Abstract
Many studies have been dedicated to the development of scaffolds for improving post-traumatic nerve regeneration. The goal of this study was to develop and test chitosan conduit to use in peripheral nerve reconstruction, either alone or combined with bone marrow mesenchymal stem cells (BMSCs). In this study, the roles of the degree of deacetylation (DD) and molecular weight of chitosan on some biological properties of chitosan films, including hydrophilicity, degradation and BMSCs affinity were investigated. The molecular weight of Chitosans used are 5 × 104 Da, 2 × 105 Da, 5 × 105 Da, 1 × 106 Da, the deacetylation degrees are 85, 95%, respectively. The affinity of eight kinds of Chitosans to the BMSCs was assessed by MTT assay, the contact angle and the degradation time of the materials in vivo were also measured. Chitosans with the molecular weight of 1 × 106 Da and DD of 95% can significantly promote the survival and outgrowth of cells, which have better hydrophilicity and can remain integrity even after 8 to 16 weeks, all of above meet the requirement of nerve engineering. The BMSCs we transplanted can differentiate into neural stem cells in vivo, and the materials we selected combined with BMSCs can bridge 8-mm-long neural gap better resulting from the differentiation effects of the BMSCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chitosan, a polysaccharide derived from chitin, exhibits numerous intriguing physicochemical and biological properties. It is composed of two subunits, D-glucosamine and N-acetyl-D-glucosamine linked together by β-(1, 4) glycosidic bonds. Due to its biocompatibility, biodegradability and bioactivity, chitosan has been known as a good biomaterial with a wide range of biomedical applications [1–4].
Chitosan is a potential material for the preparation of nerve repair conduits [5, 6]. In order to find a better chitosan for the application in peripheral nerve regeneration, the effects of degree of deacetylation (DD) and molecular weight on the physicochemical properties such as hydrophilicity, degradation time and cell affinity of chitosan films have been evaluated. Schwann Cells (SCs) and neural stem cells (NSCs) are most usually selected to evaluate the cell affinity of chitosan, because SCs play a crucial role during nerve regeneration [7] through the production of growth factors and the excretion of extracellular matrix. Interests have been directed to introduce cultured SCs into nerve regeneration as a persistent source of neurotrophic factors. NSCs can differentiate into various cell types such as neurons, astrocytes, oligodendrocytes, and Schwann cell-like supportive cells [8]. Culture of rat Schwann cells on the films showed that the chitosan films with higher DD (95%) provided better substrata for Schwann cell spreading and proliferation [9], Chitosan with higher DD (93%) and molecular weight (329000 Da) was more suitable for tissue engineering applications because of its good cell affinity to Fibroblasts and Chondrocyte [10]. In brief, slightly higher DDs, up to about 80%, allow for faster degradation and comparatively good cell adhesion and neurite outgrowth [11], all of above showed that chitosan with higher DDs maybe have higher cell affinity.
BMSCs [12] are undifferentiated multipotent cells which reside in various human tissues and have the potential to differentiate into osteoblasts, chondrocytes, adipocytes, fibroblasts and other tissues of mesenchymal origin. In the human body they could be regarded as readily available reservoirs of reparative cells capable to mobilize, proliferate and differentiate into the appropriate cell type in response to certain signals. These properties have triggered a variety of BMSCs-based therapies for pathologies including nonunions, osteogenesis imperfecta, cartilage damage and myocardial infarction, BMSCs can exhibit plastic neuro-differentiational potential in vitro [13–15], SCs co-cultured with BMSCs in vitro exhibited an increase in their proliferation compared to SCs cultured alone [16]. Compared with SCs and NSCs, BMSCs are more available, have no immunological rejection and can reduce the possibility of tumorigenesis [17], so the BMSCs are good seeding cells in nerve regeneration.
In order to exploit BMSC-based therapies in a responsible and safe manner, it is required to determine how the implanted chitosan tubes combined with BMSCs encourage peripheral nerve regeneration in vivo and convince the mechanisms by which BMSCs works. We can choose a different molecular weight and deacetylation of chitosan, in accordance with the requirements of different tissue engineering to control the speed of degradation in the body [18]. But the appropriate source of seed cells, cell cultivation, cells concentration and cell matrix need to be explored further [19], find more suitable nerve regeneration micro-environment to maximize the speed of regeneration has yet to be further completed. Previous studies have shown that NSCs can differentiate into various Schwann cell-like cells [20, 21], considering that SCs and NSCs are primary structural and functional cells in peripheral nervous system and play a crucial role in peripheral nerve regeneration, we hypothesize that using BMSCs as seeded cells might exert their efficacy via the process of differentiation from BMSCs to NSCs or NSC-like cells. To test this hypothesis, we studied the BMSCs affinity to chitosans with four different molecular weight and two higher DDs in vitro for the first time and selected a kind of chitosan with the best cell affinity. Then they are transplanted to rat model of peripheral nerve regeneration combined with BMSCs. The regeneration effect together with the differentiation of BMSCs was evaluated.
2 Materials and methods
2.1 Materials
Chitosan was purchased from Jinan Haidebei Marine Bioengineering Company (China) and was prepared from Alaska deep-sea crab shell. The molecular weight of Chitosans used are 5 × 104 Da, 2 × 105 Da, 5 × 105 Da, 1 × 106 Da (determined by Gel Permeation Chromatography), the deacetylation degrees are 85%, 95%, respectively(determined by acid-base titration). Tissue culture clusters and flasks were purchased from Costar Co. Dulbecco’s minimum essential medium (DMEM) was purchased from Gibco Co. and fetal bovine serum (FBS) was obtained from Hangzhou Sijiqing Co. All other reagents were of analytical grade.
2.2 Preparation of chitosan membrane
One gram of chitosan was dissolved in 100 ml of 2% (v/v) acetic acid solution. After stirring, the solution was filtered through a middle-pore-size nylon cloth to remove the insoluble substance. Chitosan solution was injected into the wells of tissue culture clusters (150 μl/well). Solvent was allowed to evaporate at 37°C for 24 h. The films were then soaked in a 1% (w/v) sodium hydroxide solution for 30 min to neutralize the remaining acid. Finally, the films were washed with distilled water and dried.
2.3 Contact angle tests
The Dynamic water contact angle of pure chitosan films was determined at 37°C by a program interface tensiometer [Krüss-K12, Switzerland] employing drops of pure deionized water. Each sample was measured at six different locations and the readings were averaged.
2.4 In vivo degradation test
One hundred and twenty Kunming mice (6 weeks, weighed 20 ± 2 g) were used for testing biodegradability of chitosan films. Animals were purchased from the Experimental Animal Center of Shandong University, and carried out in accordance to the ‘‘NIH Guidelines for the Care and Use of Laboratory Animals’’. The mice were randomly divided into eight groups (8 × 5 × 3 = 120), for implanting the eight kinds of films mentioned above, respectively. After anesthetized by an intraperitoneal injection of sodium pentobarbital (30 mg/kg body weight), one piece of chitosan film was interposed subcutaneous (two pieces of chitosan film for each mice). Incisions were closed using 4–0 sutures. Animals were routinely housed following surgical procedures.
After 1, 2, 4, 8 and 16 weeks, chitosan films were taken out from the mice (randomly select two mice for a group each time) by stripping organizations carefully. Tissues adhering to the films were removed with organic solvent. The chitosan films were dried under vacuum and weighed after rinsed with distilled water. The extent of in vivo degradation was expressed as percentage of weight loss of the dried films (n = 6 samples of each).
2.5 Preparation and culture of BMSCs
For isolation of rat BMSCs, tibias and femurs were dissected from adult Wistar rat (200–250 g, Animals were purchased from the Experimental Animal Center of Shandong University, and carried out in accordance to the ‘‘NIH Guidelines for the Care and Use of Laboratory Animals’’.). After the ends of the bones were cut, the marrow was extruded with 10 ml D-Hank’s solution and resuspended in DMEM solution. About 1 × 107 marrow cells were plated on 25 cm2 plastic flask in DMEM, supplemented with 15% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. All of the cells were incubated at 37°C with 5% humidified CO2. After 24 h, the nonadherent cells were removed by replacing the medium. The medium was added and replaced every 2 days for about 2 weeks. When the cells grew to confluent, they were harvested with 0.25% trypsin and 1 mM EDTA for 5 min at 37°C, replated on 25 cm2 plastic flask, again cultured.
2.6 Bone marrow mesenchymal stem cell affinity test
BMSCs were diluted to a density of 1 × 106 cells/ml and were added to 48-well tissue culture clusters embedded with polylysine or chitosan membranes. The clusters were incubated in DMEM medium containing 15% fetal bovine serum and 100 u/ml penicillin and streptomycin, in a 37°C humidified incubator with 5% CO2. Half of the culture medium was replaced every 2 days. After 1, 3, 5, 7 and 10 d incubation, the viability of every groups was assessed using MTT assay [22]. The OD of all wells were measured photometrically at 450 nm with an Multiskan MK3 reader (Thermolabsystems, Canada). A one-way ANOVA was used to compare the means of different groups, and statistical significance was accepted at the 0.05 confidence level.
2.7 Surgical procedure
Wistar female rats, weighing 200–250 g, were randomly divided into BMSCs group, autograft group and control group of 10 rats each. The animals were anesthetized by an intraperitoneal injection of 2% sodium pentobarbital before their right sciatic nerve was exposed and transected to create a 8-mm-long gap. The cells transplanted to BMSCs group were rinsed with PBS and incubated with 5 μg/ml Hoechst 33258 for 10 min at room temperature. The neural gap was then bridged using a chitosan conduit (2 mm in internal diameter and 1 cm long) we prepared by a syringe and was further administrated with the BMSCs suspension containing 107 cells/conduit (for BMSCs group) or with culture medium (for control group), autograft group was operated by bridging the gap with the nerve cutted. Both the proximal and distal stump of the transected nerve were inserted 2 mm into the conduit and connected with two stitches each using 8/0 nylon suture [23].
2.8 Functional assessment of reinnervation
Rats were tested since week 1, and every week until week 6 after above surgery. They were gently handled, and tested in a quiet environment to minimize stress levels. For the assessment of motor nerve recovery, walking track analysis was carried out as described in previous reports [24] with minor modifications. The rats were allowed conditioning trials in an 8.2 × 100 cm walking track with a piece of white paper at the bottom of the track. The hind feet were dipped in red ink, leaving prints on the white paper. The print length (PL), the toe spread (TS), and the intermediary toe spread (IT) were thus obtained. In general, the maximal value was adopted for each measurement, and the data were recorded with the prefix E for the operated side and N for the normal non-operated side. The sciatic function index (SFI), an indicator of the degree of nerve dysfunction, varies from 0 to −100, with 0 corresponding to normal function and −100 to complete dysfunction, in each walking track three footprints were analyzed by a single observer, and the average of the measurements was used in SFI calculations. It was calculated by the following formula:
2.9 Immunohistochemistry and immunofluorescence analysis
Immunohistochemistry and immunofluorescence were also applied to the regenerated sciatic nerves for all groups. The sciatic nerve was dissected out, post-fixed and cryostat sectioned after rats had been killed. The nerve sections were allowed to incubate with rabbit anti-nestin antibody (1:100 dilution, Sigma) at 4°C for 24 h, followed by further reaction with the FITC labeled secondary antibody goat anti-rabbit IgG (1:200, Gibco) for immunofluorescence analysis or secondary antibody goat anti-rabbit IgG (1:200, Gibco) for immunohistochemistry analysis at 4°C overnight. These nerve sections were processed for observation under a Leica fluorescent microscope.
3 Results and discussion
3.1 Contact angles of chitosans
The hydrophilicity of chitosan membrane can be reflected by the contact angle. The smaller the contact angle was, the better the hydrophilicity was, and it is generally believed that the hydrophilic surface is conducive to cell adhesion and growth. As is shown in Fig. 1, the chitosan with the molecular weight of 1 × 106 Da, DD of 95% has the minimum contact angle and the best hydrophilicity, while the chitosan with molecular weight of 5 × 104 Da, DD of 85% has the largest contact angle and the worst hydrophilicity. By statistical analysis of the results we can see that chitosans with molecular weight of 2 × 105 Da, 5 × 105 Da, 1 × 106 Da, DD of 95% own better hydrophilicity and maybe more conducive to cell adhesion and growth. According to Chau et al., increased surface charge could increase interfacial tension (γSL), which is a direct measurement of intermolecular forces between a solid and a contacting liquid, and the increased γSL leads to the decrease of contact angle [25, 26], the chitosan with the highest molecular weight and highest DD can significantly increase surface charge, because its –NH2 can change into –NH3 +, subsequently can more easily adsorb the proteins of cell surface.
3.2 Degradation of chitosans in vivo
The results of Chitosan membrane in vivo degradation is shown in Fig. 2. As to chitosans of the same molecular weight, DD contributed little to the degradation rate. The larger the molecular weight was, the longer the degradation time was. During the first 4 weeks, degradation rate of chitosan was slow, but speeded up during 4–8 weeks. Sixteen weeks later, the mass loss of chitosans with molecular weight of 5 × 104 Da, 2 × 105 Da reached to 80% and the appearance was not complete. However, chitosans with molecular weight of 5 × 105 Da, 10 × 106 Da, whose mass loss were 60 and 40% respectively, could still maintain the integrity of their appearance. In the process of tissue regeneration and repair, maintaining the integrity of appearance of materials is of great importance. Take 1 cm nerve injury repair for example, the time needed for repair is 12–18 weeks, therefore chitosans with molecular weight of 5 × 105 Da, 10 × 106 Da, can meet such requirement.
3.3 Bone marrow mesenchymal stem cell affinity
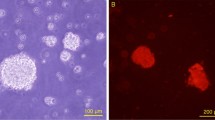
BMSCs were cultured for 10 days on films of chitosan, PLL and blank films in order to analyze the influence of the DD and molecular weight on the cell affinity and subsequently select the most suitable material for the nerve repair. Based on the light microscopy photographs (Figs. 3, 4, 5 6), all chitosan films supported BMSCs adhesion and outgrowth. The number of cells adhering to the materials was higher on chitosan derivatives having DD of 95% and molecular weight of 5 × 105 Da, 1 × 106 Da than other groups. Samples with higher DD showed cell clustering, which was particularly remarkable on films with DD of 95% and molecular weight of 1 × 106 Da, indicating a greater affinity of the cells.
Nerve engineering with the use and manipulation of BMSCs is a novel treatment modality targeting applications in a great variety of pathologies, the advantages of this approach are numerous. In order to evaluate the BMSCs affinity with the chitosan films more accurately, the results of MTT assay were shown in Figs. 7, 8. After 5 days, the cell affinity of all the chitosan groups achieved the same level to PLL group used as a control, and there were no significant differences between all the groups. Ten days later, the cell affinity of all the chitosan groups increased dramatically, and OD values of all the chitosan groups exceeded the control group. Two groups of chitosan with the molecular weight of 1 × 106 Da had a significant difference compared with the PLL group (P < 0.05). The chitosan (Mw: 1 × 106 Da) with a higher DD also had a better cell affinity, so there were significant differences between the two groups (P < 0.05). Among all the groups, the chitosan (Mw: 1 × 106 Da, DD: 95%) had the highest cell affinity, which had a significant difference compared with the PLL group (P < 0.01). From the Figs. 7, 8, it can be presumed that the proliferation time of the BMSCs on the films was about 5–10 days in vitro. MTT assay showed the same results as light microscopy photographs.
According to the results of degradation test in vivo and BMSCs affinity test, it can be summarized that the DD and molecular weight of chitosan, which can be adjusted by controlled N-acetylation or deacetylation reactions, have distinguished impacts on the material properties which are important for nerve engineering applications, including biodegradation and cell affinity [27, 28]. Prolonged degradation times and enhanced cell adhesion [29] may be achieved using chitosan with a DD close to 100%. Slightly higher DDs, up to about 95%, allow for good cell adhesion and neurite outgrowth. Fastest degradation can be achieved with chitosan having lower DDs, but at the cost of limited cell adhesion. Selecting the appropriate DD and molecular weight of chitosan provides a powerful means for controlling biodegradation and biocompatibility and can be optimized for tissue engineering applications. Finally, the chitosan (Mw: 1 × 106 Da, DD: 95%) were selected for nerve regeneration experiments in vivo combined with the BMSCs, owing to the conduit made of this chitosan can adhere more seeded BMSCs and provide a better micro-environment for cell growth and differentiation.
3.4 Functional assessment
SFI results were depicted in Fig. 9. The SFI values were evaluated from 1 to 6-week after crush injury, and all the rats were still alive during this time, as a result the risk for immunorejection and pathogen transmission appears to be very low after BMSCs transplantation. One week later, there were no significant differences between three groups, the SFI value was about −85. Two to three weeks later, the SFI value of autograft group increased faster and reached the maximum recovery rate, a significant effect of group was observed for SFI values with autograft group being different from the other two groups, and this difference remained until 6 weeks later, but there were no significant differences between the BMSCs group and control group. After 3–4 weeks, there was a significant differences between the BMSCs group and control group, and the gap between the BMSCs group and control group increased gradually, at the same time the SFI value of BMSCs group reached the maximum recovery rate, further the tendency continued to the fifth week. 5–6 weeks later, the SFI value of autograft group reached close to −10, and the sciatic nerve repair was almost completed, besides all the groups had the same recovery rate at this time.
Compared the nerve repair rate of three groups, it can be summarized that the autograft group had the fastest repair rate firstly at 2–3 weeks and reached the good level of repair after 4 weeks. BMSCs group had the fastest repair rate at the time of 3–4 weeks, 1 week later than autograft group, while the repair rate of control group was essentially unchanged. The facts that BMSCs group owned the fastest repair rate and the gap between the BMSCs group and control group was enlarged during 3–4 weeks may be associated with the proliferation and differentiation to neural cell of the BMSCs we transplanted.
3.5 Immunohistochemistry and immunofluorescence analysis
To determine whether BMSCs can engraft into the sciatic nerve, we initially injected bis-benzimide-Hoechst 33258-labeled cells into the lateral ventricle of neonatal mice. The use of Hoechst 33258 [30], a fluorescent DNA binding dye, provided a quick and convenient method to track the fate of the cells in sciatic nerve. The core of nerve regeneration engineering is to find the seeding cells and materials suitable for cell survival. Examination of cryostat sections (BMSCs group) by fluorescent microscopy revealed that the sciatic nerve contained labeled BMSCs at 6 weeks after injection (Fig. 10b), so the BMSCs we transplanted were still alive and became a part of the sciatic nerve. Nestin, an intermediate filament protein specifically expressed in neuroepithelial stem cells, may play a role in neural cell differentiation. Indeed, Nestin is a putative marker of NSCs and has even been used to identify potential neural stem cells (NSCs) [31]. Examination of cryostat sections (BMSCs group) by fluorescent microscopy revealed that the sciatic nerve contained nestin-positive cells (Fig. 10a).
Immunohistochemistry and immunofluorescence analysis of the sciatic nerve obtained at 6-week after bridging 8-mm-long neural gap (BMSCs group). a The first type of nestin-expressing cell (arrow indicated, nestin, brown in endochylema). b The second type of Hoechst 33258 (arrow indicated, blue in nucleus) labeled cell. c The third type of double staining cell (arrow 2 and 3 indicate the former two types of cells, arrow 1 indicated double positive cells, Nestin, green in endochylema and Hoechst 33258, blue in nucleus) It can be revealed that some of the seeded BMSCs had differentiated to NSCs or NSC-like cells. So the hypothesize that BMSCs might exert their efficacy via the process of differentiation from BMSCs to NSCs or NSC-like cells was certified
In order to test our hypothesis that BMSCs as seeding cells might exert their efficacy via the process of differentiation from BMSCs to NSCs, we examined the cryostat sections (BMSCs group) by fluorescent microscopy using double-straining. We found three kinds of cells under the fluorescent microscope: Hoechst 33258-labeled cells, nestin-positive cells and double-positive cells (Fig. 10c). The third kind of double-positive cells were not only transplanted by us, but also expressed nestin in vivo, so some of the BMSCs had differentiated to NSCs. According to the results of the SFI assessment, the cells which can repair the damaged nerve could only be neural cells, so we can get the conclusion that some of the seeded BMSCs had differentiated to NSCs, then the NSCs maybe differentiate to neural cells which can further promote the nerve regeneration.
4 Conclusion
In this study, the selection of chitosan materials and the evaluation of chitosan-based nerve conduit scaffold were studied. After our developing and testing both in vitro and in vivo, chitosan (Mw: 1 × 106 Da, DD: 95%) was selected to use as a scaffold combined with BMSCs. We have shown that only crushed nerve with chitosan combined with BMSCs can significantly improves nerve fiber regeneration and the degree of functional recovery, and actually some of the BMSCs had differentiated to NSCs. We thus believe that, although there was still a few differences between the BMSCs group and autograft group, BMSCs have high proliferation potential and can be handled and manipulated easily permitting differentiation prior implantation, so the BMSCs may represent as a very promising clinical tool in peripheral nerve reconstructive surgery. In our ongoing studies, we are investigating chitosan tubes for nerve regeneration and specifically the impact of BMSCs culturing matrix we injected on regenerative capacity.
References
Tanigawa J, Miyoshi N, Sakurai K. Characterization of chitosan/citrate and chitosan/acetate films and applications for wound healing. J Appl Polym Sci. 2008;110:608–15.
Chalfoun CT, Wirth GA, Evans GR. Tissue engineered nerve constructs: where do we stand. J Cell Mol Med. 2006;10(2):309–17.
Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–49.
Jianga M, Zhuge X, Yang Y, Gu X, Ding F. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci Lett. 2009;454:239–43.
Ciardelli G, Chiono V. Materials for peripheral nerve regeneration. Macromol Biosci. 2005;6(1):13–26.
Amado S, Simoes MJ, Armada da Silva PA, Luís AL, Shirosaki Y, Lopes MA, et al. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials. 2008;29:4409–19.
Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–9.
Aijun W, Qiang A, Qing H, Xiaoming G, Kai G, Yandao G, et al. Neural stem cell affinity of chitosan and feasibility of chitosan-based porous conduits as scaffolds for nerve tissue engineering. Tsinghua Sci Technol. 2006;11(4):415–20.
Wenling C, Duohui J, Jiamou L, Yandao G, Nanming Z, Xiufang Z. Effects of the degree of deacetylation on the physicochemical properties and Schwann cell affinity of chitosan films. J Biomater Appl. 2005;20(2):157–77.
Hsu S-h, Whu SW, Tsai C-L, Wu Y-H, Chen H-W, Hsieh K-H. Chitosan as scaffold materials: effects of molecular weight and degree of deacetylation. J Polym Res. 2004;11:141–7.
Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26(29):5872–8.
Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application Giannoudis. Injury. 2007;38:S23–33.
Lei Z, Yongda L, Jun M, Yingyu S, Shaoju Z, Xinwen Z, et al. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007;31(9):916–23.
Wislet-Gendebien S, Leprince P, Moonen G, Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295–302.
Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402.
Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009;1262:7–15.
Brehm M, Zeus T, Strauer BE. Stem cells-clinical application and perspectives. Herz. 2002;27:611–20.
Jing L, Yandao G, Nanming Z, Xiufang Z. Preparation of N-butyl chitosan and study of its physical and biological properties. J Appl Polym Sci. 2005;98:1016–24.
Evans GR, Facs MD. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19:312–8.
Lu L, Chen X, Zhang CW, Yang WL, Wu YJ, Sun L, et al. Morphological functional characterization of predifferentiation of myelinating glia-like cells from human bone marrow stromal cells through activation of F3/Notch signaling in mouse retina. Stem Cells. 2008;26:580–90.
Lu J, Moochhala S, Moore XL, Ng KC, Tan MH, Lee LK, et al. Adult bone marrow cells differentiate into neural phenotypes improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398:12–7.
Yang Y, Chen X, Ding F, Zhang P, Liu J, Gu X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues cells in vitro. Biomaterials. 2007;28:1643–52.
Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–910.
Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–38.
Chau LK, Porter MD. Surface isoelectric point of evaporated silver flms: determination by contact angle titration. J Colloid Interface Sci. 1991;145:283–6.
Cheng M, Cao W, Gao Y, Gong Y, Zhao N, Zhang X. Studies on nerve cell affinity of biodegradable modified chitosan flms. J Biomater Sci, Polym Ed. 2003;14(10):1155–67.
Cheng MY, Deng JG, Yang F, Gong YD, Zhao NM, Zhang XF. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24(17):2871–80.
Drllon P, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J Biomater Sci, Polym Ed. 1998;9(10):1049–69.
Yuan Y, Zhang P, Yang Y, Wang X, Gu X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25:4273–8.
Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96(19):10711–6.
Ernst C, Christie BR. The putative neural stem cell marker, nestin, is expressed in heterogeneous cell types in the adult rat neocortex. Neuroscience. 2006;138:183–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, L., Cui, HF. Use of chitosan conduit combined with bone marrow mesenchymal stem cells for promoting peripheral nerve regeneration. J Mater Sci: Mater Med 21, 1713–1720 (2010). https://doi.org/10.1007/s10856-010-4003-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4003-y