Abstract
HESA-A is an herbal-marine compound which improves the quality of life of end-stage cancer patients. The aim of the present study was to evaluate the possible protective effect of HESA-A against IR-induced genotoxicity and apoptosis in rat bone marrow. Rats were given HESA-A orally at doses of 150 and 300 mg/kg body weight for seven consecutive days. On the seventh day, the rats were irradiated with 4 Gy X-rays at 1 h after the last oral administration. The micronucleus assay, reactive oxygen species (ROS) level analysis, hematological analysis and flow cytometry were used to assess radiation antagonistic potential of HESA-A. Administration of 150 and 300 mg/kg of HESA-A to irradiated rats significantly reduced the frequencies of micronucleated polychromatic erythrocytes (MnPCEs) and micronucleated normochromatic erythrocytes (MnNCEs), and also increased PCE/(PCE + NCE) ratio in bone marrow cells. Moreover, pretreatment of irradiated rats with HESA-A (150 and 300 mg/kg) significantly decreased ROS level and apoptosis in bone marrow cells, and also increased white blood cells count in peripheral blood. For the first time in this study, it was observed that HESA-A can have protective effects against radiation-induced genotoxicity and apoptosis in bone marrow cells. Therefore, HESA-A can be considered as a candidate for future studies to reduce the side effects induced by radiotherapy in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionizing radiation (IR) can cause DNA double strand breaks, cell membrane alterations, production of reactive oxygen species (ROS) and genomic instability in the cells. These injuries will arrest the cell cycle or lead to apoptosis (Kolivand et al. 2017; Najafi et al. 2018). Normally, cells with a high proliferation rate are more sensitive to radiation. The turnover of bone marrow cells is one of the fastest. Thus, the damage to hematopoietic system as the most sensitive organ in the body to IR leads to hematopoietic dysfunction and myelosuppression. Hence, the radioprotection of hematopoietic system is an important issue in cancer treatment (Fleenor et al. 2010; Shao et al. 2014).
Amifostine is the only radioprotector that has been approved by Food and Drug Administration (FDA) for head and neck cancer patients undergoing radiotherapy. The side effects like nausea, vomiting, and hypotension associated with it restrict its use in the clinic (Cheki et al. 2018). Therefore, the screening of natural compounds, mostly from plants, for their radioprotecting ability has been an area of research in recent years (Cheki et al. 2016a, b). HESA-A as a natural drug has no side effects like nausea, vomiting, and hypotension. The HESA-A is also administered to cancer patients orally, which is one of the most preferred routes of drug administration, while amifostine is administered intravenously, and whose oral administration is not effective. HESA-A is currently used to improve the quality of life through increasing appetite, induction of weight gain, enhancing liver function, and reducing pain in case of end-stage metastatic liver, colon, and breast cancer, as well as osteosarcoma (Abbasi et al. 2015; Ahmadi et al. 2005a, b, 2009, 2010). On the other hand, HESA-A contains trace elements which are known to possess anti-oxidant and potential anti-cancer properties such as vanadium (V), nickel (Ni), titanium (Ti), zinc (Zn), strontium (Sr), and selenium (Se) (Alizadeh et al. 2009; Caruso and Rossi 2004; Guo et al. 2007; Roudkenar et al. 2012; Zeng and Combs 2008). Further, Penaeus semisulcatus, as a component of HESA-A, is a species of prawn. Prawns are a rich source of selenium, zinc, and vitamin-B12, which are important for strengthening the antioxidant and immune systems of the human body (Syama Dayal et al. 2013; Bourre and Paquotte 2008; El-Gendy et al. 2018). The several studies have shown that selenium, zinc, vanadium, and vitamin-B12 can reduce the side effects of radiation (Hosseinimehr 2015; Abou-Seif et al. 2003; Sood et al. 2011; Crescenti et al. 2011; Al-Hazzaa et al. 2007; Al-Meer et al. 2011). Hence, HESA-A could be eligible for primary evaluation as a radioprotective candidate agent. Furthermore, its antioxidant, anti-inflammatory, immunemodulatory, antiviral, free radicals scavenging and anticancer effects have been demonstrated in various in vitro and in vivo models (Ahmadian et al. 2016; Jahanban-Esfahlan et al. 2015; Mehdipour et al. 2013; Mehrbod et al. 2014; Roudkenar et al. 2012; Vahabpour et al. 2012). Since cellular and molecular damages induced by IR are mainly related to free radicals, it is expected that HESA-A as an antioxidant can inhibit these damages. Therefore, the purpose of this study is to evaluate the protective capability of HESA-A against IR-induced genotoxicity and apoptosis in rat bone marrow cells.
Materials and methods
Chemicals
HESA-A was purchased from Osvah Pharmaceutical Co., (Tehran, Iran). Phosphate buffer saline (PBS), Fetal bovine serum (FBS), May-Grunewald and Giemsa stain were obtained from Merck, (Germany). Annexin-V-FLUOS Staining Kit was obtained from Roche Diagnostics GmbH, (Penzberg, Germany). The 2′, 7′-Dichlorofluorescin diacetate (DCFH-DA) was purchased from Sigma chemicals Co. (St. Louis, USA).
Animals
Adult male Wistar rats weighing 120–180 g were used in this study. All rats were kept in a room under constant temperature (22 ± 2 °C), humidity (55–60%) and illuminated from 8:00 a.m. to 8:00 p.m. with free access to food pellets and water. The animals were accustomed to the laboratory conditions three days before the experimental session.
Drug and experimental protocol
Reagan-Shaw et al. (2008) reported a good-established formula for converting the dose of drugs from one animal species to another which has been used in numerous experimental studies. Based on this formula, the human equivalent dose (mg/kg) = animal dose (mg/kg) × animal (km)/human (km). Km for a 60 kg human adult equals 37 and for a 150 g rat equals 6. Thus, the human equivalent doses of 150 and 300 mg/kg in rat is about 25 and 50 mg/average size person of 60 kg, respectively. On the other hand, HESA-A is classically administered orally to human subjects at a dose of 25–50 mg/kg/day (Ahmadi et al. 2009, 2010). Therefore, the selected doses in this study are within the safe therapeutic range recorded in humans. Furthermore, 30 days oral administration 5000 mg/kg of HESA-A daily did not cause harm to the mice and rats (Hajhashemi et al. 2001). HESA-A was freshly dissolved in PBS (pH = 7.6) and was daily administered through oral gavage in a constant volume to rats.
In each of the 5 groups as follows, 5 rats were placed:
-
1)
Control group: rats were orally gavaged with solvent control (PBS) and exposed to sham irradiation.
-
2)
300 mg/kg HESA-A group: rats were orally gavaged with HESA-A (300 mg/kg body weight) and exposed to sham irradiation.
-
3)
Irradiation-alone (4 Gy) group: rats were orally gavaged with solvent control (PBS) and exposed to 4 Gy X-radiation.
-
4)
150 mg/kg HESA-A + 4 Gy group: rats were orally gavaged with HESA-A (150 mg/kg body weight) and exposed to 4 Gy X-radiation.
-
5)
300 mg/kg HESA-A + 4 Gy group: rats were orally gavaged with HESA-A (300 mg/kg body weight) and exposed to 4 Gy X-radiation.
All rats were gavaged with solutions, as described above respectively, from seven consecutive days. On the seventh day, the rats were irradiated to 4 Gy X-rays at 1 h after the last oral administrations. All of the rats after deep anesthetize were sacrificed at 24 h post-irradiation.
Irradiation
Irradiation was performed to the whole body of rats using X-rays (6 MV) from a medical linear accelerator (Elekta, Stockholm, Sweden) at a dose rate of 2 Gy/min and a source surface distance (SSD) of 100 cm.
Bone marrow micronucleus assay
Schmid (1975) reported a micronucleus method in bone marrow which was used in this study. Preparation of cell suspension from bone marrow was done by injection of FBS into both femurs. The cells were gathered by centrifuge at 2000 rpm for 10 min. Bone marrow smears were prepared on the slides at room temperature. After 24 h air-drying, the slides were fixed with methanol and stained with May-Grunwald/Giemsa. Based on this method, the normochromatic erythrocytes (NCEs) and polychromatic erythrocytes (PCEs) are stained orange and reddish-blue, respectively, while nuclear material is dark purple. Cells were counted on each slide using light microscopy with 1000 × magnification under oil immersion. For each experimental group, five rats were used and a total of 5000 PCEs and corresponding NCEs (1000 PCEs and 1000 NCEs per animal) were scored to determine the number of micronucleated polychromatic erythrocytes (MnPCEs), micronucleated normochromatic erythrocytes (MnNCEs), and PCE to (PCE + NCE) ratio.
ROS determination
The quantification of intracellular ROS was performed using DCFH-DA probe. The hydrolyzation of DCFH-DA and its transformation into nonfluorescent DCFH are carried out by intracellular esterases, which in the presence of ROS converted to fluorescent DCF (Halliwell and Whiteman 2004). Briefly, the suspension of bone marrow cells containing FBS was washed twice with PBS. Then, 10 μM of DCFH-DA was added to these cells and incubated in dark for 30 min to allow the formation of DCF. Fluorescence intensities were quantified using a Perkin-Elmer LS50B Fluorescence Spectrometer (Beaconsfield, UK) at an excitation wavelength of 485 nm and emission wavelength of 529 nm. ROS values were expressed as fold-difference with the control.
Hematological study
K2EDTA-coated microvette tubes were used for blood samples collected via cardiac puncture. White blood cells (WBCs), red blood cells (RBCs), platelets and hemoglobin were measured by the Sysmex KX-21 N Automated Hematology Analyzer.
Apoptosis quantification by flow cytometry
According to the manufacturer’s instruction of Annexin-V-FLUOS Staining Kit, apoptosis and necrosis were measured. Briefly, the suspension of bone marrow cells containing FBS was washed twice with PBS and incubated with Annexin-V FLUOS labeling solution (2 μl Annexin-V and 2 μl propidium iodide in 100 μl incubation buffer for each sample) at room temperature, in the dark for 15 min. A bone marrow sample without the staining procedure as negative control used for identification of quadrant. Typically, there were 1 × 106 cells/ml in each bone marrow sample. Bone marrow samples were counted by flow cytometry on a FACS Calibur flow cytometer (Becton-Dickinson, San Jose, CA, USA) at 488 nm excitation and a 515 nm bandpass filter (FL1) for Annexin-V-Fluos detection and a filter >600 nm (FL3) for PI detection. For each group, five independent bone marrow samples were analyzed. The 10000 events were counted in each sample.
Statistical analysis
The data values are rendered as means ± standard error of mean (SEM). Statistical analysis was carried out by one-way analysis of variance (ANOVA), as well as post hoc Tukey's tests (SPSS V16). P value <0.05 and < 0.001 was considered as significant and highly significant, respectively.
Results
Micronucleus assay
The frequency of MnPCE/1000PCE and MnNCE/1000NCE induced by HESA-A or in combination with 4 Gy irradiation were shown in Table 1. The treatment with 300 mg/kg of HESA-A did not lead to a significant increase in the frequency of MnPCE/1000PCE and MnNCE/1000NCE when compared to the control group (P values: 0.981 and 0.936, respectively). This shows the non-genotoxic nature of HESA-A. Significant increase in the frequency of MnPCE/1000PCE and MnNCE/1000NCE was observed in irradiated group with 4 Gy X-ray only as compared to the control group (P < 0.001). The data demonstrate that irradiated groups with HESA-A at 150 and 300 mg/kg exhibited a significant decrease in the frequency of MnPCE/1000PCE and MnNCE/1000NCE as compared to the irradiated group without HESA-A (P < 0.001). The protective effect of HESA-A on the radiation induced micronuclei formation in PCEs and NCEs significantly increased with increase in the treatment dose from 150 to 300 mg/kg (P < 0.05).
The ability of bone marrow proliferation can be measured with the PCE/(PCE + NCE ratio (Table 1). The PCE/(PCE + NCE) ratio in group treated with 300 mg/kg of HESA-A only was within the range of the control group (P value: 0.987). On the other hand, significant reduction of PCE/(PCE + NCE) ratio was found in the 4 Gy-irradiated group when compared to the non-irradiated groups (P < 0.001). The PCE/(PCE + NCE) ratio was significantly increased by about 26 and 55% in irradiated groups with HESA-A at 150 and 300 mg/kg, respectively, compared to irradiated group without HESA-A.
ROS generation
As shown in Fig. 1, ROS level did not show significant variation after treatment with 300 mg/kg of HESA-A as compared with the control group (P value: 0.999). The ROS level in the group irradiated with 4 Gy X-ray was significantly increased by about 1.7-fold, as compared with the control group (P < 0.001). Pretreatment with 150 and 300 mg/kg of HESA-A prior to radiation significantly reduced the ROS level as compared with the irradiation-alone group (P < 0.001).
Hematological analysis
As shown in Fig. 2, a significant reduction of WBCs count was found in the 4 Gy-irradiated group when compared to the control group (4480.00 ± 124.09/μl versus 5900.00 ± 141.42/μl; P < 0.001). In contrast, treatment with 150 and 300 mg/kg of HESA-A before irradiation showed a significant increase in WBCs count when compared to irradiation-alone group (5400.00 ± 122.47/μl and 5920.00 ± 86.02/μl versus 4480.00 ± 124.09/μl, respectively; P < 0.001). No difference was observed in counts of RBCs, platelets and hemoglobin after irradiation at 24 h (Fig. 2).
Apoptosis quantification by flow cytometry
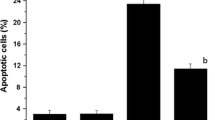
As shown in Figs. 3 and 4, irradiation with 4 Gy caused a marked increase in the percentage of apoptotic cells (Annexin V+ and PI−) as compared with the control group (18.60 ± 0.83% versus 4.65 ± 0.37%; P < 0.001). However, treatment with 150 and 300 mg/kg of HESA-A prior to irradiation demonstrated a significant decrease in the percentage of apoptotic cells when compared to irradiation-alone group (11.56 ± 0.28% and 6.41 ± 0.67% versus 18.60 ± 0.83%, respectively; P < 0.001). Also, pre-treatment with HESA-A demonstrated a strong preventive effect on the percentage of apoptotic cells induced by radiation in a dose-dependent manner (P < 0.001). The groups of control and 300 mg/kg of HESA-A alone exhibited similar results in the population of apoptotic cells. Furthermore, the percentage of necrotic cells (Annexin V+ and PI+) and necrotic cells debris or apoptotic bodies (Annexin V− and PI+) was too low and negligible in all different groups (<1.8%, Fig. 3).
Effect of HESA-A on radiation-induced apoptosis in rat bone marrow cells. Rat bone marrow cells were analyzed for Annexin V binding and for PI uptake using flow cytometry. Representative dot plots of one set of five independent experiments of Annexin V and PI staining. The lower left quadrant (Annexin V− and PI−) was considered as live cells, the lower right quadrant (Annexin V+ and PI−) was considered as apoptotic cells, the upper right quadrant (Annexin V+ and PI+) was considered necrotic cells, and the upper left quadrant (Annexin V− and PI+) was considered as necrotic cells debris or apoptotic bodies
Effect of HESA-A on radiation-induced apoptosis in rat bone marrow cells. The percentages of apoptotic cells (Annexin V+ and PI−) were shown in experimental groups. Values are expressed as mean ± SEM of five experiments in each group. *P < 0.001: 4 Gy group compared to control, #P < 0.001: 150 and 300 mg/kg HESA-A+ 4 Gy groups compared to 4 Gy alone
Discussion
During this study, we have observed that 300 mg/kg HESA-A could lead to 63% reduction in MnPCE, while previous studies have shown that the maximum administered doses of peroxiredoxin 2, peroxiredoxin 6, unmodified hydrated С60 fullerene molecules (C60UHFM), guanosine-5′-monophosphate, sesamol, melatonin, amifostine, sulfasalazine, procyanidins from lotus seedpod (LSPCs), black mulberry extract (BME), dragon’s blood (DB) and its extracts (DBE) can reduce IR-induced MnPCE by 40, 85, 58, 42, 50, 41, 51, 49, 56, 48, 30, and 45%, respectively (Asadullina et al. 2010; Duan et al. 2010; Gudkov et al. 2019; Kumar et al. 2015, 2018; Mantena et al. 2008; Ran et al. 2014; Sharapov et al. 2019; Sharapov et al. 2017; Targhi et al. 2017). We have also observed that 300 mg/kg HESA-A could lead to about 58% reduction in MnNCE, while Kumar et al. (2015), Mantena et al. (2008), Duan et al. (2010), Targhi et al. (2017), and Ran et al. (2014) have reported that sesamol, melatonin, sulfasalazine, LSPCs, BME, DB and DBE can reduce IR-induced MnNCE by 20, 8, 66, 55, 56, 25, and 41%, respectively. Comparing these results shows that HESA-A has an acceptable radioprotective effect than other compounds. In the animal study, Hajhashemi et al. (2001) observed no noxious effects as estimated by hematology, growth, clinical chemistry and histopathology in mice and rats exposed to 1250, 2500, and 5000 mg/kg of HESA-A for 30 days. Furthermore, the administration of 50, 100, and 200 mg/kg of HESA-A to pregnant mice on days 6 to 14 of gestation did not cause teratogenic effects (Moallem et al. 2011). Above results are in line with our current results, the induction of micronuclei in HESA-A-treated group were similar to those in control animals.
Normal cell metabolism requires oxygen to produce energy, which results in the generation of ROS. ROS are potentially toxic, do not pose a risk at levels produced by normal cell metabolism, however, under improper conditions, such as radiation exposure, high levels of ROS are generated and can seriously damage to the cellular genome and vital macromolecules which finally lead to secondary malignancies and genotoxicity (Zorov et al. 2014). Roudkenar et al. (2012) reported that the cytoprotective effect of HESA-A on Chinese hamster ovary and human embryonic kidney cells against H2O2 could be relate to its potent antioxidant and antiradical activity. HESA-A protects rabbits against hepatotoxicity induced by thioacetamide through increasing total antioxidant and decreasing malondialdehyde (MDA) (Ahmadi et al. 2005a, b). In the present work, HESA-A administration reduced intracellular ROS by 40% in bone marrow cells. In previous studies to intracellular ROS measurement in rodent bone marrow cells with DCFH-DA probe, it was shown that mitochondrial-targeted dihydronicotinamide (Mito-N), astaxanthin, G-003 M (combination of podophyllotoxin and rutin), and N-Acetyl-cysteine (NAC) can reduce IR-induced ROS by about 40, 33, 45, and 62%, respectively ((Jia et al. 2010; Singh et al. 2017; Xue et al. 2017; Zhang et al. 2019). This can show that HESA-A appears to be efficient in reducing ROS level induced by IR in bone marrow cells.
The hematopoietic system completely repopulate by hematopoietic stem cells from the bone marrow which is the most active and highest turnover system in the body. Hence, it is very sensitive to IR. Protection and recovery of this system against IR can help to survive and improve the quality of life (Green and Rubin 2014). In the present study, we have observed, that pre-administration of 300 mg/kg HESA-A could lead to about 32% increment in WBCs count, while Kumar et al. (2015) have shown that administration of sesamol results in an increase of about 30% in WBCs count at 24 h post-irradiation. In addition, they did not observe alteration in counts of RBCs, platelets and hemoglobin after irradiation at 24 h. Above result is in line with our current result.
Non-repair of IR-induced DNA damage can lead to cell death via apoptosis. So far, many scavengers of free radicals have been found to modulate radiation-induced apoptosis in rodent bone marrow (Mazur et al. 2003; Ormsby et al. 2014; Suman et al. 2012). In this study, it was observed that HESA-A at doses of 150 and 300 mg/kg could lead to about 38 and 65% reduction in bone marrow cell apoptosis, respectively. Suman et al. (2012) reported that treatment with ON 01210.Na (Ex-RAD) reduced apoptosis by 55% in bone marrow cells of animals irradiated with 5 Gy gamma-rays. Kumar et al. (2015) has also demonstrated that sesamol can reduce apoptosis by 65% in mouse bone marrow exposed to 2 Gy gamma-irradiation after 24 h. Furthermore, in all experimental groups, our results demonstrated that radiation-induced cell death in rat bone marrow was apoptosis, not necrosis. Mohseni et al. (2012) reported that whole body gamma-irradiation dose of 8 Gy did not cause necrotic death in rat’s peripheral blood lymphocytes. Due to the close relationship between free radicals, especially ROS, and apoptosis, this anti-apoptotic effect in our study is supposed to have resulted from the action of HESA-A as a direct free radical scavenger against ROS generated by radiation.
Conclusion
This study showed that HESA-A administration before radiation modulates the harmful effects of IR on bone marrow cells. Therefore, HESA-A can be considered as a candidate in the future to reduce radiation damage to bone marrow in cancer patients undergoing radiotherapy.
References
Abbasi MM, Helli S, Monfaredan A, Jahanban-Esfahlan R (2015) Hesa-A improves clinical outcome of oral carcinoma by affecting p53 gene expression in vivo. Asian Pac J Cancer Prev 16:4169–4172
Abou-Seif MA, El-Naggar MM, El-Far M, Ramadan M, Salah N (2003) Prevention of biochemical changes in gamma-irradiated rats by some metal complexes. Clin Chem Lab Med 41:926–933
Ahmadi A, Mohagheghi MA, Fazeli MS, Nahavandian B, Bashardoost N, Jarahi AM, Gharipoor M (2005a) HESA-A: new treatment for breast cancer and choroidal metastasis. Med Sci Monit 11:300–303
Ahmadi A, Naderi G, Asgary S (2005b) Evaluation of hepatoprotective potential of HESA-A (a marine compound) pretreatment against thioacetamide-induced hepatic damage in rabbits. Drugs Exp Clin Res 31:1–6
Ahmadi A, Mohagheghi M, Karimi M, Golestanha SA, Naseri M (2009) Anticancer effects of HESA-A in patients with metastatic colon cancer. Integr Cancer Ther 8:71–74
Ahmadi A, Mohagheghi M, Karimi M, Golestanha SA, Naseri M, Faghihzadeh S, Habibi G (2010) Therapeutic effects of HESA-A in patients with end-stage metastatic cancers. Integr Cancer Ther 9:32–35
Ahmadian N, Pashaei-Asl R, Samadi N, Rahmati-Yamchi M, Rashidi MR, Ahmadian M, Joshaghani HR (2016) Hesa-a effects on cell cycle signaling in esophageal carcinoma cell line. Middle East J Dig Dis 8:297–302
Al-Hazzaa AA, El-Habit OHM, Al-Meer RS (2007) Evaluation of the protective role of vitamin B12 on gamma radiation induced cytotoxicity in mice. J King Saud Univ Science 19:97–107
Alizadeh AM, Ahmadi A, Mohammadzadeh M, Paknejad M, Mohagheghi M (2009) The effect of HESA-A, an herbal-marine compound, on wound healing process: an experimental study. Res J Biol Sci 4:298–302
Al-Meer RS, El-Habit OHM, Al-Hazaa AA (2011) Adaptive response to ionizing radiation and the role of vitamin B12 in amelioration radiation protection standards. J King Saud Univ Science 23:197–204
Asadullina NR, Usacheva AM, Smirnova VS, Gudkov SV (2010) Antioxidative and radiation modulating properties of guanosine-5′-monophosphate. Nucleosides Nucleotides Nucleic Acids 29:786–799
Bourre JM, Paquotte P (2008) Seafood (wild and farmed) for the elderly: contribution to the dietary intakes of iodine, selenium, DHA and vitamins B12 and D. J. Nutr. Health Aging 12:186–192
Caruso F, Rossi M (2004) Antitumor titanium compounds. Mini Rev Med Chem 4:49–60
Cheki M, Mihandoost E, Shirazi A, Mahmoudzadeh A (2016a) Prophylactic role of some plants and phytochemicals against radio-genotoxicity in human lymphocytes. J Cancer Res Ther 12:1234
Cheki M, Shirazi A, Mahmoudzadeh A, Bazzaz JT, Hosseinimehr SJ (2016b) The radioprotective effect of metformin against cytotoxicity and genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Mutat Res Toxicol Environ Mutagen 809:24–32
Cheki M, Yahyapour R, Farhood B, Rezaeyan A, Shabeeb D, Amini P, Najafi M (2018) COX-2 in radiotherapy; a potential target for radioprotection and radiosensitization. Curr Mol Pharmacol 11:173–183
Crescenti EJ, Medina VA, Croci M, Sambuco LA, Prestifilippo JP, Elverdin JC, Bergoc RM, Rivera ES (2011) Radioprotection of sensitive rat tissues by oligoelements se, Zn, Mn plus Lachesis muta venom. J Radiat Res 52(5):557–567
Duan Y, Zhang H, Xie B, Yan Y, Li J, Xu F, Qin Y (2010) Whole body radioprotective activity of an acetone-water extract from the seedpod of Nelumbo nucifera Gaertn. seedpod. Food Chem Toxicol 48:3374–3384
El-Gendy AM, El-Feky F, Mahmoud NH, Elsebakhy GSA (2018) Evaluation of nutritional quality of green tiger prawn, penaeus semisulcatus from land fisheries (Alexandria) and market (India). Egypt J Hosp Med 70:924–934
Fleenor CJ, Marusyk A, DeGregori J (2010) Ionizing radiation and hematopoietic malignancies: altering the adaptive landscape. Cell Cycle 9:3077–3083
Green DE, Rubin CT (2014) Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone 63:87–94
Gudkov SV, Guryev EL, Gapeyev AB, Sharapov MG, Bunkin NF, Shkirin AV, Zabelina TS, Glinushkin AP, Sevost'yanov MA, Belosludtsev KN, Chernikov AV, Bruskov VI, Zvyagin AV (2019) Unmodified hydrated С60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomedicine 15:37–46
Guo J, Deng W, Zhang L, Li C, Wu P, Mao P (2007) Prediction of prostate cancer using hair trace element concentration and support vector machine method. Biol Trace Elem Res 116:257–272
Hajhashemi V, Ghafghazi T, Balali M, Ahmadi A, Taher M, Rajabi P, Talebi A (2001) Toxicological studies on an anticancer drug (HESA-A) with marine origin. Med J Islam Acad Sci 14:145–149
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Hosseinimehr SJ (2015) The protective effects of trace elements against side effects induced by ionizing radiation. Radiat Oncol J 33:66–74
Jahanban-Esfahlan R, Abasi M, Sani HM, Abbasi MM, Akbarzadeh A (2015) Anti-proliferative effects of hesa-A on human cancer cells with different metastatic potential. Asian Pac J Cancer Prev 16:6963–6966
Jia D, Koonce NA, Griffin RJ, Jackson C, Corry PM (2010) Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC). Radiat Res 173:579–589
Kolivand S, Motevaseli E, Cheki M, Mahmoudzadeh A, Shirazi A, Fait V (2017) The anti-apoptotic mechanism of metformin against apoptosis induced by ionizing radiation in human peripheral blood mononuclear cells. Klin Onkol 30:372–379
Kumar A, Selvan TG, Tripathi AM, Choudhary S, Khan S, Adhikari JS, Chaudhury NK (2015) Sesamol attenuates genotoxicity in bone marrow cells of whole-body γ-irradiated mice. Mutagenesis 30:651–661
Kumar A, Choudhary S, Adhikari JS, Chaudhury NK (2018) Sesamol ameliorates radiation induced DNA damage in hematopoietic system of whole body γ-irradiated mice. Environ Mol Mutagen 59:79–90
Mantena SK, Unnikrishnan MK, Uma Devi P (2008) Radioprotective effect of sulfasalazine on mouse bone marrow chromosomes. Mutagenesis 23:285–292
Mazur L, Augustynek A, Halicka HD, Deptała A (2003) Induction of apoptosis in bone marrow cells after treatment of mice with WR-2721 and gamma-rays: relationship to the cell cycle. Cell Biol Toxicol 19:13–27
Mehdipour M, Zenouz AT, Abbasi MM, Mohajeri D, Damghani H, Helli S, Abdollahi B (2013) Evaluation of the effect of two systemic doses of HESA-A on prevention of induced tongue neoplasm in rats. J Dent Res Dent Clin Dent Prospects 7:218–224
Mehrbod P, Ideris A, Omar AR, Hair-Bejo M (2014) Prophylactic effect of herbal-marine compound (HESA-A) on influenza A virus infectivity. BMC Complement Altern Med 14:131
Moallem SA, Ahmadi A, Moshafi M, Taghavi MM (2011) Teratogenic effects of HESA-A, a natural anticancer product from Iran, in mice. Hum Exp Toxicol 30:851–859
Mohseni M, Mihandoost E, Shirazi A, Sepehrizadeh Z, Bazzaz JT, Ghazi-khansari M (2012) Melatonin may play a role in modulation of bax and bcl-2 expression levels to protect rat peripheral blood lymphocytes from gamma irradiation-induced apoptosis. Mutat Res Mol Mech Mutagen 738:19–27
Najafi M, Cheki M, Rezapoor S, Geraily G, Motevaseli E, Carnovale C, Shirazi A (2018) Metformin: prevention of genomic instability and cancer: a review. Mutat Res Toxicol Environ Mutagen 827:1–8
Ormsby RJ, Lawrence MD, Blyth BJ, Bexis K, Bezak E, Murley JS, Sykes PJ (2014) Protection from radiation-induced apoptosis by the radioprotector amifostine (WR-2721) is radiation dose dependent. Cell Biol Toxicol 30:55–66
Ran Y, Wang R, Lin F, Hasan M, Jia Q, Tang B, Li Q (2014) Radioprotective effects of Dragon’s blood and its extract against gamma irradiation in mouse bone marrow cells. Phys Medica 30:427–431
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Roudkenar MH, Bahmani P, Halabian R (2012) HESA-A exerts its cytoprotective effects through scavenging of free radicals: an in vitro study. Iran J Med Sci 37:47–53
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Shao L, Luo Y, Zhou D (2014) Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal 20:1447–1462
Sharapov MG, Novoselov VI, Fesenko EE, Bruskov VI, Gudkov SV (2017) The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radic Res 51:148–166
Sharapov MG, Novoselov VI, Penkov NV, Fesenko EE, Vedunova MV, Bruskov VI, Gudkov SV (2019) Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic Biol Med 134:76–86
Singh A, Yashavarddhan MH, Kalita B, Ranjan R, Bajaj S, Prakash H, Gupta ML (2017) Podophyllotoxin and rutin modulates ionizing radiation-induced oxidative stress and apoptotic cell death in mice bone marrow and spleen. Front Immunol 8:183
Sood A, Chadha VD, Dhawan DK (2011) Radioprotective role of selenium after single-doseradioiodine (131I) exposure to red blood cells of rats. J Environ Pathol Toxicol Oncol 30:153–162
Suman S, Maniar M, Fornace AJ, Datta K (2012) Administration of ON 01210. Na after exposure to ionizing radiation protects bone marrow cells by attenuating DNA damage response. Radiat Oncol 7:6
Syama Dayal J, Ponniah AJ, Imran Khan H, Madhu Babu EP, Ambasankar K, Kumarguru Vasagam KP (2013) Shrimps–a nutritional perspective. Curr Sci 104:1487–1491
Targhi RG, Homayoun M, Mansouri S, Soukhtanloo M, Soleymanifard S, Seghatoleslam M (2017) Radio protective effect of black mulberry extract on radiation-induced damage in bone marrow cells and liver in the rat. Radiat Phys Chem 130:297–302
Vahabpour R, Sadat SM, Zabihollahi R, Ahmadi A, Keivani H, Amini S, Aghasadeghi MR (2012) In vitro inhibitory effects of the herbal-marine compound HESA-A against replication of human immunodeficiency virus-1. Jundishapur J Microbiol 5:315
Xue X, Han X, Li Y, Chu X, Miao W, Zhang J, Fan S (2017) Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res Ther 8:7
Zeng H, Combs GF Jr (2008) Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem 19:1–7
Zhang YR, Wang JY, Li YY, Meng YY, Zhang Y, Yang FJ, Xu WQ (2019) Design and synthesis a mitochondria-targeted dihydronicotinamide as radioprotector. Free Radic Biol Med 136:45–51
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950
Funding
This study was funded by grants (U-97001) from the vice chancellor of research at Ahvaz Jundishapur University of Medical Sciences (Iran).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments on rats were carried out based of the NIH Guide for Care and Use of Laboratory Animals. All study protocols were approved in Animal Ethical Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1396.699).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hazbavi, M., Zarei, M., Nazaralivand, R. et al. Protection from ionizing radiation-induced genotoxicity and apoptosis in rat bone marrow cells by HESA-A: a new herbal-marine compound. J Bioenerg Biomembr 51, 371–379 (2019). https://doi.org/10.1007/s10863-019-09808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-019-09808-5