Abstract
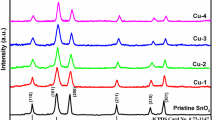
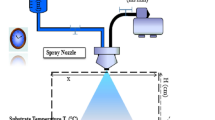
In this current work, CuO–SnO2: F mixed oxide thin films were synthesized by spray pyrolysis technique on glass substrates. The structural and optical properties were optimized and improved by varying the substrate temperature from 300 to 350 °C by a step of 25 °C. By increasing the substrate temperature, in addition to the CuO monoclinic phase we notice the appearance of the SnO2: F tetragonal phase in the XRD spectrum. Therefore, CuO–SnO2: F coupled oxide thin film, where the ratio in the spray solution (\(r=\frac{[Cu]}{[Sn]}=3)\) was successfully grown at the elevated temperature equals to 350 °C. This result was confirmed by Raman and FTIR analyses. SEM analysis of CuO–SnO2: F films elaborated at a substrate temperature equal to 350 °C endorsed the particle-like spherical shape structure with smooth surface. While EDS and Elemental mapping confirmed the presence of the expected elements. By using the transmission reflection spectra, we estimated the values of the refractive index n, the extinction coefficient k and the dielectric constant. In the visible area the refractive index (n) varies from 1.5 to 2.3 and the extinction coefficient k decreases from 0.8 for Ts = 300 °C, up to about 0.2 for Ts = 350 °C. Moreover, the photoluminescence spectra of the films was investigated and interpreted. The gas-sensing measurements revealed that CuO–SnO2: F thin films grown by spray-pyrolysis method at the substrate temperature equals to 350 °C, can detect minuscule traces of O3 gas (30 ppb) with good sensitivity, fast response and recovery times (60 s and 79 s respectively), at relatively low temperature (200 °C). This manuscript reports the effectiveness of our homemade device as promoter ozone gas sensor in many industrial applications.












Similar content being viewed by others
Data availability
Data and materials related to this research are available from the relevant author, Ghofrane Charrada within reasonable range.
References
H. Fang et al., Enhanced NO2 gas sensing performance by hierarchical CuO–Co3O4 spheres. Sens. Actuators B: Chem. 352, 131068 (2022). https://doi.org/10.1016/j.snb.2021.131068
M. Wu et al., Recent progress in chemical gas sensors based on organic thin film transistors. J. Mater. Chem. C 8(39), 13482–13500 (2020). https://doi.org/10.1039/D0TC03132A
J.F. Da Silveira Petruci et al., Analytical methods applied for ozone gas detection: a review. TrAC Trends Anal. Chem. 149, 116552 (2022). https://doi.org/10.1016/j.trac.2022.116552
M.V. Nikolic et al., Semiconductor gas sensors: Materials, technology, design, and application. Sensors 20(22), 6694 (2020). https://doi.org/10.3390/s20226694
Y. Zhou et al., MXene Ti3C2Tx-derived nitrogen-functionalized heterophase TiO2 homojunctions for room-temperature trace ammonia gas sensing. ACS Appl. Mater. Interfaces 13(47), 56485–56497 (2021). https://doi.org/10.1021/acsami.1c17429
A. Rhouati et al., MXene-based electrochemical sensors for detection of environmental pollutants: a comprehensive review. Chemosphere 291, 132921 (2022). https://doi.org/10.1016/j.chemosphere.2021.132921
S. Zhang et al., Full review: the progress and developing trends of nanosheet-based sensing applications. Coord. Chem. Rev. 433, 213742 (2021). https://doi.org/10.1016/j.ccr.2020.213742
Y. Wang et al., Horseshoe-shaped SnO2 with annulus-like mesoporous for ethanol gas sensing application. Sens. Actuators B: Chem. 240, 1321–1329 (2017). https://doi.org/10.1016/j.snb.2016.07.160
S.-H. Kwon et al., Ultraviolet light-emitting diode-assisted highly sensitive room temperature NO2 gas sensors based on low-temperature solution-processed ZnO/TiO2 nanorods decorated with plasmonic Au nanoparticles. Nanoscale 13(28), 12177–12184 (2021). https://doi.org/10.1039/D1NR01001H
K. Grossmann et al., Interplay of H2, water vapor and oxygen at the surface of SnO2 based gas sensors—an operando investigation utilizing deuterated gases. Sens. Actuators B: Chem. 166, 787–793 (2012). https://doi.org/10.1016/j.snb.2012.03.075
J. Liu et al., Size effect and comprehensive mathematical model for gas-sensing mechanism of SnO2 thin film gas sensors. J. Alloys Compd. 898, 162875 (2022). https://doi.org/10.1016/j.jallcom.2021.162875
L. Zhang et al., Facile one-step hydrothermal synthesis of SnO2 microspheres with oxygen vacancies for superior ethanol sensor. J. Alloys Compd. 814, 152266 (2020). https://doi.org/10.1016/j.jallcom.2019.152266
J.M. Xu, J.P. Cheng, The advances of Co3O4 as gas sensing materials: a review. J. Alloy. Compd. 686, 753–768 (2016). https://doi.org/10.1016/j.jallcom.2016.06.086
N.S. Ramgir et al., Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators B: Chem. 151(1), 90–96 (2010). https://doi.org/10.1016/j.snb.2010.09.043
S.I. Boyadjiev et al., Characterization of PLD grown WO3 thin films for gas sensing. Appl. Surf. Sci. 417, 218–223 (2017). https://doi.org/10.1016/j.apsusc.2017.03.212
K. Aguir et al., Electrical properties of reactively sputtered WO3 thin films as ozone gas sensor. Sens. Actuators B: Chem. 84(1), 1–5 (2002). https://doi.org/10.1016/S0925-4005(02)00003-5
M.N. Islam et al., Band gap tuning of p-type al-doped TiO2 thin films for gas sensing applications. Thin Solid Films 714, 138382 (2020). https://doi.org/10.1016/j.tsf.2020.138382
J.Y. Park et al., Surface-area-controlled synthesis of porous TiO2 thin films for gas-sensing applications. Nanotechnology 28(9), 095502 (2017). https://doi.org/10.1088/1361-6528/aa5836
Y.M. Hunge et al., A multifunctional ZnO thin film based devices for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sens. Actuators B: Chem. 274, 1–9 (2018). https://doi.org/10.1016/j.snb.2018.07.117
A. Paliwal et al., Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B: Chem. 250, 679–685 (2017). https://doi.org/10.1016/j.snb.2017.05.064
U.T. Nakate et al., Nano-bitter gourd like structured CuO for enhanced hydrogen gas sensor application. Int. J. Hydrogen Energy 43(50), 22705–22714 (2018). https://doi.org/10.1016/j.ijhydene.2018.09.162
R. Binions, A.J.T. Naik, Metal oxide semiconductor gas sensors in environmental monitoring, in Semiconductor gas sensors. (Woodhead Publishing, Duxford, 2013), pp.433–466
X. Tian et al., A review of advanced gas sensor based on sputtering SnO2 thin film challenges and opportunities. J. Environ. Chem. Eng. 11, 111516 (2023). https://doi.org/10.1016/j.jece.2023.111516
Y. Kong et al., SnO2 nanostructured materials used as gas sensors for the detection of hazardous and flammable gases: a review. Nano Mater. Sci. 4(4), 339–350 (2022). https://doi.org/10.1016/j.nanoms.2021.05.006
M. Belaqziz et al., Enhanced room temperature ozone response of SnO2 thin film sensor. Superlattices Microstruct. 71, 185–189 (2014). https://doi.org/10.1016/j.spmi.2014.03.040
G. Korotcenkov, V. Nehasil, The role of Rh dispersion in gas sensing effects observed in SnO2 thin films. Mater. Chem. Phys. 232, 160–168 (2019). https://doi.org/10.1016/j.matchemphys.2019.04.069
M. Othman et al., PM2.5 and ozone in office environments and their potential impact on human health. Ecotoxicol. Environ. Saf. 194, 110432 (2020). https://doi.org/10.1016/j.ecoenv.2020.110432
S.R. Cynthia, R. Sivakumar, C. Sanjeeviraja, Ternary CuO:SnO2:ZnO (1:1:1) composite thin film for room temperature gas sensor application. Optik 234, 166615 (2021). https://doi.org/10.1016/j.ijleo.2021.166615
A. Afzal, β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: sensing mechanisms and performance enhancement strategies. J. Materiom. 5(4), 542–557 (2019). https://doi.org/10.1016/j.jmat.2019.08.003
K. Zakrzewska, Mixed oxides as gas sensors. Thin Solid Films 391(2), 229–238 (2001). https://doi.org/10.1016/S0040-6090(01)00987-7
N. van Duy et al., Mixed SnO2/TiO2 included with carbon nanotubes for gas-sensing application. Phys. E: Low-Dimens. Syst. Nanostruct. 41(2), 258–263 (2008). https://doi.org/10.1016/j.physe.2008.07.007
W. Ge et al., In2O3–SnO2 hybrid porous nanostructures delivering enhanced formaldehyde sensing performance. J. Alloys Compd. 746, 36–44 (2018). https://doi.org/10.1016/j.jallcom.2018.02.171
N. van Toan et al., Bilayer SnO2–WO3 nanofilms for enhanced NH3 gas sensing performance. Mater. Sci. Eng. B 224, 163–170 (2017). https://doi.org/10.1016/j.mseb.2017.08.004
S.B. Dhannasare et al., Application of nanosize polycrystalline SnO2–WO3 solid material as CO2 gas sensor. Revista Mexicana de Física 58(6), 445–450 (2012)
M.K. Verma, V. Gupta, SnO2–CuO nanocomposite thin film sensor for fast detection of H2S gas. J. Exp. Nanosci. 8(3), 326–331 (2013). https://doi.org/10.1080/17458080.2012.680930
G. Charrada et al., Investigation on physical properties of CuO and SnO2:F mixed oxide sprayed thin films for photocatalytic application: coupling effect between oxides. J. Mater. Sci. Mater. Electron. 35(10), 685 (2024). https://doi.org/10.1007/s10854-024-12453-3
Q. Huang et al., Highly sensitive and selective ppb-level ozone sensor based on porous CuO nanoparticles. Sens. Actuators B: Chem. 406, 135434 (2024). https://doi.org/10.1016/j.snb.2024.135434
A. Hammoud et al., Investigation on Cu2MgSnS4 thin film prepared by spray pyrolysis for photovoltaic and humidity sensor applications. Opt. Mater. 127, 112296 (2022). https://doi.org/10.1016/j.optmat.2022.112296
G. Gordillo et al., Preparation and characterization of SnO2 thin films deposited by spray pyrolysis from SnCl2 and SnCl4 precursors. Thin Solid Films 252(1), 61–66 (1994). https://doi.org/10.1016/0040-6090(94)90826-5
J.J. Fritz, Representation of the solubility of copper (I) chloride in solutions of various aqueous chlorides. J. Phys. Chem. 85(7), 890–894 (1981)
S.K. Shinde et al., Effect of deposition parameters on spray pyrolysis synthesized CuO nanoparticle thin films for higher supercapacitor performance. J. Electroanal. Chem. 850, 113433 (2019). https://doi.org/10.1016/j.jelechem.2019.113433
M. Ajili, M. Castagné, N.K. Turki, Characteristics of CuIn1−xGaxS2 thin films synthesized by chemical spray pyrolysis. J. Lumin. 150, 1–7 (2014). https://doi.org/10.1016/J.JLUMIN.2013.12.059
K.C. Nwambaekwe et al., Crystal engineering and thin-film deposition strategies towards improving the performance of kesterite photovoltaic cell. J. Mater. Res. Technol. 12, 1252–1287 (2021). https://doi.org/10.1016/j.jmrt.2021.03.047
A. Hammoud et al., Effect of sulfur content on improving physical properties of new sprayed Cu2MgSnS4 thin films compound for optoelectronic applications. Eur. Phys. J. Plus 137(2), 232 (2022). https://doi.org/10.1140/epjp/s13360-022-02417-z
T. Shrividhya et al., Determination of structural and optical parameters of CuO thin films prepared by double dip technique. J. Mater. Sci. Mater. Electron. 25, 3885–3894 (2014). https://doi.org/10.1007/s10854-014-2103-z
J.X. Zhou et al., Raman spectroscopic and photoluminescence study of single-crystalline SnO2 nanowires. Solid State Commun. 138(5), 242–246 (2006). https://doi.org/10.1016/j.ssc.2006.03.007
N. Ahmad, S. Khan, M.M.N. Ansari, Exploration of Raman spectroscopy, dielectric and magnetic properties of (Mn, Co) co-doped SnO2 nanoparticles. Phys. B: Condensed Matter 558, 131–141 (2019). https://doi.org/10.1016/j.physb.2019.01.044
V. Perumal et al., Electron-hole recombination effect of SnO2–CuO nanocomposite for improving methylene blue photocatalytic activity in wastewater treatment under visible light. J. King Saud Univ. Sci. 35(1), 102388 (2023). https://doi.org/10.1016/j.jksus.2022.102388
A. Dieguez et al., The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Phys. 90(3), 1550–1557 (2001). https://doi.org/10.1063/1.1385573
T. Yu et al., Investigation of individual CuO nanorods by polarized micro-Raman scattering. J. Cryst. Growth 268(3–4), 590–595 (2004). https://doi.org/10.1016/j.jcrysgro.2004.04.097
N. Mukherjee et al., CuO nano-whiskers: electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity. Mater. Lett. 65(21–22), 3248–3250 (2011). https://doi.org/10.1016/j.matlet.2011.07.016
Z. Wang et al., X-ray diffraction and Raman spectroscopic study of nanocrystalline CuO under pressures. Solid State Commun. 121(5), 275–279 (2002). https://doi.org/10.1016/j.matlet.2011.07.016
H.B. Saâd et al., Investigation on thickness and annealing effects on physical properties and electrical circuit model of CuO sprayed thin films. Superlattices Microstruct. 142, 106508 (2020). https://doi.org/10.1016/j.spmi.2020.106508
C. Nefzi et al., Effect of sulfur concentration on structural, optical and electrical properties of Cu2FeSnS4 thin films for solar cells and photocatalysis applications. Superlattices Microstruct. 124, 17–29 (2018). https://doi.org/10.1016/j.spmi.2018.09.033
R.B. Ayed et al., Physical properties and Rietveld analysis of Fe2O3 thin films prepared by spray pyrolysis: effect of precursor concentration. Phys. B: Condensed Matter 563, 30–35 (2019). https://doi.org/10.1016/j.physb.2019.03.029
A. Jrad et al., Effect of copper concentration on the physical properties of ZnS: Cu alloys prepared by chemical bath deposition. J. Mater. Sci. Mater. Electron. 27, 10684–10695 (2016). https://doi.org/10.1007/s10854-016-5168-z
C. Nefzi et al., Effect of substrate temperature on physical properties of Cu2FeSnS4 thin films for photocatalysis applications. Mater. Sci. Eng. B 254, 114509 (2020). https://doi.org/10.1016/j.mseb.2020.114509
S. Nasir et al., Potential valorization of by-product materials from oil palm: a review of alternative and sustainable carbon sources for carbon-based nanomaterials synthesis. BioResources (2019). https://doi.org/10.15376/biores.14.1.Nasir
K. Thirumurugan et al., Effect of solvent volume on properties of SnO2: Al films. Surf. Eng. 29(5), 373–378 (2013). https://doi.org/10.1179/1743294412Y.0000000110
Y. Tao et al., The structural, electrical, and optical properties of SnO2 films prepared by reactive magnetron sputtering: influence of substrate temperature and O2 flow rate. Mater. Chem. Phys. 250, 123129 (2020). https://doi.org/10.1016/j.matchemphys.2020.123129
L. Dua, P.K. Biswas, Synthesis and characterization of nanostructured Mn (II) doped antimony-tin oxide (ATO) films on glass. Appl. Surf. Sci. 280, 33–41 (2013). https://doi.org/10.1016/j.apsusc.2013.04.066
M. Ajili et al., Investigation on substrate effect on physical characteristics of CuO-sprayed thin films suitable for photovoltaic application: Ag/ZnO:Sn (n)/CuO (p)/SnO2:F. Mater. Technol. 37(6), 381–396 (2022). https://doi.org/10.1080/10667857.2020.1854516
A. Bougharouat et al., Hydrophobic properties of CuO thin films obtained by sol–gel spin coating technique-annealing temperature effect. Ann. Chim. Phys. 45, 439–445 (2021). https://doi.org/10.18280/acsm.450602
S. Gandhi et al., Ultrasound assisted one pot synthesis of nano-sized CuO and its nanocomposite with poly (vinyl alcohol). J. Materials. Sci. 45, 1688–1694 (2010). https://doi.org/10.1007/s10854-016-5168-z
A.M. Abd-Elnaiem, M.A. Abdel-Rahim, S. Moustafa, Comparative investigation of electronic properties of As-70 at% Te thin films: influence of Ga doping and annealing temperature. J. Non-Cryst. Solids 540, 120062 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120062
S. Chaisitsak, Nanocrystalline SnO2: F thin films for liquid petroleum gas sensors. Sensors 11(7), 7127–7140 (2011). https://doi.org/10.3390/s110707127
C. Rodríguez et al., Graphene oxide–ZnO nanocomposites for removal of aluminum and copper ions from acid mine drainage wastewater. Int. J. Environ. Res. Public Health 17(18), 6911 (2020). https://doi.org/10.3390/ijerph17186911
L.F. da Silva et al., A novel ozone gas sensor based on one-dimensional (1D) α-Ag2WO4 nanostructures. Nanoscale 6(8), 4058–4062 (2014). https://doi.org/10.1039/C3NR05837
X. Zhou et al., Study on sensing mechanism of CuO–SnO2 gas sensors. Mater. Sci. Eng. B 99(1–3), 44–47 (2003). https://doi.org/10.1016/S0921-5107(02)00501-9
H.F. Lu et al., Amorphous TiO2 nanotube arrays for low-temperature oxygen sensors. Nanotechnology 19(40), 405504 (2008). https://doi.org/10.1088/0957-4484/19/40/405504
F. Wen-Yu et al., Preparation of CuO–SnO2 gas sensor and its sensitive effect. J. Petrochem. Univ. 24(2), 18 (2011). https://doi.org/10.3696/j.issn.1006-396X.2011.02.005
M. Sour, H.S. Amoli, Gas sensing mechanisms in ABO3 perovskite materials at room temperature: a review. Mater. Sci. Semicond. Process. 156, 107271 (2023). https://doi.org/10.1016/j.mssp.2022.107271
M. Nesa, M. Sharmin, A.H. Bhuiyan, Role of Zn dopants on the surface morphology, chemical structure and DC electrical transport properties of nanostructured p-type CuO thin films. Mater. Sci. Semicond. Process. 122, 105479 (2021). https://doi.org/10.1016/j.mssp.2020.105479
V. Saasa, B. Mwakikunga, Facile synthesis, characterization and acetone sensing properties of n-type WO3, SnO2 and VO2 semiconducting materials and their cobalt doped performance: outstanding SnO2–Co acetone selectivity and sensitivity. Mater. Res. Bull. 164, 112288 (2023). https://doi.org/10.1016/j.materresbull.2023.112288
Y. Bellal, A. Bouhank, CuO/SnO2 nanocomposite thin films: effect of solvent and precursor on optical and structural properties. Int. J. Nanosci. 20(03), 2150029 (2021). https://doi.org/10.1142/S0219581X21500290
W. Avansi, A.C. Catto, L.F. da Silva, T. Fiorido, S. Bernardini, V.R. Mastelaro, K. Aguir, R. Arenal, One-dimensional V2O5/TiO2 heterostructures for chemiresistive ozone sensors. ACS Appl. Nano Mater. 2, 4756–4764 (2019). https://doi.org/10.1021/acsanm.9b00578
C.H. Wu, G.J. Jiang, K.W. Chang, Z.Y. Deng, Y.N. Li, K.L. Chen, C.C. Jeng, Analysis of the sensing properties of a highly stable and reproducible ozone gas sensor based on amorphous In–Ga–Zn–O thin film. Sensors 18, 163 (2018). https://doi.org/10.3390/s18010163
G. Korotcenkov, B.K. Cho, Ozone measuring: what can limit application of SnO2-based conductometric gas sensors? Sens. Actuators, B Chem. 161(1), 28–44 (2012). https://doi.org/10.1016/j.snb.2011.12.003
C.H. Wu, T.-L. Chou, R.J. Wu, Rapid detection of trace ozone in TiO2–In2O3 materials by using the differential method. Sens. Actuators B: Chem. 255, 117–124 (2018). https://doi.org/10.1016/j.snb.2017.08.055
G. Korotcenkov, V. Brinzari, B.K. Cho, In2O3-and SnO2-based ozone sensors: Design and characterization. Crit. Rev. Solid State Mater. Sci. 43(2), 83–132 (2018). https://doi.org/10.1080/10408436.2017.1287661
Funding
The authors do not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors have participated to the development of this work. GC, MA, NJ, SB, KA and NK effected contribution to the experimental study, search information and results discussion. The first copy of the manuscript was written by GC and all authors contributed to the correction of this manuscript. All authors approved the final version of this article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relation with financial or non-financial interests to be reported.
Ethical approval
The authors agree with conformance with the Ethical standards of Material science Journal: Materials in Electronics. The authors report that this article is compliant with ethical standards and does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Charrada, G., Ajili, M., Jebbari, N. et al. Improvement of ozone sensing parameters by CuO–SnO2: F mixed oxide sprayed thin films. J Mater Sci: Mater Electron 35, 1120 (2024). https://doi.org/10.1007/s10854-024-12873-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12873-1