Abstract
With the use of a recently created chitosan neutral hydrogel, we have been able to create various mixtures of chitin and chitosan without changing their characteristics even at room temperature. The aim of this study was the initial comparison of various mixtures of β-chitin and chitosan as a scaffold for rabbit chondrocyte culture. We created five types of sponges: pure β-chitin, pure chitosan, 3:1, 1:1, and 1:3 β-chitin-chitosan. The absorption efficiencies of chondrocytes in all five types of sponges were found to be around 98%. The mean concentrations of chondroitin sulfate were statistically different neither at week 2 nor at week 4 postculture between the types of sponges. The content of hydroxyproline in the β-chitin sponge was significantly greater than in other sponges at week 4 postculture. From the histochemical and immunohistochemical findings, the cartilage-like layer in the chondrocytes-sponge composites of all five types of sponges was similar to hyaline cartilage. However, only immunohistochemical staining of type II collagen in the pure β-chitin sponge was closer to normal rabbit cartilage than other types of sponges. The pure β-chitin sponge was superior to other sponges concerning the content of extracellular matrices of collagen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It has been established that full-thickness defects of articular cartilage do not repair spontaneously. In particular, the healing of hyaline cartilage is limited because of the low mitotic activity of chondrocytes and the avascular nature of cartilage. Transplantation of cultured chondrocytes has been shown to be effective for repairing cartilage. However in a monolayer culture, chondrocytes change their phenotype, have fibroblastic morphology, synthesize type I collagen and lose the ability to accumulate to produce a cartilageous matrix [1, 2]. It has been reported that chondrocytes maintained their phenotype in three-dimentional cultures embedded in type I collagen gels [1–3]. Recently, various scaffolds, including collagen gel [1–4], fibrin [5], agarose [6], alginate beads [7], collagen sponge [8, 9], and synthetic biodegradable polymer [10, 11], were used for cell suspensions after an in vitro chondrocytes expansion and stabilized the phenotype of chondrocytes during culture.
Chitin is a natural polysaccharide found particularly in the shells of crustaceans such as crab and shrimp, the cuticles of insects, and the cell walls of fungi, and is one of the most abundant biopolymers next to cellulose. Chitin and its deacetylated derivatives, chitosan have chemical structures corresponding to the series of linear copolymers of linked β, (1–4) N-acetylglucosamine and N-glucosamine, respectively [12]. Chitin and chitosan and their carboxymethyl derivatives have recently received a lot of attention in the fields of biomass research because of their special properties and inexpensive, abundant supply.
Because chitin is a highly biocompatible [13], bioresorbable [14, 15], and bioactive [16] carbohydrate biopolymer, it has been used in experimental studies as a delivery vehicle for growth factor [17, 18], a hybrid vehicle with another polymer and protein [19–22], and a covering vehicle for wound healing [23–25]. α-Chitin, also called regular chitin, from crab and shrimp shells, is known to be sparingly soluble and to have poor reactivity due to its rigid crystalline structure. β-Chitin, on the other hand, from squid bone, forms slurry easily when it is ground with water due to its loose crystalline structure. Also, the softness and hydrophilicity of β-chitin is superior to α-chitin, which can be explained by the crystalline structural difference between them [26]. Moreover, chitin foam prepared by lyophilization is especially absorbent of anionic dye [27]. Characteristics of chitin are expected to function advantageously for maintaining chondrocytes and extracellular matrix that are synthesized by chondrocytes. From the characteristics of different types of chitin, we thought that β-chitin is superior to α-chitin. Therefore, in our previous study, we reported that β-chitin was useful as a scaffold for three-dimensional chondrocytes cultures by producing cartilage-scaffold composites with a cartilage-like layer on its surface [28].
Chitosan was also reported to be an effective substrate for the growth and continued function of human chondrocytes in an in vitro culture on chitosan-coated coverslips [29], and to be a useful alternative as a scaffold for porcine chondrocyte culture [30]. It also has an advantage in chondrocyte cultures, because chitosan has the capability to form insoluble ionic complexes with anionic polysaccharides such as aggrecan which chondrocytes synthesize [31]. It was also reported that the relationship of attachment and growth of any cells with a percentage of acetylation of chitosan followed a general trend with the higher deacetylated chitosan supporting attachment and subsequent growth of the cells [32]. It was therefore determined that the higher deacetylated chitosan was suitable for culture.
Although there has been concern over which, chitin or chitosan, is the better material for cartilage tissue engineering, and whether there is a synergy by mixing them, the results are thought to be influenced by the type of chitin, the percentage of the deacetylation of chitosan, the method of mixing them, and so on. A mixed hydrogel of chitin and chitosan had scarcely been considered for comparing the two. Though chitosan became water-soluble following the formation of salt with organic acids [33], it is difficult to maintain the molecular weight, the viscosity, and its antimicrobial activity at room temperature. However, a superior method of preparing chitosan hydrogel has recently been established; a sodium hydroxide aqueous solution is added to the chitosan acetic solution and then extensive rinsed with distilled water to remove sodium acetate, to make it acid smell free. This method maintains the molecular weight, the viscosity, and its antimicrobial activity at room temperature [34].
Although there is a report comparing α-chitin and 85% deacetylated chitosan as a scaffold for chondrocyte culture [35], it is better to compare β-chitin and the higher deacetylated chitosan from above the characteristics of chitin and chitosan, and to use a recently created chitosan hydrogel for more accurately investigating the usefulness of various mixtures of chitin and chitosan as a scaffold for chondrocyte culture. The aim of the present study is to compare scaffolds of β-chitin, chitosan, and various mixtures of β-chitin and chitosan.
2 Materials and methods
2.1 Preparation of sponges
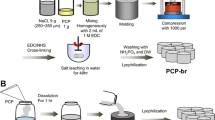
β-Chitin hydrogel was prepared as follows: 5.0 g β-chitin powder (molecular weight 2.0 × 105) and 40 ml distilled water were blended in a mixer, and then 20 ml distilled water was added and the mixture further blended. Chitosan neutral hydrogel was prepared as follows: 10.0 g chitosan powder (molecular weight 1.5 × 105, degree of deacetylation 98%) and 1 liter distilled water were blended in a mixer, and then 4 w/v% acetic acid aqueous solution added followed by filtration to remove insoluble material and the mixture further blended. The chitosan solution was then brought to pH 10–12 by the addition of 10 w/v% sodium hydroxide aqueous solution and was dialyzed to be neutral [34].
Pure β-chitin, pure chitosan, 3:1, 1:1, and 1:3 β-chitin-chitosan sponges were created from mixtures of β-chitin hydrogel with chitosan hydrogel. The hydrogels were each placed in 5 mm diameter dishes (Iwaki-Glass, Chiba, Japan), and frozen and vacuum-dried for 24 h to form sponges in a pillar shape (5 mm diameter × 10 mm height). Pore sizes of five types of sponges were measured by scanning electron microscopy (model S-4800; Hitachi, Tokyo, Japan).
2.2 Isolation and culture of chondrocytes
Twenty four 10-week-old Japanese white rabbits with a mean body weight of 2.0 kg were used for gathering articular cartilage. Slices of articular cartilage from their knees, hips and shoulders were gathered and cut into small pieces. The fragments were rinsed three times with sterile 0.9% sodium chloride and digested with 0.25% trypsin (GIBCO, Grand Island, NY) in sterile saline for 30 min followed by 0.25% collagenase (collagenase Type II; GIBCO) in Dulbecco’s modified Eagle’s medium (DMEM; Nissui, Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Trace, Melbourne, Australia) and 1% penicillin (100 IU/ml)/streptomycin (100 μg/ml)/amphotericin B (0.25 μg/ml) (GIBCO) for 4 h at 37 °C in a culture bottle. After filtration with 100 μm (pore size) cell strainers (Falcon, BD Biosciences Discovery Labware, Franklin Lakes, NJ), the isolated cell suspensions were centrifuged for 5 min at 1,500 rpm. Collected cells were washed three times with the culture medium. The final cell density was adjusted to 5.0 × 107 cells/ml. Approximately 400 μl of DMEM cell mixture was able to be produced from one rabbit. Therefore, we could make ten samples from one rabbit on average. DMEM cell mixture (40 μl) was placed in each of 12 well dishes of a non-treated microplate (Sumitomo Bakelite Co. Ltd., Tokyo, Japan), and then the flat surface of each pillar-shaped sponge was placed onto the DMEM cell mixture. When the sponge had fully absorbed the mixture, it was laid on its side in the dish. After 30 min of incubation at 37 °C and 5% CO2-air, 3.0 ml of culture medium containing l-ascorbic acid phosphate magnesium salt n-hydrate (Wako Pure Chemical Industries, Osaka, Japan) (20 μl/ml) was added to the cell-sponge composites and laid lengthwise. The cell cultures were then incubated at 37 °C and 5% CO2-air for 24 h, the cell-sponge composites were removed to another 12 well dishes of a non-treated microplate (Sumitomo Bakelite Co. Ltd.) and the number of cells remaining in solution was counted with a hemocytometer. This number was then used to calculate the rate of cell absorption by the each sponge. Culture medium (3.0 ml) was added to the dish and replaced with fresh DMEM containing l-ascorbic acid phosphate magnesium salt n-hydrate (Wako Pure Chemical Industries) (20 μl/ml) twice per week. In this manner, we produced a total of 240 chondrocytes-sponge composites, yielding 48 per type of sponge, at week 2 and 4 postculture (6 for analysis of chondroitin, 12 for analysis of hydroxyproline, 6 for histochemical and immunohistochemical examination).
2.3 Analysis of chondroitin sulfate
For the measurement of chondroitin sulfate (CS) content, 6 chondrocytes-sponge composites of each type of sponge at weeks 2 and 4 postculture were stored at −80 °C in a deep freezer until use. At the time of assay, samples were digested with Actinase E (Kaken Pharmaceutical Company, Tokyo, Japan), Chondroitinase ABC and Chondroitinase AC-II (Seikagaku Corporation, Tokyo, Japan). The levels of unsaturated disaccharides, which were derived from isomers of chondroitin 4-sulfate (C4S) and chondroitin 6-sulfate (C6S) in samples, were measured by high performance liquid chromatography (HPLC) [36, 37].
2.4 Analysis of hydroxyproline contents
At weeks 2 and 4 postculture, the upper one-third of 12 chondrocytes-sponge composites of each type of sponge, including the cell layer, were stored at −80 °C in a deep freezer until use. At the time of assay, each sample was freeze-dried under vacuum, and then hydrolyzed in 1.0 ml of 6N HCl at 110 °C for 24 h in screw-top hydrolysis glass tubes (Iwaki-Glass). The hydroxyproline content was measured by means of an automated amino acid analyzer system (Model 835-50; Hitachi, Tokyo, Japan), using an aliquot of hydroxylate of the samples [38].
2.5 Histochemical and immunohistochemical analysis
Histochemical and immunohistochemical evaluations of chondrocytes-sponge composites were performed at weeks 2 and 4 postculture. After fixation with 10% phosphate-buffered formalin for 24 h, specimens were embedded in paraffin and sectioned at a thickness of 5 μm. Using standard histochemical techniques, sections were stained with hematoxylin-eosin (H&E), safranin O, and toluidine blue. Immunohistochemical stainings were performed with anti-type II collagen antibody (F-57, Fuji Pharmaceutical Laboratories, Toyama, Japan), anti-type I collagen antibody (F-56, Fuji Pharmaceutical Laboratories), and anti-aggrecan ARGxx antibody (ab3773, ABcam, Cambridge, UK). Specimens for aggrecan immunohistochemical stainings were deglycosylated using Chondriotinase ABC, Keratanase, and Keratanase II (Seikagaku Corporation) for optimal BC-3 epitope recognition. The histologic evaluation of the staining was performed by a semi-quantitative method. The degree of staining was classified into three categories: “++” indicates almost the same staining degree as that of normal rabbit hyaline cartilage, “+” indicates similar staining to that of normal rabbit hyaline cartilage, but the degree of staining was the different, and “−”indicates no staining.
2.6 Statistical analysis
An ANOVA statistical analysis was made for the five types of sponges. Analysis was performed using StatView J 5.0 software on a Macintosh computer. p Values < 0.05 were considered to be statistically significant.
3 Results
3.1 Pore size of sponges
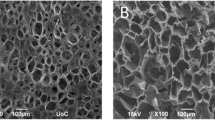
Pore sizes of the five types of scaffolds ranged from 50 to 150 μm. The holed surface of the pure β-chitin scaffold was smooth while the surfaces of scaffolds containing chitosan were rough (Fig. 1).
3.2 Absorption efficiency of chondrocytes in sponges
The absorption efficiencies of chondrocytes were 98.25 ± 1.47% (means ± SD) in the pure β-chitin sponges, 98.75 ± 0.61% in the β-chitin:chitosan = 3:1 sponges, 98.50 ± 0.95% in the β-chitin:chitosan = 1:1 sponges, 98.75 ± 1.13% in the β-chitin:chitosan = 1:3 sponges, and 98.25 ± 0.61% in the pure chitosan sponges. There were no significant differences in the absorption efficiency among the five types of scaffolds.
3.3 Analysis of chondroitin sulfate
The mean concentration values of C4S and C6S in chondrocytes-sponge composites and total CS (C4S + C6S), at weeks 2 and 4 postculture, are shown in Fig. 2. C4S, C6S and total CS content of all five types of sponges had increased at week 4 postculture more than at week 2 postculture. There were no significant differences in C4S, C6S, and total CS among the five types of sponges at weeks 2 and 4 postculture. The content of C6S was more than that of C4S at both weeks 2 and 4 postculture. The means of C6S:C4S ratios were not different among the five types of sponges at weeks 2 and 4 postculture (Fig. 3).
Changes in mean concentrations of chondroitin sulfate in chondrocytes-sponge composites at weeks 2 (dotted columns) and 4 (slashed columns) postculture. (A): total CS (C4S + C6S). (B): C6S. (C): C4S. Each column represents the mean of six samples. There was statistically difference among the five types of sponges neither at week 2 nor at week 4 postculture
3.4 Analysis of hydroxyproline contents
The hydroxyproline contents at week 2 and week 4 postculture, are shown in Fig. 4. The content of hydroxyproline in chondrocytes-sponge composites of the five types of sponges increased at week 4 postculture more than at week 2 postculture. Although there was no difference in the content of hydroxyproline in chondrocytes-sponge composites of the five types of sponges at week 2 postculture, the content of hydroxyproline in the chondrocytes-pure β-chitin sponge composite was significantly greater than in composites of other sponges at week 4 postculture (p < 0.05).
3.5 Histochemical and immunohistochemical findings
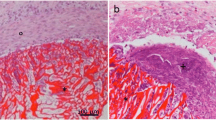
Figures 5 and 6 show the histology of the cartilage-like layer of a cultured chondrocytes-sponge composite at week 4 postculture, and Table 1 summarizes the histologic evaluation results for all the stainings. H&E staining revealed the cell layer at the surface of each type of sponge, and round cells without complete tissue formation were seen in the layer at week 2 (data not shown). At week 4, abundant extracellular matrix accumulations around round cells in all five types of sponges, and a few spindle shape cells were seen in the superficial layer of the matrix in all five types of sponges (Fig. 5A). Although staining with Safranin O revealed the presence of sulfated proteoglycan-rich extracellular matrix even at week 2 postculture, the cell layer of all five types of sponges at week 4 postculture were stained almost to the same degree, but not stained up to the degree of normal rabbit cartilage (Fig. 5B). When stained with toluidine blue, there were metachromatic matrices in the cell layer of all five types of sponges at both weeks 2 and week 4 postculture, but still not to the degree of normal rabbit cartilage (Fig. 5C). Although immunostainings for type II collagen of the cell layer of all five types of sponges were positive at weeks 2 and week 4 postculture (Fig. 6A), immunostainings for Type I collagen were positive in the cell layer of all five types of sponges neither at week 2 nor week 4 postculture (data not shown). The presence of type II collagen was uniform over the whole layer in normal rabbit cartilage, but was restricted to only around the cells in the cartilage-like layer, except with the pure β-chitin sponge. For type II collagen, the cell layer of the pure β-chitin sponge was stained closer to that of normal rabbit cartilage than other types of sponges. Immunostainings for aggrecan of the cell layer of all five types of sponges were also positive at weeks 2 and week 4 postculture (Fig. 6B). The presence of aggrecan was noted around the cells in normal rabbit cartilage, and was similar in the cell layer of all five types of sponges.
4 Discussion
In the present study, we compared β-chitin and chitosan as a scaffold for chondrocyte culture by creating porous sponge scaffolds from various mixtures of β-chitin hydrogel and chitosan hydrogel. Chitosan hydrogel, the preparation method of which is a recent development and used in the present study, is stable and can maintain the viscosity, molecular weight, and antibacterial activity for a long period even at room temperature [34]. Therefore, we were able to mix β-chitin hydrogel with chitosan hydrogel and create various desired shapes of scaffolds at room temperature. The method of culturing chondrocytes using a pillar-shape β-chitin sponge developed in our previous study [28] makes it possible to fit the composites into an articular cartilage defect without covering the periosterium or suturing the implant, similar to the method of mosaic plasty, in which the autologous osteochondral graft is fitted into the articular cartilage defect. Our previous study also demonstrated that chondrocytes migrated and distributed uniformly, and then proliferated, synthesized an extracellular matrix, and formed a hyaline-like cartilage layer in the β-chitin sponge [28].
In the present study, the absorption efficiencies of chondrocytes in all five types of sponges were great, reaching approximately 98%. This is the result of the common characteristics of their cationic nature and the porous structure of their scaffold, and it shows the abilities of β-chitin and highly acetylated chitosan for supporting cell attachment are not different.
We measured isomers of CS in order to evaluate glycosaminoglycan (GAG) synthesis in this culture system, because most GAG in articular cartilage exists in the form of CS-GAG [39]. C6S occupies more than 90% of the CS isomer in human adult cartilage [40], suggesting that C6S reflects a mature cartilage. In contrast, C4S is detected predominantly in fetal human cartilage and osteoarthritis cartilage, suggesting that C4S reflects an immature or degenerated cartilage [40, 41]. The CS content in all five types of sponges increased at week 4 postculture greater than week 2 postculture, with C6S increasing more than C4S. The CS content at week 4 postculture was not different among the five types of sponges. This result indicates that chondrocytes in all five types of sponges synthesized a mature proteoglycan similar to articular cartilage. Therefore, the effects of β-chitin and highly acetylated chitosan on the proteoglycan synthesis were not different.
Hydroxyproline content in chondrocytes-sponge composites were measured in order to evaluate collagen synthesis. The content of hydroxyproline in all five types of sponges increased at week 4 postculture more than week 2 postculture. Although there was no difference in the content of hydroxyproline among the five types of sponges at week 2 postculture, it was much more in the pure β-chitin sponge than in other sponges at week 4 postculture. This result indicates that chondrocytes in all five types of sponges synthesized collagen during the culture period, and it shows the ability of the pure β-chitin sponge for synthesizing collagen is superior to the abilities of other types of sponges.
In the immunohistochemical analysis, we detected positive staining of the pericellular and the interceller with type II collagen and aggrecan, and no positive staining with type I collagen in all five types of sponges. Therefore, increasing hydroxyproline in chondrocytes-sponge composites meant that chondrocytes synthesized type II collagen existing predominantly in articular cartilage. Histological observation suggested that most of the cells cultured in all five types of sponges maintained their spherical morphology and produced an abundant amount of proteoglycans and were embedded in them. Of the five types of sponges, type II collagen was stained in the pure β-chitin sponge, which was closer to normal rabbit cartilage compared with other types of sponges. The results of the biochemical analysis, along with the histochemical and immunohistochemical findings, indicate that the cartilage-like layers in the chondrocytes-sponge composites of all five types of sponges were similar to hyaline cartilage and that of the pure β-chitin sponge was closer to normal rabbit cartilage than other types of sponges. From these results, the pure β-chitin sponge was thought to be more suitable than other types of sponges as a scaffold for cartilage tissue engineering.
Kuo et al. previously reported on the effect of chitin and chitosan scaffolds with genipin-crosslinking and hydroxyapatite modifications. They compared four types of scaffolds as following: 1:1, 1:2, 1:5 chitin-chitosan scaffolds, and pure chitosan scaffold with a porous sponge structure [35]. However, they did not statistically analyze their data, rather, they showed the tendency of the sponge with increased volume of chitin to have a greater number of cells, more glycosaminoglycans, and more collagen over a 28-day cultivation of bovine knee chondrocytes. In the present study, only the pure β-chitin sponge had the advantage over other sponges concerning the synthesis of collagen, that is not identified in their report. We believe their results to be caused by the different sources of the scaffolds, such as the types of chitin, the degrees of deacetylation of chitosan, the methods of preparing chitosan hydrogel, chitin-chitosan crosslinking, and hydroxyapatite modifications between theirs and ours. They also used different types of chitin and chitosan from the present study; they used α-chitin and 85% deacetylated chitosan, whereas we used β-chitin and 98% deacetylated chitosan. In addition, they prepared chitosan hydrogel from chitosan-acetic acid solution, which is thought to be unstable and to immediately decrease its molecular weight, its viscosity, and its antibacterial activity during the mixing of chitin hydrogel with chitosan hydrogel. On the other hand, we prepared chitosan hydrogel using the method that progresses its stability in aqueous solution on standing for long time at room temperature [34]. It is possible that the differences in percentages of deacetylation and the preparations of chitosan influenced the results more than the differences in the types of chitin. Moreover, in their study, crosslinking by genipin and modifications by hydroxyapatite may influence chitin and chitosan crystalline structure characteristics, the size of the molecular weight, or the degree of deacetylation. Therefore, the present study can be regarded as the first report comparing β-chitin with chitosan as a scaffold for chondrocyte culture, and the method of the present study is superior to previous methods for comparing chitin with chitosan using various mixed hydrogels of chitin and chitosan.
References
N. YASUI, S. OSAWA, T. OCHI, H. NAKASHIMA and K. ONO, Exp. Cell Biol. 50 (1982) 92
T. KIMURA, N. YASUI, S. OSAWA and K. ONO, Clin. Orthop. 186 (1987) 231
S. WAKITANI, T. KIMURA, A. HIROOKA, T. OCHI, M. YONEDA, N. YASUI, K. OWAKI and K. ONO, J. Bone Joint Surg. Br. 71 (1989) 74
K. KATSUBE, M. OCHI, Y. UCHIO, S. MANIWA, M. MATSUSAKI, M. TOBITA and J. IWASA, Arch. Orthop. Trauma. Surg. 120 (2000) 121
D. HENDRICKSON, A. NIXON, H. ERB and G. LUST, Am. J. Vet. Res. 55 (1993) 410
B. RAFFOTH, J. WEISSER, F. STERNKOPF, T. AIGNER, K. VAN DER MARK and R. BRÄUER, Osteoarthritis cartilage 6 (1998) 50
J. GUO, G. W. JOURDIAN and D. K. MACCALLUM, Connect. Tissue Res. 19 (1989) 277
S. NEHRER, H. A. BREINAN, A. RAMAPPA, S. SHORTKROFF, G. YOUNG, T. MINAS, C. B. SLEDGE, I. V. YANNAS and M. SPECTOR, J. Biomed. Mater. Res. (Appl. Biomater.) 38 (1997) 95
J. D. PIEPER, P. M. VAN DER KRAAN, T. HAFMANS, J. KAMP, P. BUMA, J. L. C. VAN SUSANTE, W. B. VAN DEN BERG, J. H. VEERKAMP and T. H. VAN KUPPEVELT, Biomaterials 23 (2002) 3183
L. E. FREED, J. C. MARQUIS, A. NOHRIA, J. EMMANUAL, A. G. MIKOS and R. LANGER, J. Biomed. Mater. Res. 27 (1993) 11
M. HONDA, T. YADA, M. UEDA and K. KIMATA, J. Oral Maxillofac. Surg. 58 (2000) 767
P. R. AUSTIN, C. J. BRINE, J. E. CATLE and J. P. ZIKAKIS, Science 212 (1981) 749
G. FRADET, S. BRISTER, D. MULDER, J. LOUGH and B. AVERBACH, in “Chitin in Nature and Technology”, Edited by: R. Muzzarelli, C. Jeuniaux and G. W. Gooday (Plenum Press, New York, USA, 1986) p. 443
M. TACHIBANA, A. YAITA, H. TANIURA, K. FUKASAWA, N. NAGASUE and T. NAKAMURA, Jpn. J. Surg. 18 (1988) 533
K. Y. LEE, W. S. HA and W. H. PARK, Biomaterials 16 (1995) 1211
R. OSLEN, D. SCHWARTZMILLER, W. WEPPNER and R. WINANDY, in “Chitin and Chitosan”, Edited by: G. Skjak-Braëk, T. Anthonsen and P. Sandford (Kluwer Academic, Dordrecht, Netherlands, 1988) p. 813
M. MATTIOLI-BELMONTE, A. GIGANTE, R. A. A. MUZZARELLI, R. POLITANO, A. DE BENEDITTIS, N. SPECCHIA, A. BUFFA, G. BIAGINI and F. N. GRECO, Med. Biol. Eng. Comp. 37 (1999) 130
J. Y. LEE, S. H. NAM, S. Y. IM, Y. J. PARK, Y. M. LEE, Y. J. SEOL, C. P. CHUNG and S. J. LEE, J. Control. Release 78 (2002) 187
Y. HU, X. JIANG, Y. DING, H. GE, Y. YUAN and C. YANG, Biomaterials 23 (2002) 3193
A. DENUZIERE, D. FERRIER, O. DAMOUR and A. DOMARD, Biomaterials 19 (1998) 1275
T. TANABE, N. OKITSU, A. TACHIBANA and K. YAMAUCHI, Biomaterials 23 (2002) 817
M. L. GONZÁLEZ-RODÍGUEZ, M. A. HOLGADO, C. SÁNCHEZ-LAFUENTE, A. M. RABASCO and A. FINI, Int. J, Pharm. 232 (2002) 225
S. MINAMI, Y. OKAMOTO, A. MATSUHASHI, H. SASHIWA, H. SAIMOTO, Y. SHIGEMASA, T. TANIGAWA, Y. TANAKA and S. TOKURA, in “Advances in Chitin and Chitosan” Edited by: C. J. Brine, P. A. Sandford and J. P. Zikakis (Elsevier, New York, USA, 1992) p. 61
Y. OKAMOTO, S. MINAMI, A. MATSUHASHI, H. SASHIWA, H. SAIMOTO, Y. SHIGEMASA, T. TANIGAWA, Y. TANAKA and S. TOKURA, in “Advances in chitin and Chitosan” Edited by: C. J. Brine, P. A. Sandford and J. P. Zikakis (Elsevier, New York, USA, 1992) p. 70
Y. M. LEE, S. S. KIM, M. H. PARK, K. W. SONG, Y. K. SUNG and I. K. KANG, J. Mater. Sci. Mater. Med. 11 (2000) 817
M. TAKAI, F. NONOMURA, Y. SHIMIZU, J. HAYASHI, S. TOKURA, M. OGAWA, T. KOHRIYAMA, M. SATAKE, T. FUJITA and T. URAGAMI, in “Proceedings of the International Symposium on Chitin Derivatives in Life Science, held in Sapporo, Japan, 1990”, Edited by: S. Tokura and I. Azuma (1992) p. 162
S. TOKURA, H. TAMURA, T. DOHBA, K. TAKAHASHI, N. SAKAIRI and N NISHI, in, “ACS Symposium Series 737: Polysaccharide Applications-Cosmetics and Pharmaceuticals”, Edited by: M. A. E-Nokaly and H. A. Soini (Oxford University Press, New York, USA, 1999) p. 85
M. ABE, M. TAKAHASHI, S. TOKURA, H. TAMURA and A. Nagano, Tissue Eng. 10 (2004) 585
A. LAHIJI, A. SOHRABI, D. S. HUNGERFORD and C. G. FRONDOZA, J. Biomed. Mater. Res. 51 (2000) 586
D. L. NETTLES, S. H. ELDER and J. A. GILBERT, Tissue Eng. 8 (2002) 1009
J. K. F. SUH and H. W. T. MATTHEW, Biomaterials 21 (2000) 2589
M. PRASITSILP, R. JENWITHISUK, K. KONGSUWAN, N. DAMRONGCHAI and P. WATTS, J. Mater. Sci.-Mater. Med. 11 (2000) 773
M. V. SHAMOV, S. Y. BRATSKAYA and V. A. AVRAMENKO, J. Colloid Interface Sci. 249 (2002) 316
H. TAMURA, K. WADA, R. RUJIRAVANIT and S. TOKURA, J. Metals Mater. Miner. 15 (2005) 19
Y. C. KUO and C. Y. LIN, Biotech. Bioeng. 95 (2006) 132
H. TOYODA, K. SHINOMIYA, S. YAMANASHI, I. KOSHIISHI and T. IMANARI, Anal. Sci. 4 (1989) 381
M. SHINMEI, S. MIYAUCHI, A. MACHIDA and K. MIYAZAKI, Arthritis Rheum. 35 (1992) 1304
A. UCHIYAMA, T. OHISHI, M. TAKAHASHI, K. KUSHIDA, T. INOUE, M. FUJIE and K. HORIUCHI, J. Biochem. 110 (1991) 714
M. T. BAYLISS, C. DAVIDSON, S. M. WOODHOUSE and D. J. OSBORNE, Acta Orthop. Scand. Suppl. 266 (1995) 22
P. A. S. MOURANO, Arthritis Rheum. 31 (1988) 1028
T.E. HARDINGHAM and A.J. FONSANG, FASEB J. 6 (1992) 861
Acknowledgments
We express our deep gratitude to Mr. Yuichi Kaneko and Ms. Ayako Okamoto for their technical help for obtaining chondrocytes from rabbits and the measurements of hydroxyproline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, D., Takahashi, M., Abe, M. et al. Comparison of various mixtures of β-chitin and chitosan as a scaffold for three-dimensional culture of rabbit chondrocytes. J Mater Sci: Mater Med 19, 1307–1315 (2008). https://doi.org/10.1007/s10856-007-3245-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3245-9