Abstract
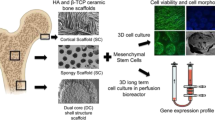
The main principle of a bone tissue engineering (BTE) strategy is to cultivate osteogenic cells in an osteoconductive porous scaffold. Ceramic implants for osteogenesis are based mainly on hydroxyapatite (HA), since this is the inorganic component of bone. Rapid Prototyping (RP) is a new technology in research for producing ceramic scaffolds. This technology is particularly suitable for the fabrication of individually and specially tailored single implants. For tissue engineering these scaffolds are seeded with osteoblast or osteoblast precursor cells. To supply the cultured osteoblastic cells efficiently with nutrition in these 3D-geometries a bioreactor system can be used. The aim of this study was to analyse the influence of differently fabricated HA-scaffolds on bone marrow stromal cells. For this, two RP-techniques, dispense-plotting and a negative mould method, were used to produce porous ceramics. The manufactured HA-scaffolds were then cultivated in a dynamic system (bioreactor) with an osteoblastic precursor cell line. In our study, the applied RP-techniques give the opportunity to design and process HA-scaffolds with defined porosity, interconnectivity and 3D pore distribution. A higher differentiation of bone marrow stromal cells could be detected on the negative mould fabricated scaffolds, while cell proliferation was higher on the dispense-plotted scaffolds. Nevertheless, both scaffold types can be used in tissue engineering applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the past decades bone tissue engineering (BTE) has undergone considerable progress and demonstrates a great potential for improved reconstruction of damaged bone in comparison with conventional therapies. The approach of BTE includes the development of strategies for cultivation of porous scaffolds, which provide an appropriate microenvironment to create ideal conditions for bone cells [1, 2]. Porous materials for this application can be manufactured from calcium phosphate ceramics. These materials are biocompatible and have osteoconductive properties because of their similarity to the inorganic phase of the human bone [3]. The fabrication of individually and specially tailored single implants is possible with rapid prototyping (RP), a new technology for producing ceramic scaffolds [4, 5]. For example, 3D-printing, which is based on an ink-jet printing of binder onto a ceramic powder bed, was used to produce hydroxyapatite (HA) scaffolds for tissue engineering [6] or β-tricalciumphosphate (TCP) implants [7]. Customised HA implants [8] and scaffolds [9] have also been fabricated by robocasting, an extrusion-based robotic deposition method. Indirect RP techniques, where the RP is used to fabricate negative moulds, which are then transferred into ceramic, are also being developed for the production of calcium phosphate scaffolds [10]. Other methods, such as stereolithography have been used to fabricate implants from other ceramics, as alumina [11], or from polymer/ceramic composites (polycaprolactone and HA [12] or polypropylene and TCP [13]).
For tissue engineering these scaffolds are seeded with osteoblast precursor cells or osteoblasts. Bone marrow stromal cells are progenitors of skeletal tissue components such as bone, cartilage, the hematopoiesis supporting stroma and adipocytes. Bone formation results from a complex cascade of events that involves proliferation of mesenchymal cells, differentiation into osteoblast precursor cells, maturation of osteoblasts, formation of the extra cellular matrix and finally mineralisation. To supply the cultured cells efficiently with nutrition in these 3D-geometries a bioreactor system can be used. These bioreactors, allowing direct perfusion of culture medium through tissue-engineered constructs, may overcome diffusion limitations associated with static culturing and may provide flow-mediated mechanical stimuli [14–16].
The aim of this study was to analyse the influence of differently fabricated HA scaffolds on bone marrow stromal cells. For this, two RP techniques, dispense-plotting and a negative mould technique, were used to produce porous ceramics. These HA-scaffolds were then cultivated in a dynamic system (bioreactor) with an osteoblastic precursor cell line.
2 Materials and methods
2.1 Scaffold fabrication
HA-scaffolds for the dynamic cultivation experiments were produced by two different processing techniques, a direct and an indirect RP method. With the direct RP technique the scaffolds are manufactured directly from ceramic, while with the indirect method a negative mould is fabricated by a RP technique and afterwards transferred into a ceramic scaffold.
The direct RP technique used in this work is called dispense-plotting, where a ceramic scaffold is produced layer by layer out of rods. A paste-like ceramic slurry is filled into a cartridge and extruded by pressurized air through a fine nozzle while the cartridge moves computer-controlled in x- and y-direction. Essential for this method is a ceramic slurry with a high solids loading and a thixotropic flow behaviour. In that way the slurry can be extruded easily through the fine nozzle, but remains a stable rod when deposited onto the building platform. By rotating the direction of the extruded rods from layer to layer by a certain angle 3D-scaffolds can be produced. In this work we fabricated scaffolds with a 0/90° lay-down-pattern. The rod diameter is controlled by the nozzle diameter and the deposition speed, while the pore size is determined by the CAD-data, which controls the plotter. After fabrication and drying the scaffolds were sintered at 1,300 °C.
The indirect RP technique was realised with wax negative moulds , which were designed with a CAD-program (SolidWorks, Version 2006, U.S.). We used a wax-printer (BT66, Solidscape, U.S.) to produce the negative moulds. Two different waxes, a build and a support wax, were printed dropwise onto the building platform. The support wax enables the fabrication of intersections or overhangs in the negative mould. After printing each layer was milled to a thickness of 13 μm. From the finished negative mould the support wax was resolved at 50 °C with a kerosene derivate. The porous wax negative mould was then impregnated with an HA-slurry in vacuum. After impregnation the part was dried and sintered at 1,300 °C. During this step the wax was burnt out.
The two different scaffold types were characterised by helium pycnometry (AccuPyk 1330, micromeritics GmbH, Germany) regarding bulk density and total porosity (calculated with the help of the geometrical density, measured with a calliper). Pore size and strut diameter were measured from SEM images (Quanta 200, FEI, The Netherlands) with an analysing software (AnalySIS, Soft Imaging System, Germany). The SEM images were also used for the assessment of pore geometry and surface roughness of the scaffolds. The specific surface area was measured by gas adsorption—desorption by Brunauer–Emmett–Teller with krypton gas (ASAP 2010, micromeritics, Germany). The phase purity of the HA powder was examined by X-ray diffraction (3000 P, Seifert, Germany). Before cell seeding the scaffolds were cleaned by soaking in Extran (Merck, Germany) and SDS (sodium dodecyl sulphate, Sigma–Aldrich, Germany) solutions. Afterwards, they were autoclaved (Systec, Germany).
2.2 Cell culture
ST-2 cells (Deutsche Sammlung für Mikroorganismen und Zellkultur, Braunschweig), a clonal stromal cell line isolated from bone marrow of BC8 mice [17], were cultured on the different scaffolds. Cells were maintained in RPMI 1640 medium (Gibco, Germany) containing 10 vol.% FBS (Sigma–Aldrich, Germany), 1 vol.% penicillin/streptomycin (Sigma–Aldrich, Germany) and 1 vol.% Glutamax (Gibco, Germany). For osteogenic stimulation the culture medium was supplemented with 50 μg/ml ascorbic acid (Sigma–Aldrich, Germany), 100 nM dexamethasone (Sigma–Aldrich, Germany) and 10 mM β-glycerophosphate (Sigma–Aldrich, Germany). The scaffolds were seeded from two opposite sides with 1 million ST-2 cells and incubated for three days allowing the cells to adhere in the scaffolds before they were transferred into a perfusion bioreactor system. The bioreactor (Perfusionskammer 1301, Minucells and Minutissue Vertriebs GmbH, Germany) was connected by silicone tubings with a medium reservoir for the back flow loop. The reactor and the medium containing flask were placed into an incubator (conditions: 37 °C, 5% CO2). The silicon tubing was long enough to ensure sufficient gas exchange. The adjusted medium flow rate was 5 ml/min and was realised by a peristaltic pump (BCP Standard, Ismatech, Germany). The dynamic cultivation period was 14 days.
2.3 Cell proliferation
Cell proliferation was measured by determining the lactate dehydrogenase (LDH) activity in cell lysis with a commercially available kit (Sigma–Aldrich, Germany). Cell viability was analysed using the WST-1 test (Roche, Germany) with a working concentration of WST-1 of 10 μl/ml.
2.4 Cell differentiation
Alkaline phosphatase (ALP) activity is an important marker for osteoblastic differentiation. After lysing the cells the ALP-activity was determined colourimetrically using an ALP kit (Sigma–Aldrich, Germany). The specific activity was calculated referring to the protein concentration of the lysates. The protein content was determined using a commercially available kit (BCA, Sigma–Aldrich, Germany). Content of collagen I of the ST-2 cells was determined by Sirius Red solution (Chroma, Germany). Samples and collagen standards were dried, fixed with Bouins fluid (Sigma–Aldrich, Germany) and incubated with Sirius Red solution. Sodium hydroxide was used to dissolve the stained collagen. The resulting solution was measured at 560 nm in an ELISA-reader (BMG, Germany), and the collagen I content was quantified by the determined standard curve. Furthermore, expression of genes for collagen I, osteocalcin (OC) and bone sialoprotein (BSP) were examined by RT-PCR. After RNA isolation reverse transcription was carried out before polymerase chain reaction was done to amplify the gene products.
2.5 Cell morphology
Distribution of the cells in the scaffolds was determined by using a fluorescence microscope (Zeiss, Germany). Samples were fixed with 3.7 vol.% paraformaldehyde (Merck, Germany), and afterwards stained with DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride, Molecular Probes, Germany). For SEM-characterisation samples were fixed in 3 vol.% paraformaldehyde, 3 vol.% glutaraldehyde (Sigma–Aldrich, Germany) and 0.2 M sodiumcacodylate (Sigma–Aldrich, Germany). After a dehydration through a series of graded acetone, the samples were critical point dried with CO2 (Parr Instrument Company Moline, USA) and sputtered with gold (Cressington, UK). The cell morphology was analysed by SEM (FEI, The Netherlands).
Each experiment was repeated with three samples. Results are presented as mean ± SD of three replicates and evaluated by one-way analysis of variance (ANOVA). The level of statistical significance was defined at p < 0.05.
3 Results and discussion
The produced scaffolds consisted of phase-pure HA, which was proved by X-ray diffraction. Both scaffold types fabricated by dispense-plotting and negative mould technique had a three-dimensional interconnecting porosity with pore sizes of 300 μm and strut diameters of approx. 500 μm. Although both scaffold types had parallel pore channels in a 90° angle, the geometry of the pores differed a little between the two scaffold types. The negative mould scaffolds had rectangular pores with rounded edges in all three spatial directions. As the dispense-plotted scaffolds are built of crossing rods, only the pores in x/y-direction are rectangular, while the edges of the pores in z-direction have acute angles. Total porosity was 37 (dispense-plotted scaffolds) and 44 vol.% (negative mould scaffolds), while a bulk density of 96% th.d. was achieved for both scaffold types. The surface of the dispense-plotted scaffolds had a low roughness. Due to the milling steps in the negative mould production the surface of the wax negative moulds and, as a consequence, the surface of the ceramic scaffolds produced by this technique had a very high surface roughness. This result is confirmed by the specific surface area of the two ceramic scaffold types (dispense-plotted: 0.02 m2/g, negative mould scaffold: 0.03 m2/g).
Cell proliferation measured by LDH-activity was higher in cells grown on the negative mould scaffolds than on the dispense-plotted scaffolds. However, cell viability indicated no significant difference (Fig. 1). Collagen I content and ALP-activity results showed differences (Fig. 2). The collagen I content of the cells was higher on the negative mould fabricated scaffolds. In contrast, the ALP-activity of the cells on these scaffolds was significantly lower than on the dispense-plotted ones. For this reason the expression of typical osteoblast markers were analysed by RT-PCR to clarify the differentiation stadium of the ST-2 cells on the scaffolds. GAPDH, as control, and collagen I were expressed on all scaffolds, but OC was only found in cells grown on the negative mould fabricated scaffolds, while BSP was absent in all cells (Fig. 3). The positive detection of collagen I showed, that all cells were osteogenic differentiated [18]. The differentiation progress was clarified by the ALP-activity and the expression of BSP. As BSP is a marker of the late osteoblast phenotype, the lack of BSP showed that cells on both scaffold types were not yet mature osteoblasts. During osteogenesis the ALP-activity is known to raise in the beginning with ongoing differentiation, but decreases while cells differentiate to early osteoblasts and has a final maximum for the phenotype of the mature osteoblast [19]. Therefore, mRNA expression of OC, the lack of BSP, the higher content of collagen I and the lower ALP-activity indicated, that the bone marrow stromal cells were differentiated into precursor-osteoblasts on the negative mould fabricated scaffolds. The missing of the osteocalcin lane in the case of the dispense-plotted scaffolds might be due to a slower differentiation of the cells on these scaffolds. The lower collagen I content and higher ALP-activity confirmed this presumption. These differences in cell differentiation on the two scaffold types may be the consequence of surface roughness and pore geometry. The initial attachment of the bone marrow stromal cells on the scaffolds is necessary for proliferation, and finally, for osteoblastic differentiation. On both scaffold types a homogenous seeding and attachment throughout the whole scaffold took place. Images of the fluorescence microscopy of defined broken scaffolds showed cells on the top, in the centre and on the bottom of the scaffolds after a dynamic cultivation of 17 days. Nuclei staining of bone marrow stromal cells cultivated on dispense-plotted and negative mould fabricated scaffolds from the top layer are shown in Fig. 4. The cells in the centre of the scaffolds are not shown in this figure. In a previous study we cultivated the fabricated HA-scaffolds in a static system (cell culture plate) with the same cell line for 17 days. In contrast to the dynamic cultured scaffolds the static cultured scaffolds revealed a very inhomogeneous cell growth. The measurement of cell vitality and cell differentiation showed significantly lower values than in this study with the use of a bioreactor. This also proves the advantage of a bioreactor system for BTE-applications.
Cell morphology was investigated by SEM in the centre of the scaffolds. After 17 days of dynamic cultivation with stimulated ST-2 cells, a completely closed monolayer and even multilayer were observed on the struts throughout the scaffolds (Fig. 5). The analysed cells had highly structured cell membranes, indicating a high metabolic activity and viability. Although both scaffolds had pore sizes of only 300 μm, no pore bridging of the cells could be seen. The cells migrated from layer to layer without closing the pores. This gives evidence that the cultivated scaffolds could in principle be vascularised when implanted into a bone defect. The phenotype of the ST-2 cells was both fibroblastic and osteoblastic. However, on the negative mould fabricated scaffolds more osteoblast-like cells were found. This finding is in accordance with the previously mentioned results and is a further evidence for the presumption that ST-2 cells on the dispense-plotted scaffolds differentiated more slowly.
4 Summary
The main principle of a BTE-strategy is to cultivate osteogenic cells in an osteoconductive porous scaffold. Scaffold optimisation, cell seeding, cell stimulation and cultivation are necessary for tissue engineering. In our study, the two applied RP-techniques give the opportunity to design and process HA-scaffolds with defined porosity, interconnectivity, 3D pore distribution and different pore morphology. It could be shown that, as expected, the employment of a bioreactor system is beneficial for BTE. Comparing the cell behaviour on the differently fabricated scaffolds, a higher differentiation of bone marrow stromal cells into precursor osteoblasts could be detected on the negative mould fabricated scaffolds, while cell proliferation was higher on the dispense-plotted scaffolds. Nevertheless, both scaffold types are suitable for tissue engineering applications.
References
U. KNESER, D. SCHAEFER, J. POLYKANDRIOTIS and E. HORCH, J. Cell. Mol. Med. 10 (2006) 7
D. LOGEART-AVRAMOGLOU, F. ANAGNOSTOU, R. BIZIOS and H. PETITE, J. Cell. Mol. Med. 9 (2005) 72
R. LANGER and J. P. VACANTI, Science 260 (1993) 920
D. W. HUTMACHER, M. SITTINGER and M. V. RISBUD, Trends Biotechnol. 22 (2004) 354
W. Y. YEONG, C. K. CHUA, K. F. LEONG and M. CHANDRASEKARAN, Trends Biotechnol. 22 (2004) 643
B. LEUKERS, H. GÜLKAN, S. IRSEN, S. MILZ, C. TILLE, M. SCHIEKER and H. SEITZ, J. Mat. Sci.: Mat. Med. 16 (2005) 1121
A. KHALYFA, S. VOGT, J. WEISSER, G. GRIMM, A. RECHTENBACH, W. MEYER and M. SCHNABELRAUCH, J. Mater. Sci.: Mater. Med. 18 (2007) 909
J. CESARANO, J. G. DELLINGER, M. P. SAAVEDRA, D. D. GILL, R. D. JAMISON, B. A. GROSSER, J. M. SINN-HANLON and M. S. GOLDWASSER, Int. J. Appl. Ceram. Technol. 2 (2005) 212
E. SAIZ, L. GREMILLARD, G. MENENDEZ, P. MIRANDA, K. GRYN and A. P. TOMSIA, Mater. Sci. Eng. C27 (2007) 546
A. WOESZ, M. RUMPLER, J. STAMPFL, F. VARGA, N. FRATZL-ZELMAN, P. ROSCHGER, K. KLAUSHOFER and P. FRATZL, Mater. Sci Eng. C25 (2005) 181
K. PHAM-GIA, M. SCHWARZ, M. SCHAEFER and B. WESSLER, Rapid prototyping process to fabricate ceramic surgical implant with ceramic powder and binding agent suspension devoid of solvent, DE102005058116
D. W. HUTMACHER, I. ZEIN and S. H. TEOH, Processing of bioresorbable scaffolds for tissue engineering of bone by applying rapid prototyping technologies, in: Proceedings of the symposium Processing and Fabrication of Advanced Materials VIII, ed. K.A. Khor, T.S. Srivatsan. M. Wong, W. Zhou and F. Boey. World Scientific Publishing Co. Pte. Ltd., Singapore (2000) 201
S. J. KALITA, S. BOSE, H. L. HOSICK and A. BANDYOPADHYAY, Mater. Sci. Eng. C23 (2003) 611
X. YU, E. A. BOTCHWEY, E. M. LEVINE, S. R. POLLACK and C. T. LAURENCIN, Proc. Natl. Acad. Sci. USA. 31 (2004) 11203
H. SINGH, S. H TEOH, H. T. LOW and D. W. HUTMACHER, J. Biotechnol. 119 (2005) 181
G. ALTMAN, R. HORAN, I. MARTIN, J. FARHADI, P. STARK, V. VOLLOCH, J. RICHMOND, G. VUNJAK-NOVAIKOVIC and D. L. KAPLAN, FASEB J. 16 (2002) 270
P. BIANCO, M. RIMINUCCI, S. GRONTHOS and P. G. ROBEY, Stem Cells 19 (2001) 180
J. B. LIAN and G. S. STEIN, Iowa Orthop. J. 15 (1995) 118
N. JAISWAL, S. E. HAYNESWORTH, A. I. CAPLAN and S. P. BRUDER, J. Cell. Biochem. 64 (1997) 295
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Detsch, R., Uhl, F., Deisinger, U. et al. 3D-Cultivation of bone marrow stromal cells on hydroxyapatite scaffolds fabricated by dispense-plotting and negative mould technique. J Mater Sci: Mater Med 19, 1491–1496 (2008). https://doi.org/10.1007/s10856-007-3297-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3297-x