROS, MDA, and SOD levels. At first, DCFH-DA staining cellular ROS assay kit (ab113851, Abcam) was employed to examine the ROS level in a dark room, followed by the analysis using flow cytometry. For the measurement of MDA and SOD levels, harvested SK-N-SH cells were lysed. Then, obtained cell extract was used to detect their levels using the MDA kit (S0131S, Beyotime) and the SOD kit with WST-8 (S0103, Beyotime).
Cell transfection
For CBLN4 or METTL14 knockdown, shRNAs targeting the CBLN4 or METTL14 sequence were inserted into pLKO.1 lentiviral vector to obtain sh-CBLN4 or sh-METTL14 lentivirus plasmid. Then, these vectors were transfected into HEK293T cells (Invitrogen) with packing and helper plasmids, followed by enrichment, filtration, and quantification. After being infected into SK-N-SH cells with 8 µg/mL polybrene for 48 h, stable cells were selected with 4 µg/mL puromycin for 2 weeks.
For the upregulation system, 50 ng of CBLN4 (NM_080617.6) or METTL14 (NM_020961.4) overexpression plasmids (pcDNA-CBLN4 or METTL14, GenePharma, Shanghai, China) were transfected into SK-N-SH cells, with pcDNA empty vector used as the negative control.
Methylated RNA immunoprecipitation (MeRIP)
In this assay, the m6A existence in CBLN4 were examined using a Magna MeRIP m6A kit (Millipore, Molsheim, France). Briefly, sh-NC or sh-METTL14-transfected SK-N-SH cells were extracted based on TRIzol (Invitrogen) and then fragmented. After that, one-ninth of the fragmented RNA was aliquot for the “Input” sample. In parallel, four-ninths were mixed with anti-m6A and anti-IgG antibodies on magnetic protein A/G beads. After being eluted and purified, the immunoprecipitated RNA from each sample was subjected to RT-qPCR analysis.
RNA immunoprecipitation (RIP)
To verify the relationship between CBLN4 and METTL14, RIP assay was conducted. In short, SK-N-SH cells at 80% confluency were collected and lysed in the ice-cold RIP lysis buffer (Millipore). Then, cell lysates were incubated with anti-IgG or anti-METTL14 antibodies. After being digested and isolated, coprecipitated RNA was determined using RT-qPCR.
Dual-luciferase reporter assay
To further validate their interaction, this experiment was conducted in SK-N-SH cells. In brief, the wild-type CBLN4 (CBLN4 WT) and mutant CBLN4 (CBLN4 MUT) were synthesized and cloned into pmirGLO plasmid (Promega, Madison, WI, USA), which were transfected into SK-N-SH cells with sh-NC or sh-METTL14. The luciferase activities in cell lysates were assessed at 48 h post-transfection with the Dual-Luciferase Reporter Assay System.
Statistical analysis
Statistical significance was set at P < 0.05. Data in this study were obtained from at least three independent experiments and expressed as mean ± standard deviation (SD). Data were in normal distribution with uniform variance. The statistical significance of differences was evaluated by Student’s t-test and one-way ANOVA with Tukey’s tests. Statistical analyses were conducted using GraphPad Prism7.
Results
CBLN4 expression was decreased in AD tissues and Aβ1-42-induced neuroblastoma cells
Firstly, three microarray datasets related to AD, containing GSE33000 (https://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE33000), GSE122063 (https://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE122063), and GSE48350 (https://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE48350), were downloaded from the Gene Expression Omnibus (GEO) database and selected to characterize CBLN4 expression in AD samples and normal brain samples. As shown in Fig. 1A-I, the CBLN4 mRNA level was clearly reduced in AD samples relative to normal tissues. Meanwhile, we found that CBLN4 expression was associated with the age and sex of AD samples in all three databases. Moreover, we further verified that CBLN4 mRNA level and protein level were lower expressed in Aβ1-42-treated SK-N-SH cells compared with the control group (Fig. 1J and K). Together, these results implied the involvement of CBLN4 in AD progression.
Expression patterns of CBLN4 in AD tissues and Aβ1-42-induced SK-N-SH cells. A-C GSE33000 (310 AD and 314 no-demented) dataset was applied to assess the expression level of CBLN4 in AD samples and normal samples. D-F GSE122063 (56 AD and 44 no-demented) dataset was used to assess the CBLN4 expression level in AD samples and normal samples. G-I GSE48350 (80 AD and 173 normal) dataset assessed the expression level of CBLN4 in AD samples and normal samples. J CBLN4 level was determined in SK-N-SH cells-treated with Aβ1–42 or without (control group) using RT-qPCR assay. (K) Western blot assay was used to detect CBLN4 protein level in in SK-N-SH cells-treated with Aβ1–42 or without (control group). **P < 0.01, ***P < 0.001, n = 3
Aβ1–42 could induce SK-N-SH cell damage in vitro
Then, to further check the effects of Aβ1–42 on SK-N-SH cell biological behaviors, SK-N-SH cells were treated with 20 µM Aβ1–42 for 24 h. After that, MTT assay exhibited that SK-N-SH cell viability was significantly hindered after Aβ1–42 treatment (Fig. 2A). Moreover, Aβ1–42 exposure could promote SK-N-SH cell apoptosis rate (Fig. 2B). In parallel, the effects of Aβ1–42 on Bax (pro-apoptosis marker), Bcl-2 (anti-apoptosis marker), and caspase 3 (a key mediator of apoptosis) expression were determined. As shown in Fig. 2C, Aβ1–42 treatment elicited an obvious enhancement in Bax and C-caspase 3/total-caspase 3 protein levels and a substantial decline in Bcl-2 expression in SK-N-SH cells. In terms of inflammatory responses, secretions of pro-inflammatory cytokines IL-1β and TNF-α were highly induced in response to Aβ1–42 in SK-N-SH cells (Fig. 2D and E). Furthermore, Aβ1–42 exposure could induce oxidative stress, as evidenced by increased ROS and MDA levels (Fig. 2F and G), and decreased SOD activity (Fig. 2H). In addition, our data found that Aβ1–42 treatment could clearly upregulate the expression of endoplasmic reticulum (ER) stress marker (CHOP and GRP78) in SK-N-SH cells (Fig. 2I), implying the promotion of Aβ1–42 on ER stress. Collectively, these findings suggested that Aβ1–42 triggered neuronal apoptosis, inflammation, oxidative stress, and ER stress.
Aβ1–42 could induce SK-N-SH cell damage in vitro. SK-N-SH cells were treated with 20 µM Aβ1–42 or without (control) for 24 h. A Cell viability was assessed using MTT assay in Aβ1-42-treated SK-N-SH cells. B Cell apoptosis rate was examined using flow cytometry assay. C Bax, Bcl-2, total-caspase 3, and C-caspase 3 protein levels were measured in Aβ1-42-treated SK-N-SH cells by western blot. D and E The concentrations of IL-1β and TNF-α in Aβ1-42-treated SK-N-SH cells were checked using matched ELISA kits in Aβ1-42-treated SK-N-SH cells. F-H The levels of ROS, MDA, and SOD were examined to assess oxidative stress. I CHOP and GRP78 protein levels (markers of ER stress) were detected using western blot. **P < 0.01, ***P < 0.001, n = 3
Overexpression of CBLN4 could relieve Aβ1-42-induced SK-N-SH cell injury
Subsequently, gain-of-function experiments were performed to determine the influences of CBLN4 in the cell model of AD. Firstly, western blot results presented that the CBLN4 protein level was improved in pcDNA-CBLN4-transfected SK-N-SH cells compared with cells with pcDNA (Fig. 3A), suggesting that overexpression efficiency is available. Then, Aβ1-42-induced SK-N-SH cells were transfected with CBLN4 or pcDNA, and western blot assay displayed that the transfection of pcDNA-CBLN4 partly abolished the repression of Aβ1–42 treatment on CBLN4 protein level in SK-N-SH cells (Fig. 3B). Functionally, the upregulation of CBLN4 effectively abrogated Aβ1-42-mediated SK-N-SH cell viability inhibition (Fig. 3C). Meanwhile, Aβ1-42-induced SK-N-SH cell apoptosis was significantly ameliorated by CBLN4 overexpression (Fig. 3D), accompanied by decreased Bax and C-caspase 3/total-caspase 3, and increased Bcl-2 (Fig. 3E and F). Beyond that, the forced expression of CBLN4 remarkably attenuated Aβ1-42-triggered promotion of inflammation and oxidative stress in SK-N-SH cells, as described by decreased IL-1β and TNF-α levels (Fig. 3G and H), lower ROS and MDA levels (Fig. 3I and J), and higher SOD activity (Fig. 3K). Besides, ER stress was highly stimulated in response to Aβ1–42 in SK-N-SH cells, which was also relieved by CBLN4 upregulation, as depicted by lower CHOP and GRP78 (Fig. 3L). Overall, the above results indicated that Aβ1-42-mediated neuronal injury could be partially mitigated by CBLN4 overexpression.
Effects of CBLN4 upregulation on Aβ1-42-induced SK-N-SH cell injury. A The overexpression efficiency of CBLN4 in SK-N-SH cells were assessed using western blot. (B-L) SK-N-SH cells were treated with Control + pcDNA, Aβ1–42 + pcDNA, or Aβ1–42 + CBLN4. B CBLN4 protein level was determined using western blot in treated SK-N-SH cells. C and D MTT and flow cytometry assays were used to examine SK-N-SH cell viability and apoptosis. E and F Bax, Bcl-2, total-caspase 3, and C-caspase 3 protein levels were analyzed by western blot. G and H The concentrations of IL-1β and TNF-α were detected using ELISA kits. (I-K) ROS, MDA, and SOD levels were examined to evaluate oxidative stress. L CHOP and GRP78 protein levels were determined using western blot. **P < 0.01, ***P < 0.001, n = 3
METTL14 sustains RNA stability and CBLN4 expression by regulating the m6A modification
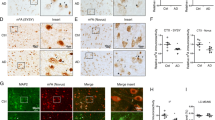
Next, RT-qPCR results found that METTL14 mRNA level was clearly decreased in Aβ1-42-treated SK-N-SH cells (Fig. 4A), indicating that METTL14 was involved in AD processes. Considering that METTL14 is essential for the m6A catalytic process, we further detected the m6A modification of CBLN4 in SK-N-SH cells by using the sequence-based RNA adenosine methylation site predictor (SRAMP) website. As presented in Fig. 4B, there are m6A modification sites on the mRNA of CBLN4. Therefore, we hypothesized that METTL14 could mediate the m6A methylation of CBLN4. Then, MeRIP assay showed that METTL14 knockdown significantly reduced the m6A modification level of CBLN4 in SK-N-SH cells (Fig. 4C). As expected, CBLN4 was found to have binding site with METTL14 using the RBPsuite website (Fig. 4D). Moreover, RIP and dual-luciferase reporter assays further validated the interaction between CBLN4 and METTL14 in SK-N-SH cells (Fig. 4E and F). In addition, the knockdown or overexpression of METTL14 in SK-N-SH cells was detected and exhibited in Fig. 4G. Subsequently, RT-qPCR and Western blot results showed that CBLN4 mRNA levels and protein levels were significantly suppressed by METTL14 silencing, while CBLN4 mRNA levels and protein levels were remarkably increased by METTL14 transfection of SK-N-SH cells (Fig. 4H and I). Collectively, the above data illuminated that METTL14 promoted CBLN4 expression by regulating the RNA m6A modification.
CBLN4 is regulated by METTL14-mediated m6A modification. A METTL14 mRNA level was detected in SK-N-SH cells treated with Aβ1–42 or without (Control group) using RT-qPCR assay. B SRAMP website predicted that CBLN4 mRNA has an m6A site. C Changes in the m6A methylation level of CBLN4 after METTL14 deficiency were analyzed using MeRIP assay. D RBPsuite website was used to analyze the binding site between CBLN4 and METTL14. E and F Their interaction were verified using RIP and dual-luciferase reporter assays in SK-N-SH cells. G METTL14 mRNA level was assessed in SK-N-SH cells transfected with sh-NC, sh-METTL14, pcDNA, or METTL14 using RT-qPCR. (H and I) Effect of METTL14 knockdown or overexpression on CBLN4 protein levels were measured using RT-qPCR and western blot in SK-N-SH cells. **P < 0.01, ***P < 0.001, n = 3
Upregulation of CBLN4 could abolish the repression of METTL14 on Aβ1-42-triggered SK-N-SH cell damage
Considering the regulatory role of METTL14 in CBLN4 expression in SK-N-SH cells, whether the impact of METTL14 in Aβ1-42-induced cell injury was correlative with CBLN4 was further investigated. Then, western blot results presented that the upregulation of METTL14 strikingly enhanced the protein level of CBLN4 in Aβ1-42-treated SK-N-SH cells, which were partly counteracted after sh-CBLN4 co-transfection (Fig. 5A). Functional analysis discovered that METTL14 overexpression-mediated cell viability increase and apoptosis reduction in Aβ1-42-treated SK-N-SH cells were markedly ameliorated by CBLN4 deficiency (Fig. 5B and C). Simultaneously, Bax and C-caspase 3/total-caspase 3 were lowly expressed and Bcl-2 was highly expressed with METTL14 overexpression in Aβ1-42-treated SK-N-SH cells, whereas these effects were partly reversed through CBLN4 silencing (Fig. 5D). Apart from that, Aβ1-42-evoked inflammatory response and oxidative stress were obviously alleviated through METTL14 addition, and this protection was partly overturned after sh-CBLN4 co-transfection, as described by enhanced IL-1β and TNF-α (Fig. 5E and F), higher ROS and MDA levels (Fig. 5G and I), and lower SOD activity (Fig. 5J). Additionally, the lack of CBLN4 partly weakened the inhibitory impact of METTL14 upregulation on ER stress in Aβ1-42-treated SK-N-SH cells, as evidenced by increased CHOP and GRP78 (Fig. 5K). All these results demonstrated that METTL14 could suppress Aβ1-42-induced SK-N-SH cell injury by targeting CBLN4.
METTL14/CBLN4 regulated Aβ1-42-induced SK-N-SH cell injury. SK-N-SH cells were treated with Control + pcDNA + sh-NC, Aβ1–42 + pcDNA + sh-NC, Aβ1–42 + METTL14 + sh-NC, or Aβ1–42 + METTL14 + sh-CBLN4. A Western blot analysis of CBLN4 protein level in treated SK-N-SH cells. B and C Cell viability and apoptosis were examined using MTT and flow cytometry assays. D Bax, Bcl-2, and C-caspase 3/total-caspase 3 protein levels were was monitored by western blot assay. E and F Specific ELISA kits was used to analyze the concentrations of IL-1β and TNF-α. G-J Oxidative stress was analyzed by measuring ROS, MDA, and SOD levels. K Western blot assay was applied to detect the protein levels of CHOP and GRP78. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3
Discussion
Current evidence has described amyloid plaques as a distinctive feature of AD, which are deposits of excess accumulated Aβ peptides, a initiator of pathogenic cascade ultimately leading to AD (Hampel et al. 2023; , Liu and Wang 2023). Furthermore, Aβ is a proteolytic derivative of the transmembrane glycoprotein amyloid precursor protein (APP), especially Aβ1–42, which plays a key role in all forms of AD (Citron 2010). Based on the amyloid cascade hypothesis, Aβ1–42 oligomers could induce a neurotoxic cascade with kinase activation, resulting in neuronal death, free radical production, and neuroinflammation (Hugon et al. 2017). Several studies have suggested that in vitro AD models are established by culturing fibrillar Aβ1–42 (Wang et al. 2021; , Wang et al. 2018). As a member of a small family of secreted synaptic proteins, CBLN4 has been reported to exert an important role in the formation and maintenance of synaptic connections. Also, the upregulation of CBLN4 could improve inhibitory activity and resistance of neurons to Aβ toxicity (Chacón et al. 2015). Consistent with the former report, the current work indicated that CBLN4 level was decreased in Aβ1-42-treated SK-N-SH cells. Microarray datasets also exhibited the downregulation of CBLN4 in AD samples. In the chapter, functional analysis discovered that Aβ1–42 induced a series of SK-N-SH cell damages, such as neuronal apoptosis, inflammation, and oxidative stress, which were partly relieved by CBLN4 upregulation. Beyond that, recent literature presented that activated ER stress could facilitate the activation of unfolded protein response, a signaling pathway that initiates irreversible apoptosis in damaged cells (Gerakis and Hetz 2018). In this work, our data validated that Aβ1–42 treatment-triggered ER stress was significantly ameliorated after CBLN4 addition. Overall, the above observation provides strong evidence that CBLN4 plays a protective role in Aβ1-42-mediated SK-N-SH cell injury.
Regarding the molecular mechanism, increasing evidence has suggested that m6A methylation performs vital roles in modulating gene expression in different diseases by controlling RNA splicing, stability, and translation (He and He 2021). Some studies indicated that the expression levels of m6A regulators, such as writers, erasers, and readers, are frequently dysregulated in AD and involved in AD progression (Xia et al. 2023; , Shafik et al. 2021). Previous studies have suggested that the major determination of m6A levels is attributed to the catalyzing of methyltransferase complexes (METTL3, METTL14, and WTAP) (Zhang et al. 2022). Interestingly, their expression levels were reduced in the Hippocampus of Tyrobp-/-mice (Lv 2022). It has been reported that METTL3 could enhance STUB1 mRNA expression via m6A methylation, thus improving autophagic clearance of p-Tau in Aβ1-42-treated cells and ameliorating AD (Tang et al. 2023). Therefore, we inferred that m6A methyltransferases could participate in the regulation of CBLN4 in Aβ1-42-treated cells. Herein, CBLN4 was a latent target of METTL14. Beyond that, METTL14 could enhance CBLN4 mRNA stability and protein expression in SK-N-SH cells. As expected, rescue experiments verified that METTL14 overexpression could effectively abate Aβ1-42-evoked SK-N-SH cell injury, whereas these effects were partially abolished by CBLN4 silencing. In total, these above findings provide first-hand evidence of the regulatory effects of METTL14/CBLN4 in Aβ1-42-induced neuronal damage. However, there are still some shortcomings in this research. First, our study is limited to immortalized human neuroblastoma cells which likely have altered biological characteristics than in vivo neurons. Second, Aβ1-42-treated SK-N-SH cells cultured in vitro cannot completely simulate the in vivo environment with continuous interactions among multiple cells and cytokines.
Conclusion
In this project, our results revealed that METTL14 could ameliorate Aβ1-42-stimulated SK-N-SH cell apoptosis, inflammation, oxidative stress, and ER stress by naturally increasing CBLN4 expression (Fig. 6), providing a novel avenue for AD management.
Data availability
No datasets were generated or analysed during the current study.
References
(2023) Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2023, 19(4):1598–1695
Breijyeh Z, Karaman R (2020) Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 25(24)
Bourgeron T (2015) From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci 16(9):551–563
Chacón PJ, del Marco Á, Arévalo Á, Domínguez-Giménez P, García-Segura LM, Rodríguez-Tébar A (2015) Cerebellin 4, a synaptic protein, enhances inhibitory activity and resistance of neurons to amyloid-β toxicity. Neurobiol Aging 36(2):1057–1071
Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discovery 9(5):387–398
Deng Y, Zhu H, Xiao L, Liu C, Liu YL, Gao W (2021) Identification of the function and mechanism of m6A reader IGF2BP2 in Alzheimer’s disease. Aging 13(21):24086–24100
Gerakis Y, Hetz C (2018) Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J 285(6):995–1011
Hampel H, Hu Y, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M et al (2023) The amyloid-β pathway in Alzheimer’s disease: a plain language summary. Neurodegenerative Disease Manage 13(3):141–149
He PC, He C (2021) M(6) a RNA methylation: from mechanisms to therapeutic potential. EMBO J 40(3):e105977
Hugon J, Mouton-Liger F, Dumurgier J, Paquet C (2017) PKR involvement in Alzheimer’s disease. Alzheimers Res Ther 9(1):83
Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR (2001) Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng 17(6):157–165
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X et al (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10(2):93–95
Liu X, Wang Y (2023) Elucidating the molecular targets and mechanisms of Chlorogenic Acid against Alzheimer’s Disease via Network Pharmacology and Molecular Docking. Lett Drug Des Discovery 20(9):1329–1342
Lv Z, Xu T, Li R, Zheng D, Li Y, Li W, Yang Y, Hao Y (2022) Downregulation of m6A methyltransferase in the Hippocampus of Tyrobp (-/-) mice and implications for learning and memory deficits. Front NeuroSci 16:739201
Murakami S, Jaffrey SR (2022) Hidden codes in mRNA: control of gene expression by m(6)a. Mol Cell 82(12):2236–2251
Meyer KD, Jaffrey SR (2014) The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol 15(5):313–326
Meng S, Wang B, Li W (2022) CircAXL Knockdown alleviates Aβ(1–42)-Induced neurotoxicity in Alzheimer’s Disease via repressing PDE4A by releasing miR-1306-5p. Neurochem Res 47(6):1707–1720
Salaikumaran Raj M, Prasad Burra Laxmi Siva V (2023) <i > in silico design of novel SAM analogs as potential inhibitors against N2G966 16s rRNA methyltransferase (RsmD)</i >. Lett Drug Des Discovery 20(12):1898–1910
Sanfilippo C, Musumeci G, Kazakova M, Mazzone V, Castrogiovanni P, Imbesi R, Di Rosa M (2021) GNG13 is a potential marker of the state of Health of Alzheimer’s Disease patients’ Cerebellum. J Mol Neuroscience: MN 71(5):1046–1060
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet (London England) 397(10284):1577–1590
Sehar U, Rawat P, Reddy AP, Kopel J, Reddy PH (2022) Amyloid Beta in Aging and Alzheimer’s Disease. Int J Mol Sci 23(21)
Seigneur E, Südhof TC (2017) Cerebellins are differentially expressed in selective subsets of neurons throughout the brain. J Comp Neurol 525(15):3286–3311
Śledź P, Jinek M (2016) Structural insights into the molecular mechanism of the m(6)a writer complex. eLife 5
Shafik AM, Zhang F, Guo Z, Dai Q, Pajdzik K, Li Y, Kang Y, Yao B, Wu H, He C et al (2021) N6-methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer’s disease. Genome Biol 22(1):17
Tang Z, Cao J, Yao J, Fan X, Zhao J, Zhao M, Duan Q, Han B, Duan S (2023) KDM1A-mediated upregulation of METTL3 ameliorates Alzheimer’s disease via enhancing autophagic clearance of p-Tau through m6A-dependent regulation of STUB1. Free Radic Biol Med 195:343–358
Wang H, Jiang T, Li W, Gao N, Zhang T (2018) Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol Lett 282:100–108
Wang J, Zheng B, Yang S, Wang F, Wang Z, Wang J (2021) The protective effects of agomelatine against Aβ1–42 oligomers-induced cellular senescence mediated by SIRT6 and Agomelatine’s potential in AD treatment. Hum Cell 34(6):1734–1743
Wei P, Pattarini R, Rong Y, Guo H, Bansal PK, Kusnoor SV, Deutch AY, Parris J, Morgan JI (2012) The Cbln family of proteins interact with multiple signaling pathways. J Neurochem 121(5):717–729
Xia L, Zhang F, Li Y, Mo Y, Zhang L, Li Q, Luo M, Hou X, Du Z, Deng J et al (2023) A new perspective on Alzheimer’s disease: m6A modification. Front Genet 14:1166831
Yang Y, Hsu PJ, Chen YS, Yang YG (2018) Dynamic transcriptomic m(6)a decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res 28(6):616–624
Zhang N, Ding C, Zuo Y, Peng Y, Zuo L (2022) N6-methyladenosine and neurological diseases. Mol Neurobiol 59(3):1925–1937
Zhao F, Xu Y, Gao S, Qin L, Austria Q, Siedlak SL, Pajdzik K, Dai Q, He C, Wang W et al (2021) METTL3-dependent RNA m(6)a dysregulation contributes to neurodegeneration in Alzheimer’s disease through aberrant cell cycle events. Mol Neurodegeneration 16(1):70
Zhang TP, Li R, Wang LJ, Huang Q, Li HM (2022) Roles of the m6A methyltransferases METTL3, METTL14, and WTAP in pulmonary tuberculosis. Front Immunol 13:992628
Zhang XX, Tian Y, Wang ZT, Ma YH, Tan L, Yu JT (2021) The epidemiology of Alzheimer’s Disease Modifiable Risk factors and Prevention. J Prev Alzheimer’s Disease 8(3):313–321
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology: Jiangpeng Jing and Ruichun Li; Formal analysis and Data curation: Ruichun Li and Chuankun Li; Validation and Investigation: Chuankun Li and Bin Mu; Writing - original draft preparation and Writing - review and editing: Bin Mu, Jiangpeng Jing and Ruichun Li; Approval of final manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. CBLN4 expression was downregulated in Aβ1-42-induced SK-N-SH cells.
2. CBLN4 overexpression suppressed Aβ1-42-induced SK-N-SH cell injury.
3. METTL14 improved the stability and expression of CBLN4 mRNA via m6A methylation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mu, B., Jing, J., Li, R. et al. METTL14 inhibits Aβ1-42-induced neuronal injury through regulating the stability of CBLN4 mRNA in Alzheimer’s disease. J Bioenerg Biomembr (2024). https://doi.org/10.1007/s10863-024-10036-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10863-024-10036-9