Abstract
In order to investigate bone tissue reaction to the low rigidity titanium alloy of TNTZ in bone plate fixation, animal experiment with rabbit was performed with X-ray follow-up and histological observation. Experimental fractures were made in rabbit tibiae, and fixed by different bone plates of SUS316L, Ti–6Al–4V and TNTZ. Although there was no significant difference in fracture healing, bone atrophy was observed in cortical bone especially under the bone plate, which was different in time course among three materials. The bone atrophy under the bone plate was confirmed as porous or poor bone tissue in histological observation. In addition, the diameter of the tibia bone was increased in TNTZ as the result of bone remodeling with a new cortical bone. It is confirmed that the elastic modulus of the bone plate will naturally influence bone tissue reaction to the bone plate fixation according to the Wolff’s law of functional restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Conventional high rigidity metal implant can induce bone atrophy due to the absence of mechanical stress, so-called stress shielding. And setting aside the amount, metal ion release is unavoidable in any metal implants. As a solution of these problems, a low rigidity titanium alloy of Ti–29Nb–13Ta–4.6Zr (TNTZ) was developed where all elements are considered as non-toxic. The developed TNTZ is beta type alloy (crystal structure of body center cubic) with remarkably low elastic modulus and high fatigue strength exceeding conventional SUS316L and Ti–6Al–4V [1–2]. The biocompatibility was already confirmed as bioinert showing the contact osteogenesis [3]. The most interested and significant question is whether the low rigidity titanium alloy can reasonably avoid the stress shielding and prevent bone atrophy or not. In this study, animal experiment on fracture fixation was performed with TNTZ bone plate, and bone tissue reaction was investigated by post-operative X-ray images and histological observation.
2 Materials
Based on the design of commercial AO mini dynamic compression plate (DCP) for human finger (Fig. 1), the bone plate with eight holes (42 × 5 × 1.5 mm) and screws of 10 mm and 12 mm were made of TNTZ (Elastic Modulus E = 58 GPa).
The commercial AO mini DCP and screws of Ti–6Al–4V (E = 108 GPa) and SUS316L (E = 161Gpa) were also provided as control. The important mechanical properties were shown in Table 1. The elastic modulus of TNTZ is remarkably lower as compared with those of SUS316L and Ti–6Al–4V. Although the ultimate tensile strength of TNTZ is lower, the fatigue strength of TNTZ is higher as compared with those of SUS316L and Ti–6Al–4V.
As experimental animal, mature New Zealand white rabbits (all male, weight about 3 kg) were used.
3 Methods
3.1 Experimental fracture fixation
Prior to this experimental study, the location of the bone plate fixation was considered by putting the bone plate on a real tibia sample (Fig. 2). The medial surface of proximal tibia was decided as the location with an adequate flat surface.
The experimental fracture fixation was performed by well-experienced orthopedic surgeon. Under venous anesthesia by pent barbiturate, the left proximal tibia was exposed with medial incision. First the bone plate was provisionally placed and fixed with six screws where the screw length was decided by using a depth gauge, and screw thread was made by tapping as the same as clinical standard procedure. Second, the bone plate was once removed, and upper one third of tibia was cut through by using oscillating saw (Fig. 3a) where the cut position was corresponding to the center of the bone plate. However, fibula was kept intact. Finally, the bone plate was tightly fixed (Fig. 3b), and soft tissue was sutured.
Furthermore, in order to avoid faulty union and dislocation, the rabbit was kept in a retention appliance for 3 weeks, and then moved to breeding cage.
3.2 X-ray follow-up
Under sedation by ketamine hydrochloride, X-ray observation was made in lateral and AP views at every week to 10 weeks, subsequently every 4 weeks until 48 weeks after the fixation. The X-ray pictures were scanned by flatbed image scanner with translucent light unit and stored in PC as digital image file.
3.3 Histological observation
At 48 weeks after the fixation, both tibiae were extracted with the bone plate, and externally observed the bone formation around the bone plate.
Regarding the tibiae with Ti–6Al–4V and TNTZ bone plates which were prepared in time for histological observation, the tibiae were dehydrated and stained by 80%, 90% and 100% ethyl alcohol with fuchsine. Then the tibiae were embedded in methyl meth-acrylate. After complete hardening, thin slice specimens of 300 μm at proximal, middle and distal parts of the bone plate were made from the embedded tibia by using cutting band saw, then grinded into 130 μm for histological observation and Contact Micro Radiogram (CMR).
After taking CMR, the thin slice specimens were mounted on slide glasses. Microscopic images were taken by the attached digital camera, and the microscopic images were stored in PC as digital image file.
4 Results
4.1 X-ray follow-up
Regarding fracture healing, there was no significant difference among SUS316L, Ti–6Al–4Vand TNTZ. As a general time course, callus formation was observed at 2 weeks, which became distinct at 3 weeks. Then bone union was obtained at 4 weeks, and the fracture line was hardly observed around 8 weeks. The trace of the experimental fracture was completely disappeared at 16–20 weeks.
However, bone atrophy (thinning of cortical bone) was observed under the bone plate, which was different in time course among the materials. In SUS316 (Fig. 4a), the thinning of cortical bone had begun from 7 weeks, and the cortical bone had almost disappeared at 12 weeks. In Ti–6Al–4V (Fig. 4b), the thinning had begun from 7 weeks, and almost disappeared at 14 weeks. In TNTZ (Fig. 4c), the thinning had begun from 10 weeks, and almost disappeared at 18 weeks after the fixation.
4.2 Histological observation
As for external findings of the extracted tibiae at 48 weeks after the fixation (Fig. 5, 6), all bone plate was partially covered by thin bone tissue. With regard to TNTZ and Ti–6Al–4V bone plates, CMR findings were described as follows.
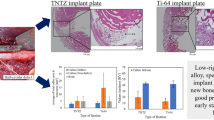
In Ti–6Al–4V, thinning of cortical bone was observed in all proximal, middle and distal levels (Fig. 7). The bone plate seemed to be buried in bone tissue in proximal and distal levels. In middle level, poor bone tissue was observed under the bone plate. In distal level, porous bone was observed under the bone plate. In addition, direct contact of the bone tissue to the plate was little and partial in all levels (Figs. 8).
In TNTZ, thinning of cortical bone was also observed in all levels (Fig. 9). Porous bone was observed under the bone plate in proximal level. In middle and distal levels, poor bone tissue was observed under the bone plate, the diameter of the tibia bone was increased, where thin bone tissue was observed in the medullary cavity. In addition, direct contact of the bone tissue to the plate was confirmed as greater than that of Ti–6Al–4V (Fig. 10).
5 Discussion
It is considered that the difference of bone tissue reaction was derived from the difference of material elasticity in the bone plate. The bone atrophy was observed in all materials as thinning and subsequent disappearance of bone tissue under the bone plate in X-ray follow-up.
Although an influence of devascularization due to the bone plate can be considered, there is however a certain time difference in the progress of the bone atrophy among the materials. In addition, the bone atrophy under the bone plate was confirmed as porous or poor bone tissue in histological observation at 48 weeks after the fixation in Ti–6Al–4V and TNTZ.
As Wollf’s law of the functional restoration, it is well known that the mechanical stress will influence the bone remodeling. According to this theory, the elastic modulus of the bone plate will naturally influence the bone tissue reaction to the bone plate fixation. However, since bone formation and remodeling is faster in small animals as compared with human and large animals, such remarkable bone atrophy was probably caused in a short periods of 48 weeks after the fixation [4–7].
Regarding the increase of tibia diameter and the intramedullary bone tissue in TNTZ, the double wall structure was observed with different X-P densities and a clear boundary line at the middle and distal levels, where the shape of the inner wall was close to the original cortical bone. Therefore, it seemed that the outer cortical bone was newly formed, and the intramedullary bone tissue was the remains of old cortical bone (Fig. 11), which is a possible result of the bone remodeling with the low rigidity bone plate. As the reason for such remodeling, it is considered that the increase of the tibia diameter will increase the bending rigidity of the tibia bone, which may reduce excessive stress around the fixation.
From these results, it is suggested that the low rigidity titanium alloy can delay or minimize the bone atrophy and promote the bone reorganization due to the mechanical stress, which is clinically applicable for long term or no extractive implantation in the aged patients and physically high-risk patients with sever complications.
6 Conclusion
In order to investigate bone tissue reaction to the low rigidity titanium alloy of TNTZ in bone plate fixation, animal experiment with rabbit was performed with X-ray follow-up and histological observation.
Experimental fractures were made in rabbit tibiae, and fixed by different bone plates of SUS316L, Ti–6Al–4V and TNTZ. Although there was no significant difference in fracture healing, bone atrophy was observed in cortical bone especially under the bone plate, which was different in time course among three materials. The bone atrophy under the bone plate was confirmed as porous or poor bone tissue in histological observation.
In addition, the diameter of the tibia bone was increased in TNTZ as the result of bone remodeling with a new cortical bone. It is confirmed that the elastic modulus of the bone plate will naturally influence bone tissue reaction to the bone plate fixation according to the Wolff’s law of functional restoration.
It is suggested that low rigidity titanium alloy is promising and applicable metal material which provides a new option for fracture fixation.
References
M. Niinomi, Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 243, 231–236 (1998)
M. Niinomi, D. Kuroda et al., in Non-Aerospace Application of Titanium and Its Alloys, ed. by F.H. Froes, P.G. Allen, M. Niinomi. New Beta Type Titanium Alloys with High Biocompatibility (The Minerals, Metals and Materials Society, 2001), pp. 217–233
M. Niinomi, T. Hattori, S. Niwa, in Biomaterials In Orthopedics, ed. by M.J. Yaszemski, D.J. Trantolo. Material Characteristics and Bio-compatibility of Low Rigidity Titanium Alloys for Biomedical Applications (Marcel Dekker, New York, 2003), pp. 55–60
J. Wolff, The Law of Bone Remodeling, (trans: P. Maquet, R. Furlong) (Berlin, Springer-Verlag, 1986)
R. Huiskes et al., Adaptive bone-modeling theory applied to prosthetic-design analysis. J. Biomech. 20, 1135–1150 (1987)
C.T. Rubin et al., Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J. Orthop. Res. 5, 300–310 (1987)
T.P. Ruedi, W.M. Murphy, AO Principles of Fracture Management, 1st edn. (AO Publishing, Davos, 2003). First Japanese edition 2003, Igaku-Shoin Ltd., Tokyo
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sumitomo, N., Noritake, K., Hattori, T. et al. Experiment study on fracture fixation with low rigidity titanium alloy. J Mater Sci: Mater Med 19, 1581–1586 (2008). https://doi.org/10.1007/s10856-008-3372-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3372-y