Abstract
Osteoporosis is a chronic disease that impairs proper bone remodeling. Guided bone regeneration is a surgical technique that improves bone defect in a particular region through new bone formation, using barrier materials (e.g. membranes) to protect the space adjacent to the bone defect. The polytetrafluorethylene membrane is widely used in guided bone regeneration, however, new membranes are being investigated. The purpose of this study was to evaluate the effect of P(VDFTrFE)/BT [poly(vinylidene fluoride-trifluoroethylene)/barium titanate] membrane on in vivo bone formation. Twenty-three Wistar rats were submitted to bilateral ovariectomy. Five animals were subjected to sham surgery. After 150 days, bone defects were created and filled with P(VDF-TrFE)/BT membrane or PTFE membrane (except for the sham and OVX groups). After 4 weeks, the animals were euthanized and calvaria samples were subjected to histomorphometric and computed microtomography analysis (microCT), besides real time polymerase chain reaction (real time PCR) to evaluate gene expression. The histomorphometric analysis showed that the animals that received the P(VDF-TrFE)/BT membrane presented morphometric parameters similar or even better compared to the animals that received the PTFE membrane. The comparison between groups showed that gene expression of RUNX2, BSP, OPN, OSX and RANKL were lower on P(VDF-TrFE)/BT membrane; the gene expression of ALP, OC, RANK and CTSK were similar and the gene expression of OPG, CALCR and MMP9 were higher when compared to PTFE. The results showed that the P(VDF-TrFE)/BT membrane favors bone formation, and therefore, may be considered a promising biomaterial to support bone repair in a situation of osteoporosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Osteoporosis is a disease in which density and quality of bone are reduced, increasing porosity and fragility as well as the risk of fractures. Bone loss occurs in a silent and progressive way, and commonly there are no symptoms until the first fracture occurs. Worldwide, 1 in 3 women over age 50 will experience osteoporotic fractures, with a fracture occurring every 3 s [1].
In dentistry, osteoporosis has brought great concern to health professionals since this disease is associated with periodontal disease, tooth loss [2–5], and interference with the repair of critical size bone defects. One technique used for bone repair is guided bone regeneration (GBR). This technique is a surgical procedure that utilizes barrier membranes creating a protected space around the bone defect. The graft material/barrier created space is filled with the blood clot, allowing the osteogenic cells to colonize the augmentation area without the competition of the overlying soft tissue cells [6].
The polytetrafluoroethylene (PTFE) membrane is considered the gold standard in guided bone regeneration technique [6]. However, it is essential the investigation and development of new biomaterials for bone repair.
Although absorbable and non-absorbable membranes have been developed and widely investigated, ongoing research is evaluating these novel membranes, aiming to establish an ideal membrane for clinical application [7, 8]. Thus, a membrane of the composite poly (vinylidene fluoride-trifluoroethylene)/barium titanate (P(VDF-TrFE)/BT) was created. This composite combines the mechanical characteristics of the polymers with the biocompatibility of ceramics [9].
Previous in vitro studies [10–12] showed that the phenotypic expression of osteoblasts, periodontal ligament fibroblasts, and keratinocytes are higher in cultures grown on P(VDF-TrFE)/BT membrane when compared to PTFE membrane, and its utilization may be a beneficial alternative in procedures that require bone regeneration.
In addition, in vivo studies that used PTFE and P(VDF-TrFE)/BT membrane in healthy animals also showed better results with the latter membrane [13].
Considering the previous results obtained in in vitro and in vivo studies, our hypothesis is that the P(VDF-TrFE)/BT membrane can also favor the formation of bone tissue in rats subjected to an experimental model of osteoporosis and bone loss may interfere with the repair of critical defects. Thus, this study aimed to evaluate the effect of P(VDF-TrFE)/BTmembrane on in vivo bone formation in the calvaria of ovariectomized rats using histological, microtomographic and molecular parameters.
2 Materials and methods
2.1 Membranes
P(VDF-TrFE) copolymer was supplied by Piezotec S.A. (Saint Louis, France). Commercial BaTiO3 powder (Aldrich) was sintered at 1380 °C for 5 h and milled at a planetary ball mill at 400 RPM during 2 h in agate jars and isopropanol medium. The powders were dried under vacuum at room temperature and sieved using a mesh size of 25 μm.
2.2 Fabrication of P(VDF-TrFE)/BaTiO3 membranes
Composites were obtained by dissolving the PVDF-TrFE pellets, as received, in dimethyl formamide (DMF) at 50 °C using a polymer/solvent ratio of 15 g/100 mL. BaTiO3 powder was added in the polymer solution and homogenized using an ultrasonic vibration (VCX750, Sonics and Materials, Newtown, USA). The viscous precursor dispersion obtained was precipitated in demineralized water. The precipitate was dried under vacuum at 90 °C for 24 h, and uniaxially pressed at 170 °C for 5 min. After the cooling at room temperature, the membranes were cut into disks of 10 mm diameter and 100 μm of thickness. The commercially available PTFE membranes were used as control (Bionnovation, Bauru, SP, Brazil). Membranes were cut into 5-mm-diameter discs and sterilized using autoclave. Both membranes presented a thickness of 0.25 mm and surface roughness ranging from 0.15 to 0.20 [12].
2.3 Animals
Twenty-eight female Wistar rats that weighed approximately 300 g provided by the Central Animal Facility of the USP campus at Ribeirão Preto were used in this study. Three animals were housed in each Polystyrene box, day and night cycles of 12 h and a controlled average temperature of 23 °C. Food and water were given ad libitum to all the animals. All procedures were approved by the Ethics Committee on Animal Experiments of the Ribeirão Preto School of Dentistry (Protocol N. 2014.1.157.58.4).
2.4 Animal treatment
After a week, 23 animals were bilaterally ovariectomized. The animals were weighted and anesthetized by an intraperitoneal injection of ketamine (75 mg/Kg) (União Química Farmacêutica Nacional S/A—Embu-Guaçu, SP, Brazil) and xylazine (10 mg/Kg) (Hertape Calier—Juatuba, MG, Brazil). Afterwards the animals were submitted to trichotomy, antisepsis and bilateral incisions, exposure and excision of the ovaries. The incisions were sutured with 4.0 silk thread (Ethicon, Johnson & Johnson, São José dos Campos, SP, Brazil). After surgery, the animals were given intramuscular injection with a single dose of Banamine painkiller-1.1 mg/Kg (MSD Saúde Animal, São Paulo, SP, Brazil) and Pentabiotico Veterinário Pequeno Porte—0.1 mL/ 100 g (Fort Dodge®, Campinas, SP, Brazil). To evaluate the success of ovariectomy, an analysis of the estrous cycle was made 2 weeks after surgery. During the euthanasia, the animals were inspected by looking for the characteristic atrophy normally found within the uterine horns.
In the sham group (n = 5), the same procedures were carried out, however, the ovaries were exposed and repositioned. These animals also received the same medications given to the ovariectomized group.
2.5 Bone graft surgery
After 150 days, bone defects were created in both ovariectomized and sham animals. They were weighted and anesthetized by an intraperitoneal injection of ketamine (75 mg/Kg) (União Química Farmacêutica Nacional S/A—Embu-Guaçu, SP, Brazil) and xylazine (10 mg/Kg) (Hertape Calier—Juatuba, MG, Brazil). Afterwards, the animals were submitted to trichotomy, antisepsis and a sagittal incision (1 cm long) to expose the intended bone area. A critical bone defect was made in the central region of the left parietal bone using a trephine drill (Neodent, Curitiba, PR, Brazil) and an electric implant motor (Dentscler, Ribeirão Preto, SP, Brazil) at 3000 rpm. The bone defect was made under constant irrigation with sterile saline solution (0.9 %).
Subsequently, the animals were divided to create the following groups: bone defects in five sham and five ovariectomized animals without membrane, bone defects filled with P (VDF-TrFE)/BT membrane in nine animals and bone defects with PTFE membrane in more nine animals. The incisions were then sutured with 4.0 silk thread (Ethicon, Johnson & Johnson, São José dos Campos, SP, Brazil). All animals received intramuscular injection with a single dose of Banamine painkiller-1.1 mg/Kg (MSD Saúde Animal, São Paulo, SP, Brazil) and Pentabiotico Veterinário Pequeno Porte—0.1 mL/ 100 g (Fort Dodge®, Campinas, SP, Brazil).
The animals were sacrificed after 4 weeks and the calvaria were collected and processed for histological, morphometry by microCT and gene expression analysis (Table 1).
2.6 MicroCT morphometric analysis
The calvaria were fixed in 4 % buffered formalin solution (pH = 7) for 2 days and transferred to a 70 % ethanol solution for 3 days.
The microtomographic analysis was performed using a Skyscan 1172 microCT scanner (Skyscan, Kontich, Belgium) operating with 100 kV x-rays detected by a 11-megapixel camera with a resolution of up to 1 µm. Data were acquired through the reconstruction of the two-dimensional (2D) projection images into a 3D volumetric image stack performed using the software NRecon (Bruker microCT, Kontich, Belgium). After the reconstruction, the bone defect area was analyzed according to the following parameters: tissue volume (mm3), bone surface (mm2), specific bone surface (mm2/mm3), trabecular number (1/mm), trabecular thickness (mm), trabecular separation (mm) and connectivity density (1/mm3) [14].
2.7 Histology
After the microtomographic analysis, the specimens were prepared to obtain non-decalcified histological sections. The samples were dehydrated in a graded series of ethanol solutions and then embedded in acrylic resin (LR White Hard Grade, London Resin Company Ltd, United Kingdom) and sectioned using Exakt Cutting System (Exakt, Norderstedt, Germany). The sections obtained were polished and mounted on acrylic slides using Exakt Grinding System (Exakt, Norderstedt, Germany). For visualization of the different tissue structures, the serial sections were stained with Stevenel’s blue and Alizarin red S, according to Maniatopoulos et al. [15]. The analysis was carried out using a Leica DM4000B light microscope (Leica, Bensheim, Germany) outfitted with a Leica DFC310FX digital camera (Leica, Bensheim, Germany).
2.8 Osteoblastic and osteoclastic marker gene expressions
The real time PCR technique was used to evaluate gene expression of RUNX2, ALP, BSP, OC, OPN, OSX, OPG and RANKL (osteoblastic markers) and RANK, CTSK, MMP-9 and CALCR (osteoclastic markers).
After 4 weeks, the bone fragments of the calvaria were removed with a trephine drill of equal size to the membranes previously inserted in the bone defect. The removal of calvaria was performed with constant and cooled irrigation of phosphate buffered of PBS (phosphate buffered saline) (Life Technologies, Carlsbad, CA, USA) to minimize a possible total RNA degradation due to heat caused by the drill instrument. After the removal, the fragments were immediately frozen in liquid nitrogen and stored in a freezer at −80 °C until the time of total RNA extraction.
For the total RNA extraction, the calvaria were macerated utilizing TRIzol ® reagent (Life Technologies-Invitrogen, Carlsbad, CA) followed by SV Total RNA Isolation System (Promega, Madison, WI, USA), both used according to manufacturer’s instructions.
The concentration and purity of total RNA were determined using NanoVue Plus spectrophotometer (GE Healthcare, Uppsala, Uppsala County, Sweden). The measures were achieved through wavelengths of 260 nm to obtain the concentration of RNA/µ L, as well as any trace of contamination by proteins and phenol (280 and 230 nm respectively).
The complementary DNA (cDNA) was synthesized to obtain sufficient cDNA from 1 µg total RNA by reverse transcription reaction using a High Capacity cDNA Reverse Transcription Kit (Life Technologies), according to the manufacturer’s instructions.
The real time polymerase chain reactions were carried out using the TaqMan reverse transcription kit (Life Technologies) on a StepOnePlus ™ Real-Time PCR System (Life Technologies). Real time PCR reactions were performed four times with a final volume of 10 µL correspondent to 11.25 ng of total RNA. Thermal cycling parameters for the RT reactions were as follows: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles in 15 s at 95 °C, and 1 min at 60 °C (denaturation and extension). The relative expression was normalized using GAPDH constitutive gene.
2.9 Statistical analysis
The microtomographic and gene expression data were tabulated and submitted to statistical analysis using Sigma Plot 11.0. Statistical significance was set at p < 0.05. The Kolmogorov-Smirnov test showed that data followed a normal distribution. Anova test was applied, followed by the Holm-Sidak test used in histomorphometric data, when appropriate. The t-test was used in gene expression data.
3 Results
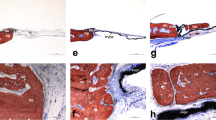
The histological analysis of the calvaria of sham animals without the the membranes showed a small bone formation in the peripheral region of the defect while the remaining part was filled by connective tissue (Fig. 1a-b). In the ovarietomized animals, it was possible to observe greater spaces between the neoformed bone trabeculae, culminating with a large amount of connective tissue in this region. The connective tissue occupying the area of the bone defect created in OVX animals was thinner when compared to that in sham group (Fig. 1c-d).
Photomicrographs (light microscopy) of rat calvaria bone defects without treatment (control a, b), OVX (c, d), with PTFE (e, f) or P(VDF-TrFE)BT (g, h) membranes. It was observed the presence of connective tissue in control (a, b) and OVX groups (c, d) and newly formed bone tissue in PTFE (e, f) and P(VDF-TrFE)BT (g, h) groups. Alizarin red S and Stevenel’s blue stain. OVX: ovariectomized; PTFE: polytetrafluoroethylene; P(VDF-TrFE)BT: poly(vinylidene fluoride-trifluoroethylene)/barium titanate
In animals that received the PTFE and the P(VDF-TrFE)/BT membranes, a new bone formation was observed in surgically-created defects, with no inflammatory process nearby or in the adjacent tissues (Fig. 1e-h).
The PTFE membrane showed direct contact with the newly formed bone tissue (Fig. 1e). A great number of structures, such as blood vessels in the medullary connective tissue could be seen interwoven with the bone trabeculae (Fig. 1f).
The P(VDF-TrFE)/BT membrane promoted the growth of new bone trabeculae above and below its structure. However, there was intimate direct contact with the newly formed bone as observed with the PTFE membrane (Fig. 1g). A higher augmentation shows the presence of connective tissue with blood vessels between the newly formed bone trabeculae interwoven with medullary connective tissue (Fig. 1h).
After image reconstruction by microCT, it was possible to observe that bone formation in the defects of the ovariectomized and control groups was minimal. The images also showed bone formation on the PTFE membrane as well as on the PVDF membrane (Fig. 2a-h).
Three-dimensional reconstructed micro-computed tomography (micro-CT) images of rat calvaria bone defects without treatment (control a, b), OVX (c, d), with PTFE (e, f) or P(VDF-TrFE)BT (g, h) membranes. It was observed new bone formation with both membranes (e, h). Scale bar: 1 mm. OVX: ovariectomized; PTFE: polytetrafluoroethylene; P(VDF-TrFE)BT: poly(vinylidene fluoride-trifluoroethylene)/barium titanate
Both the P(VDF-TrFE)/BT and the PTFE membranes exhibited significantly higher bone volume when compared to control and OVX groups (PTFE × Control: p < 0.001; PTFE × OVX: p < 0.001; P(VDF-TrFE)BT × Control: p < 0.001; P(VDF-TrFE)BT × OVX: p = 0.002). There was no statistical difference between the groups that used both the P(VDF-TrFE)/BT and PTFE membranes. The groups that received the PTFE membrane and P(VDF-TrFE)/BT membrane showed significantly higher bone surface when compared to control and OVX groups (PTFE × Control: p < 0.001; PTFE × OVX: p < 0.001; P(VDF-TrFE)BT × Control: p < 0.001; P(VDF-TrFE)BT × OVX: p < 0.001). The group that received the P(VDF-TrFE)/BT membrane exhibited significantly higher specific bone surface when compared to PTFE, OVX and control groups (P(VDF-TrFE)BT × Control: p < 0.001; P(VDF-TrFE)BT × OVX: p = 0.001; P(VDF-TrFE)BT × PTFE: p < 0.001). For the number of trabeculae, there was no statistical difference between the groups that used both the P(VDF-TrFE)/BT and PTFE membranes. There was statistically significant difference between the groups that received the membranes and those that did not (PTFE × Control: p < 0.001; PTFE×OVX: p < 0.001; P(VDF-TrFE)BT × Control: p < 0.001; P(VDF-TrFE)BT × OVX: p < 0.001). The trabecular thickness was not affected by the use of membranes. Thus, there was no statistical difference between the groups that received different types of membrane and those that did not (p = 0.084). For the trabecular separation, the values were significantly lower for the PTFE and P(VDF-TrFE)/BT groups compared to the control and OVX groups (PTFE × Control: p < 0.001; PTFE × OVX: p < 0.001; P(VDF-TrFE)BT × Control: p < 0.001; P(VDF-TrFE)BT × OVX: p < 0.001). For connectivity density, the P(VDF-TrFE)/BT group presented significantly higher value when compared to the PTFE, control and OVX groups. The PTFE group also had significantly higher value when compared to control and OVX groups (PTFE × Control: p = 0.001; PTFE × OVX: p = 0.003; P(VDF-TrFE)BT × Control: p = 0.001; P(VDF-TrFE)BT × OVX: p = 0.001; P(VDF-TrFE)BT × PTFE: p = 0.001) (Fig. 3).
Morphometric parameters obtained from three-dimensional reconstructed micro-computed tomography images on rat calvaria bone defects without treatment (control), OVX, with PTFE or P(VDF-TrFE)BT membranes. *Indicates statistically significant difference (p < 0.05). OVX: ovariectomized; PTFE: polytetrafluoroethylene; P(VDF-TrFE)BT: poly(vinylidene fluoride-trifluoroethylene)/barium titanate
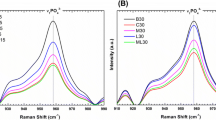
Gene expression was affected regarded to the use of different types of membrane (Fig. 4). The results indicated lower mRNA levels of RUNX2 (p < 0.001), BSP (p < 0.001), OPN (p < 0.001) and OSX (p < 0.001) in the cells of animals treated with the P(VDF-TrFE)/BT membrane. The expressions of ALP and OC genes in the P (VDF-TrFE)/BT group was similar (p = 0.600 and p = 0.174, respectively), when compared to the PTFE group. The OPG modulation was significantly higher (p < 0.001) in the P(VDF-TrFE)/BT group compared to the PTFE group. The expression of RANKL gene was significantly lower (p = 0.001) in the P(VDF-TrFE)/BT group compared to the PTFE group. The expression of RANK and CTSK genes in the P(VDF-TrFE)/BT group was similar (p = 0.262 and p = 0.291, respectively), when compared to the PTFE group. The expression of MMP-9 and CALCR genes were significantly higher (p = 0.009 and p = 0.003, respectively), in the P(VDF-TrFE)/BT group compared to the PTFE group. The RANK/OPG ratio was significantly lower (p < 0.001) in the P(VDF-TrFE)/BT group compared to PTFE group.
Gene expression of RUNX2, BSP, OPN, OSX, ALP, OC, OPG, RANKL, RANK, CTSK, MMP9, CALCR and ratio RANKL: OPG of cells from new bone tissue formed on rat calvaria bone defect with PTFE or P(VDF-TrFE)BT membranes. Gene expression was normalized by GAPDH. *Indicates statistically significant difference (p < 0.05). PTFE: polytetrafluoroethylene; P(VDF-TrFE)BT: poly(vinylidene fluoride-trifluoroethylene)/barium titanate
4 Discussion
The results obtained in the present study showed bone neoformation in defects filled with the PTFE and the P(VDF-TrFE)/BT membranes in calvaria of ovariectomized rats. Our study used the most adequate experimental model for the research of osteoporosis [16] because the characteristics of the bone loss in the ovariectomized rats resemble those found in postmenopausal women [17].
The results of histological and microCT morphometrical evaluation obtained in this study made it possible to observe new bone formation using both types of membranes [13, 18, 19]. It is worth mentioning that P(VDF-TrFE)/BT membrane favored the growth of new bone trabeculae above and below its structure, which was not observed in defects filled with PTFE membrane [13]. Differently from the results found by Lopes et al. [13], there was no connective tissue between the P(VDF-TrFE)/BT membrane and bone tissue, which suggests a good integration between this biomaterial and the bone tissue [20]. No inflammatory response was detected in the presence of the P(VDF-TrFE)/BT membrane, allowing proper de novo bone formation observed in MicroCT and histological analysis. This was not observed in other in vivo studies that used, for example, Bio-Gide(®)-ALP membranes [21].
The quantification of regenerated bone was performed through tridimensional images from micro-computed tomography (microCT), which is a traditional gold standard technique of evaluation [14]. The in vivo experiments comparing the PTFE and P(VDF-TrFE)/BT membranes revealed similar results with respect to bone volume, bone surface, trabecular number, trabecular thickness and trabecular separation, which indicates that the P(VDF-TrFE)/BT membrane was effective in bone formation [13].
Other research groups that investigate the biocompatibility of materials also observed significant bone formation in ovariectomized rats after the use of PTFE membranes combined with zolendronic acid (ZA) [22] and bone substitutes such as the Biosilicate ® and Bio-Oss ® [23]. In contrast, the use of bovine collagen membranes in OVX animals presented significantly lower bone volume [24]. In our study, for the bone surface and number of trabeculae, the groups that received the membranes showed significantly higher values when compared to those that did not receive the biomaterial. Weber et al. [25] reported that the PVDF-TrFE membrane is cytocompatible and in a three-dimensional format could be a promising material able to influence cell proliferation. Even though these authors have performed only in vitro experiments, they suggest that PVDF-TrFE is a potential biomaterial to be used in tissue engineering also for in vivo investigations. In a previous study on cell culture, Teixeira et al. [11] showed that the P(VDF-TrFE)/BT membrane favored cell adhesion, proliferation and differentiation of fibroblasts and keratinocytes compared to the PTFE membrane. Despite the fact that our results are from in vivo experiments, future in vitro studies could elucidate if P(VDF-TrFE)/BT would also favor the same cellular events in osteoporotic animals.
Specific bone surface, which is an important parameter from microCT analysis, is the ratio between bone surface and bone volume [14]. Higher values of this parameter were found in the group that used the P(VDF-TrFE)/BT membrane, suggesting that its presence favors bone formation in a situation of osteoporosis. In this study, the connectivity density showed significantly higher values for the P(VDF-TrFE)/BT group compared to all the other groups. Odgard and Gundersen [26] stated that the connectivity is a topologic measure counting the number of objects like marrow cavities fully surrounded by bone as well as the number of connections that must be broken to split the structure in two parts. It should be divided by the total volume, being more appropriate called connectivity density. Therefore, it can be inferred that the bone tissue formed by the P(VDF-TrFE)/BT membrane is more resistant than the one formed by the PTFE membrane.
Osteoporosis promotes changes in the metabolism of bone cells due to the action of a diverse set of proteins modulated by the differential expression of their respective genes. The goal of genomic research is to understand the functional roles that different genes play and in which biological processes they participate regarded to different physiological, pathological or environmental conditions [27]. The present study evaluated gene expression of cells present in the bone defects. The main osteoblastic bone markers investigated were RUNX2, ALP, BSP, OC, OPN, OSX, OPG and RANKL and the osteoclastic bone markers were RANK, CTSK, MMP-9 and CALCR.
Our results were not consistent with those of other studies that have observed a greater expression of RUNX2, OPN, ALP, BSP and OC in alveolar bone derived cells cultivated on the P(VDF-TrFE)/BT membrane [12]. This occurred because when the two membranes were compared, there was a lower expression of RUNX2, BSP, OPN, OSX and RANKL in the group that received the P(VDF-TrFE)/BT membrane. Our results also did not corroborate with those obtained from healthy animals that used the same type of membranes in calvaria bone defects, where similar gene expression was observed and/or was significantly higher in the same group [13]. Lower gene expression of osteoblastic markers may have occurred not because of the membrane itself, but due to systemic changes induced by osteoporosis that were observed not only in the initial period of bone repair but also in more advanced stages [28, 29], interfering with the proliferation rate of undifferentiated mesenchymal stem cells and osteoblasts [30].
Along with other authors that have used bone substitutes [23], however, a higher quantitative expression of OPG was observed, combined with the repression of RANKL, suggesting that the PVDF membrane prevents bone resorption due to a lower activation of osteoclasts, and consequently, lower rate of bone remodeling. Probably, the resorption activity of osteoclasts was suppressed by high concentrations of OPG, at the molecular level, and OPG decreased the expression of osteoclastic bone resorption-related genes [31]. In our study, the RANKL: OPG ratio confirmed the influence of membrane in gene modulation, as seen in the study of Lima et al. [32]. It is noteworthy that the expression of ALP and OC genes was similar in both groups, suggesting that the bone mineralization below the P(VDF-TrFE)/BT membrane was similar to the one in the PTFE membrane.
In our study, although there was a similar and positive bone formation with the use of both membranes evaluated, different behaviors were observed in gene modulation, with increased repression of genes associated to bone formation on PVDF membrane when compared with PTFE membrane. Other studies have emphasized that the relative gene expression was increased with Biosilicate®, without significant bone formation [23]. The exact mechanism of this effect has yet to be clarified. However, it is believed that this discrepancy may be related to the inherent properties of the materials used. The results of the microtomographic analysis showed that bone formation parameters were similar, possibly due to the presence of barium titanate on the PVDF membrane, which has piezoelectric properties. According to Baxter et al. [33], the piezoelectric properties have no single mode of action, although the preferential adsorption of proteins, ions, and other molecules onto surfaces of differing electrical states is probable and may have effects on the osteogenesis process.
Our results showed significantly higher expressions of genes associated to bone resorption, such as MMP9 and CALCR in the presence of the P(VDF-TrFE)/BT membrane compared to PTFE membrane and similar values in the expression of RANK and CTSK genes. The higher expression of CALCR may have contributed to the greater amount of bone tissue detected through microCT analysis in animals that received the P(VDF-TrFE)/BT membrane.
5 Conclusion
Based on the results obtained, it can be concluded that P(VDF-TrFE)/BT membrane can be explored as a promising alternative in guided tissue regeneration treatment that involves the use of biomaterials and with beneficial effects on systemic bone disorders such as osteoporosis.
References
International Osteoporosis Foundation. http://www.iofbonehealth.org/what-is-osteoporosis. Accessed 31 May 2016.
Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–8.
Siéssere S, de Albuquerque Lima N, Semprini M, de Sousa LG, Paulo Mardegan Issa J, Aparecida Caldeira, Monteiro S, Cecílio Hallak Regalo S. Masticatory process in individuals with maxillary and mandibular osteoporosis: electromyographic analysis. Osteoporos Int. 2009;20:1847–51.
Vishwanath SB, Kumar V, Kumar S, Shashikumar P, Shashikumar Y, Patel PV. Correlation of periodontal status and bone mineral density in postmenopausal women: a digital radiographic and quantitative ultrasound study. Indian J Dent Res. 2011;22:270–6.
Scalize PH, de Sousa LG, Regalo SC, Semprini M, Pitol DL, da Silva GA, de Almeida Coelho J, Coppi AA, Laad AA, Prado KF, Siessere S. Low-level laser therapy improves bone formation: stereology findings for osteoporosis in rat model. Lasers Med Sci. 2015;30:1599–607.
Ronda M, Rebaudi A, Torelli L, Stacchi C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: a prospective randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:859–66.
Dimitriou R, Mataliotakis GI, Calori GM, Giannoudis PV. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med. 2012;10:81
Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57:3–14.
Gimenes R, Zaghete MA, Bertolini M, Varela JA, Coelho LO, Silva NF Jr. Composites PVDF-TrFE/BT used as bioactive membranes for enhancing bone regeneration. In: Bar-Cohen Y, (ed.) Proceedings of SPIE: Smart Structures and Materials. Bellinghan, WA: SPIE; 2004;5385:539–47.
Beloti MM, de Oliveira PT, Gimenes R, Zaghete MA, Bertolini MJ, Rosa AL. In vitro biocompatibility of a novel membrane of the composite poly(vinylidene-trifluoroethylene)/barium titanate. J Biomed Mater Res A. 2006;79:282–8.
Teixeira LN, Crippa GE, Trabuco AC, Gimenes R, Zaghete MA, Palioto DB, de Oliveira PT, Rosa AL, Beloti MM. In vitro biocompatibility of poly(vinylidene fluoride-trifluoroethylene)/barium titanate composite using cultures of human periodontal ligament fibroblasts and keratinocytes. Acta Biomater. 2010;6:979–89.
Teixeira LN, Crippa GE, Gimenes R, Zaghete MA, de Oliveira PT, Rosa AL, Beloti MM. Response of human alveolar bone-derived cells to a novel poly(vinylidene fluoride-trifluoroethylene)/barium titanate membrane. J Mater Sci Mater Med. 2011;22:151–8.
Lopes HB, Santos TD, de Oliveira FS, Freitas GP, de Almeida AL, Gimenes R, Rosa AL, Beloti MM. Poly(vinylidene-trifluoroethylene)/barium titanate composite for in vivo support of bone formation. J Biomater Appl. 2014;29:104–12.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86.
Maniatopoulos C, Rodriguez A, Deporter DA, Melcher AH. An improved method for preparing histological sections of metallic implants. Int J Oral Maxillofac Implants. 1986;1:31–7.
Frost HM, Jee WS. On the rat model of human osteopenias and osteoporosis. Bone Miner. 1992;18:227–36.
Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:171–92.
Crump TB, Rivera-Hidalgo F, Harrison JW, Williams FE, Guo IY. Influence of three membrane types on healing of bone defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:365–74.
Salata LA, Hatton PV, Devlin AJ, Craig GT, Brook IM. In vitro and in vivo evaluation of e-PTFE and alkali-cellulose membranes for guided bone regeneration. Clin Oral Implants Res. 2001;12:62–8.
Amano Y, Ota M, Sekiguchi K, Shibukawa Y, Yamada S. Evaluation of a poly-l-lactic acid membrane and membrane fixing pin for guided tissue regeneration on bone defects in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:155–63.
Oortgiesen DA, Plachokova AS, Geenen C, Meijer GJ, Walboomers XF, van den Beucken JJ, Jansen JA. Alkaline phosphatase immobilization onto Bio-Gide® and Bio-Oss® for periodontal and bone regeneration. J Clin Periodontol. 2012;39:546–55.
Mardas N, Busetti J, de Figueiredo JA, Mezzomo LA, Scarparo RK, Donos N. Guided bone regeneration in osteoporotic conditions following treatment with zoledronic acid. Clin Oral Implants Res. 2016. doi:10.1111/clr.12810
van Houdt CI, Tim CR, Crovace MC, Zanotto ED, Peitl O, Ulrich DJ, Jansen JA, Parizotto NA, Renno AC, van den Beucken JJ. Bone regeneration and gene expression in bone defects under healthy and osteoporotic bone conditions using two commercially available bone graft substitutes. Biomed Mater. 2015. doi:10.1088/1748-6041/10/3/035003
Hirata HH, Munhoz MA, Plepis AM, Martins VC, Santos GR, Galdeano EA, Cunha MR. Feasibility study of collagen membranes derived from bovine pericardium and intestinal serosa for the repair of cranial defects in ovariectomized rats. Injury. 2015;46:1215–22.
Weber N, Lee YS, Shanmugasundaram S, Jaffe M, Arinzeh TL. Characterization and in vitro cytocompatibility of piezoelectric electrospun scaffolds. Acta Biomater. 2010;6:3550–6.
Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone. 1993;14:173–82.
Donate PB, Fornari TA, Macedo C, Cunha TM, Nascimento DC, Sakamoto-Hojo ET, Donadi EA, Cunha FQ, Passos GA. T cell post-transcriptional miRNA-mRNA interaction networks identify targets associated with susceptibility/resistance to collagen-induced arthritis. PLoS One. 2013;8:e54803
Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, Kitajima I, Semba I, Hirasawa Y. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol. 1999;68:197–202.
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–6.
Torricelli P, Fini M, Giavaresi G, Giardino R. Human osteoblast cultures from osteoporotic and healthy bone: biochemical markers and cytokine expression in basal conditions and in response to 1.25(OH)2 D3. Art Cells Blood Subs Immob Biotech. 2002;30:219–27.
Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY, Yuan Y, Liu XZ, Bian JC, Liu ZP. Osteoprotegerin influences the bone resorption activity of osteoclasts. Int J Mol Med. 2013;31:1411–7.
Lima LL, Gonçalves PF, Sallum EA, Casati MZ, Nociti FH Jr Guided tissue regeneration may modulate gene expression in periodontal intrabony defects: a human study. J Periodontal Res. 2008;43:459–64.
Baxter FR, Bowen CR, Turner IG, Dent AC. Electrically active bioceramics: a review of interfacial responses. Ann Biomed Eng. 2010;38:2079–92.
Acknowledgments
Sebastião C. Bianco and Milla S. Tavares are acknowledged for technical assistance during the experiments.
Funding
This work was supported by São Paulo Research Foundation—FAPESP [grant number 2014/02984-0], National Council for Scientific and Technological [CNPq] and Coordination for the Improvement of Higher Education Personnel (CAPES). The synthesis of the PVDF-TrFE/BaTiO3 membranes was supported by Minas Gerais State Research Foundation (FAPEMIG, Brazil) under the Grant TEC - APQ-03013-15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Scalize, P.H., Bombonato-Prado, K.F., de Sousa, L.G. et al. Poly(Vinylidene Fluoride-Trifluorethylene)/barium titanate membrane promotes de novo bone formation and may modulate gene expression in osteoporotic rat model. J Mater Sci: Mater Med 27, 180 (2016). https://doi.org/10.1007/s10856-016-5799-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5799-x