Abstract
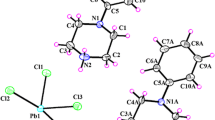
t-butyl ammonium picrate (TBAP) crystallized in a P-1 space group with two molecules per unit cell. The solid has hydrogen bonding (1.86 and 1.88 Å) in the b-axis between the phenolate oxygen and one of the –NH3+ hydrogen. In the unit cell, if one picrate ion is on the left and the cation is on the right along the b-axis. The other molecule is just below along the b-axis with cation on the left and picrate ion on the right disposition. Each phenolate ion forms H-bonding with two different t-butyl ammonium ions. Thus the two cations and two anions are held together by H-bonding. The IR spectrum had characteristic bands due to ν(C−O) phenolic, ν(NO2), ν(NH3+) and ν(C–H) peaks. The 1H NMR spectrum showed a broad peak at 7.7 ppm due to NH3+ protons along with the characteristic aromatic and –CH3 protons. The 13C NMR showed peaks due to tertiary (51.15 ppm), aromatic (124–160 ppm) and methyl carbons (27.15 ppm). The peaks at 235 and 380 nm are due to π → π* and n → π* observed from the UV–Visible spectrum and having a band gap of 2.82 eV. The crystal melts at 476.5 K followed by decomposition. The dielectric constant and dielectric loss at 323 K were found to be maximum at lower frequencies. The values are lesser at 373 K and 423 K due to phase changes as shown by DTA. AC conductivity studies indicated that the conductance is maximum at 323 K and lesser at both 373 K and 423 K due to phase changes. The I–V studies showed a negative effect, that is the photocurrent is lower than the dark current due to Fermi energy gaps nearer to VB and CB. The polarizability was found to be 8.342 × 10–23 cm3 which indicated that the molecule can be used as an optoelectronic material.














Similar content being viewed by others
Data availability
CCDC: 1009718 contains the supplementary crystallographic data for this paper. This data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Tel: + 44 (0)1223 336408.
References
DC Santra MK Bera PK Sukul S Malik 2016 Chem. Eur. J. 22 2012
M Amudha J Madhavan P Praveen Kumar 2017 J. Opt. 46 382
JU Maheswari C Krishnan S Kalyanaraman P Selvarajan 2016 Phys. B Condens. Matter 502 32
M Manonmani C Balakrishnan SR Ahamed G Vinitha SP Meenakshisundaram RM Sockalingam 2019 J. Mol. Struct. 1190 1
N Sudharsana B Keerthana R Nagalakshmi V Krishnakumar L Guru Prasad 2012 Mater. Chem. Phys. 134 736
R Bharathikannan A Chandramohan MA Kandhaswamy J Chandrasekaran R Renganathan V Kandavelu 2008 Cryst. Res. Technol. 43 683
S Suguna D Anbuselvi D Jayaraman KS Nagaraja B Jeyaraj 2014 Spectrochim. Acta A Mol. Biomol. Spectrosc. 132 330
RM Jauhar V Viswanathan P Vivek G Vinitha D Velmurugan P Murugakoothan 2016 RSC Adv. 6 57977
G Anandha Babu A Chandramohan P Ramasamy G Bhagavannarayana B Varghese 2011 Mater. Res. Bull. 46 464
V Kalaipoonguzhali S Surendarnath M Vimalan K SenthilKannan 2023 J. Mater. Sci. Mater. Electron. 34 1
K SenthilKannan N Balamurugapandian T Jayanalina GA Vincy MG Prasath M Vimalan P Sasikumar 2023 J. Mater. Sci. Mater. Electron. 34 1
H Ganesan KS Radha M Vimalan K SenthilKannan 2024 Polycycl. Aromat. Compd. 44 1850
P Kalhor ZW Yu 2020 J. Mol. Struct. 1215 128257
K Elangovan KE Ingle R Dhanasekaran M Mahadevan M Dhilip 2024 Spectrochim. Acta A Mol. Biomol. Spectrosc. 308 123680
C Indumathi TC Sabari Girisun K Anitha S Alfred Cecil Raj 2017 J. Phys. Chem. Solids 106 37
K Senthilkumar N Kanagathara V Natarajan V Ragavendran T Srinivasan MK Marchewka 2020 J. Mol. Struct. 1220 128764
GM Sheldrick 2007 Acta Crystallogr. A Found. Crystallogr. 64 112
OV Dolomanov LJ Bourhis RJ Gildea JAK Howard H Puschmann 2009 J. Appl. Crystallogr.Crystallogr. 42 339
A Szumna J Jurczak Z Urbańczyk-Lipkowska 2000 J. Mol. Struct. 526 165
B Chidambaranathan S Sivaraj S Selvakumar V Jancik 2023 Acta Crystallogr. E Crystallogr. Commun. 79 8
YJ Li 2009 Acta Crystallogr. Sect. E Struct. Rep. Online 65 o2566
S Ramalingam E John David RC Ramachandra P Jobe Prabakar 2014 J. Theor. Comput. Sci. 1 1
S Selvaraj P Rajkumar M Kesavan S Gunasekaran S Kumaresan 2019 Vib. Spectrosc. 100 30
C Karnan KS Nagaraja S Manivannan A Manikandan V Ragavendran 2021 J. Mol. Model. https://doi.org/10.1007/s00894-021-04842-w
A Ponnuvel S Nivithaa A Kala GR Ramkumaar KS Nagaraja C Karnan 2023 J. Chem. Crystallogr.Crystallogr. https://doi.org/10.1007/s10870-023-00989-x
S Nishanth S Nivithaa C Sridhar KS Nagaraja C Karnan 2023 J. Mol. Struct. 1285 135501
R Gandhimathi S Dheivamalar R Dhanasekaran 2015 Eur. Phys. J. Appl. Phys. 69 10202
A Ponnuvel AP Kala KS Nagaraja C Karnan 2021 Acta Crystallogr. E Crystallogr. Commun. 77 1019
R Samui AK Bhunia S Saha 2023 J. Mater. Sci. Mater. Electron. 34 1
TN Ghosh SS Pradhan SK Sarkar AK Bhunia 2021 J. Mater. Sci. Mater. Electron. 32 19157
KD Mandal AK Rai L Singh O Parkash 2012 Bull. Mater. Sci. 35 433
CP Smyth 1955 Dielectric Behaviour and Structure McGraw-Hill New York
KM Chauhan SK Arora 2009 Cryst. Res. Technol. 44 189
D Kalaiselvi R Jayavel 2012 Appl. Phys. A Mater. Sci. Process. 107 93
AK Bhunia SS Pradhan K Bhunia AK Pradhan S Saha 2021 J. Mater. Sci. Mater. Electron. 32 22561
KV Rao A Smakula 1966 J. Appl. Phys. 37 319
A Bera D Basak 2008 Appl. Phys. Lett. https://doi.org/10.1063/1.2968131
VN Joshi 1990 Photoconductivity Marcel Dekker New York
RH Bube 1981 Photoconductivity of Solids Wiley New York
QH Li T Gao YG Wang TH Wang 2005 Appl. Phys. Lett. 86 1
AK Bhunia S Sen PK Guha S Saha 2023 Eur. Phys. J. Plus https://doi.org/10.1140/epjp/s13360-023-04244-2
NM Ravindra RP Bhardwaj KS Kumar VK Srivastava 1981 Infrared Phys. 21 369
DR Penn 1962 Phys. Rev. 128 2093
NM Ravindra VK Srivastava 1980 Infrared Phys. 20 67
RR Reddy YN Ahammed 1996 Infrared Phys. Technol. 37 505
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S. Suguna: formal analysis, investigation, methodology, data curation, writing—original draft. K.S. Nagaraja: conceptualization, resources, writing—original draft, writing—review and editing. C. Karnan: visualization, validation, writing—review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suguna, S., Nagaraja, K.S. & Karnan, C. Structural, optical, dielectric, conductivity and solid-state behaviour of 2-methylpropan-2-ammonium 2,4,6-trinitrophenolate (TBAP) single crystal. J Mater Sci: Mater Electron 35, 1173 (2024). https://doi.org/10.1007/s10854-024-12916-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12916-7