Abstract
The effects of Mn-deficiency on the structure, Mn ions valence, vacancy defects, magnetic and dielectric characteristics of GdMn1−xO3 ceramics synthesized by solid-state reaction were investigated. The results revealed that Mn-deficiency caused the lattice deformation, but had no effect on the orthorhombic perovskite structure of all GdMn1−xO3 samples. Raman analysis showed that Mn-deficiency induced octahedral distortions and lattice disorder and affected < Mn–O > length. XPS analysis implied that the valence state of Mn ion evolved in GdMn1−xO3 samples owing to the charge compensation. Positron annihilation lifetime spectroscopy revealed that the vacancy size and concentration increased with increasing Mn-deficiency concentration x. The properties measurements showed that the Mn-deficiency increased the relative dielectric permittivity and magnetization of GdMnO3 system. The relationship between structure and performances was discussed in this paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a new type of multifunctional materials, multiferroic materials with magnetoelectric coupling between magnetic and ferroelectric orders exhibit new physical effects, which have broad potential application in transducers and other fields [1, 2]. They have drawn a great deal of interest recently. Among the many multiferroic materials, GdMnO3 with perovskite structure has unique magnetic structure, ferroelectricity induced by magnetic orders, and significant intrinsic magnetoelectric coupling. GdMnO3 shows an antiferromagnetism in the temperature of 46–22 K and a canted antiferromagnetism (weak ferromagnetism) at 7 K < T < 22 K due to the evolution of Mn3+ ordering, the long-range ordering of Gd moments begins to appear at ∼7 K [3,4,5]. Although researchers have conducted a lot of studies on the structure and physical behavious of GdMnO3 system, the physical mechanism of magnetic, ferroelectric, and magnetoelectric coupling is still up for debate, and the ferroelectric and magnetic phase-transition temperature needs to be further improved. The physical properties of GdMnO3 system are regulated by lattice structure, chemical composition, spin structure, vacancy defects, etc. In the process of exploring the generation and regulation mechanism of ferroelectricity and magnetism, and optimizing the physical properties of GdMnO3, it has been proved that adjusting the composition of B-site ions is an effective approach to effectively regulate its microstructure and properties. Tiwari et al. [6] reported Cr doping on the evolution of structure and magnetic transitions in GdMnO3, and found that the magnetization reversal with spin reorientation was associated with the competition between Mn, Cr and Gd ions. Pal et al. [7] explored the evolution of different magnetic orderings in GdMnO3 systems by Fe3+ ions doping, and revealed that the magnetic structure of the systems was relegated by Fe3+–Fe3+ interaction, Gd3+–Fe3+ interaction, Mn magnetic structure, and Jahn–Teller distortion. In the W-substituted GdMnO3 studied by Li et al. [8], W6+ ion substitution enhanced the polarization and magnetization of GdMnO3 system through regulating ion valence state, grain/grain boundary resistivity, structural distortion, and microdefect. Previous research revealed that a slight adjustment of B-site ions in GdMnO3 might greatly influence the electric and magnetic characteristics. Considering that GdMnO3 was placed at the phase boundary between the A-type antiferromagnetic order and the cycloidal spin order in the phase diagram of rare-earth manganites, the chemical disorder and defects caused by B-site deficiency may have some important influence on the multiferroicity of GdMnO3 system. In this study, Mn-deficiency GdMnO3 ceramics (GdMn1−xO3) were synthesized, and the impact of Mn-deficiency on the structure characteristics and physical performances of GdMnO3 system was investigated.
Vacancy-type defects, which are unavoidable in materials, have distinct influence on the physical characteristics of materials [9]. Perovskite materials can tolerate certain level of structural defects. GdMnO3 with perovskite structure could accommodate certain concentration of anion and cation vacancies. Positron annihilation technology can clearly provide detailed information about the concentration, size, and type of vacancy defects at the atomic scale of materials. Therefore, positron annihilation technique was employed in this work to derive the characteristics of vacancy-type defects in GdMn1−xO3 with Mn-deficiency, and the relation between structure and physical properties of GdMn1−xO3 ceramic was studied.
2 Experimental details
GdMn1−xO3 ceramics (x = 0.00, 0.02, 0.05, 0.08, and 0.10) were synthesized by the solid-state reaction. Using alcohol as the medium, the dried powders Gd2O3 (99.99%) and Mn3O4 (99.99%) with proper stoichiometric ratio were ball milled for 24 h at 150 rpm for homogenous mixing. The ball-milled mixtures were calcined twice at 1000 and 1250 °C for 24 h, respectively. After each calcination, the calcined powders were ball milled for 2 h at 150 rpm to obtain powders with finer particles. Finally, the twice calcined powders were pressed into a wafer (φ11mm × 1.5 mm) under 10 MPa.
To investigate the phase structure of the GdMn1−xO3 ceramics, X-ray powder Diffraction with a Bruker D8 diffractometer using Cu-kα radiation within a 2θ range of 20°-70° was performed. To obtain the vibrational modes information, a Raman microscope (Renishaw, United Kingdom) with 532 nm excitation laser was employed. The element composition, valence state and relative content were determined by X-ray photoelectron spectroscopy (XPS, Esca lab 250XI). To determine the cation vacancy information in the samples, positron annihilation lifetime spectroscopy (PALS) measurement was carried out utilizing 13 Ci 22Na as the positron source. An Agilent HP 4194 A impedance analyzer was employed to analyze the dielectric properties in the 100 Hz-10 MHz frequency range. The magnetic behaviors of the GdMn1−xO3 were measured using a Physical property measuring system (Quantum Design).
3 Results and discussion
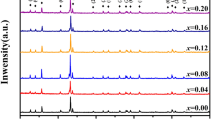
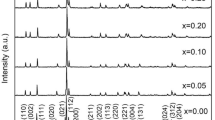
X-ray powder diffraction patterns of GdMn1−xO3 (x = 0.00–0.10) ceramics were shown in Fig. 1. The patterns for all ceramics matched perfectly with the standard card of GdMnO3 without any other impurity phases [10], indicating that a series of GdMn1−xO3 polycrystalline samples with single-phase orthorhombic perovskite structure (space group Pbnm) were synthesized. The results proved that the deficiency of Mn ions in GdMnO3 had no influence on the type of crystal structure. The dominant peaks in all ceramics exhibited the characteristics of high intensity and narrow width, which demonstrated the GdMn1−xO3 ceramics possessed excellent crystallinity.
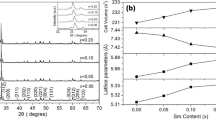
To precisely analyze the influence of Mn-deficiency on lattice properties, the XRD data was refined using the GSAS program. Table 1 displayed the lattice parameters and cell volume of the GdMn1−xO3. The weighted difference sum of squares (χ2) was within the accepted level, demonstrating the credible of refinement results. The lattice parameters of the stoichiometric GdMnO3 samples (x = 0.00) deviated from the published ICSD values by less than 0.5 percent, showing that the refinement results of the GdMn1−xO3 samples were reliable. Compared with the x = 0.00 samples, the cell volume of Mn-deficiency samples was slightly reduced, indicating Mn-deficiency induced slight lattice shrinkage. This might be due to the introduction of Mn vacancies and the generation of Mn4+ (0.53 Å) ions with a smaller ionic radius than that of Mn3+ (0.58 Å) [11].
Raman spectroscopy measurements were performed to investigate the impact of Mn-deficiency on the vibrational modes information for GdMn1−xO3 samples. Figure 2 showed the Raman spectra of GdMn1−xO3 compounds. It has been predicted that the GdMnO3 with orthorhombic perovskite structure had a total of 24 Raman modes (\(7A_{{\text{g}}} \; + \;7B_{{1{\text{g}}}} \; + \;5B_{{2{\text{g}}}} \; + \;5B_{{3{\text{g}}}}\)) [12, 13]. Nevertheless, since the majority of Raman vibrational modes were too weak to be detected, as shown in Fig. 2, only four Raman vibrational modes were observed within the frequency range of 300–800 cm−1. The Raman vibration peaks situated at ~ 484.3 and ~ 610.3 cm−1 were assigned to the antisymmetric and symmetric Jahn–Teller stretching modes, respectively. The other peaks located at ~ 365.3 and ~ 504.6 cm−1 were associated to the tilt and bending of the MnO6 octahedron, respectively. The positions of Raman vibrational modes matched to the values in earlier research [12, 13]. Compared with the stoichiometric GdMnO3 (x = 0.00), the positions of Raman modes located at ~ 365.3 and 504.6 cm−1 were slightly moved towards the higher wave number, the mode at ~ 484.3 cm−1 corresponding to the < Mn–O > length also shifted towards the higher wave number, while the mode at 610.3 cm−1 arised from < Gd–O > vibration did not change significantly. The results showed that Mn-deficiency had obvious effect on the structure of the MnO6 octahedron and < Mn–O > length, but had little effect on the < Gd–O > bond. This might be attributed to the stress and the change of the Mn3+/Mn4+ mixed structure in the samples caused by Mn-deficiency. It could also been detected the broadening of Raman peaks caused by the deficiency of manganese. The broadening characteristic indicated that Mn-deficiency induced lattice disorder in GdMn1−xO3, because the width of Raman peaks was related to the lattice disorder in GdMnO3 [14]
The influence of Mn-deficiency on the chemical states of elements in GdMn1−xO3 ceramics was investigated by utilizing XPS. Figure 3 illustrated the XPS survey spectrum of GdMn0.95O3 samples. Carbon peaks located at binding energy of 284.8 eV were due to the adventitious carbon from atmosphere, which could be utilized for calibrating each spectrum. The XPS survey spectrum showed in Fig. 3 revealed that elements Gd, Mn, O, and C could be identified in the samples devoid of impurity elements. Figure 4 illustrated the high-resolution spectra of Mn 2p3/2 for GdMn0.95O3 ceramics. The Mn 2p3/2 XPS spectra for all samples were fitted into two sub-peaks at ~ 640.5 and 642.2 eV, which were corresponding to Mn3+ and Mn4+ ions [4, 15], respectively. The peak area could be used to calculate the relative amount of Mn ions with different valences. The relative content of Mn4+ in the GdMn1−xO3 samples was shown in Table 2 and Fig. 5. With the increase of Mn-deficiency content, Mn4+ content increased gradually. It revealed that the deficiency of Mn ions resulted in the formation of high-valent Mn ions (Mn4+) due to the charge compensation [16]. The O1s spectra of all GdMn1−xO3 shown in Fig. 4 could also be deconvoluted into two sub-peaks. The fitted peaks with binding energies of ~ 529.3 (OI) and ~ 531.4 eV (OII) represented the lattice oxygen and oxygen vacancies [4, 15], correspondingly. As shown in Table 2, the ratio of OII/OI in the x = 0.00, 0.02, 0.05, 0.08, and 0.10 samples were approximately 0.321, 0.334, 0.310, 0.342, and 0.314, respectively. This signified that the deficiency of Mn ions had no significant influence on the oxygen vacancy concentration for the x = 0.00–0.10 samples. The change trends of Mn4+ and oxygen vacancies concentration showed that deficiency of Mn mainly causes the conversion of Mn valence states.
The feature of vacancy defect in GdMn1−xO3 ceramic samples was characterized by positron annihilation spectroscopy. Typically, positron annihilation lifetime spectroscopy of ceramics was decomposed into three lifetime components τ1, τ2, and τ3 with the corresponding intensities I1, I2, and I3 (I1 + I2 + I3 = 1) after subtracting source composition and background. The intensity I3 was relatively small (I3 < 0.9%), it will be ignored in the following discussion, and I1 and I2 were re-normalized (I1 + I2 = 1). The positron annihilation lifetime parameters of GdMn1−xO3 samples were listed in Fig. 6. The short lifetime τ1 represented the positrons annihilation in perfect lattice. The long lifetime τ2, representing the open volume of vacancy defects, was due to the annihilation of positrons at defects [16,17,18]. The corresponding intensities I1, I2 reflected the relative concentrations of the components. According to the two-state trapping model, the average positron lifetime τav, which also characterized the defect concentration in materials, could be calculated using the following equation [16]:
The local electron density ne was calculated using the following formula [16]:
where r0 is the classical electron radius, c is the velocity of light.
Figure 6 depicted the values of τ1, τ2, τav, and I2 with Mn-deficiency content x. It was clear that τ1 decreased with Mn-deficiency content x. This was compatible with the XRD analysis. The positron annihilation lifetime was inversely proportional to the electron density of the positron annihilation, and the XRD results indicated that Mn-deficiency induced lattice shrinkage in GdMn1−xO3. Therefore, the increase of electron density in the lattice caused the decrease of τ1. The reported theoretical positron lifetimes for Tb-site and Mn-site monovacancy defects in TbMnO3 are 259 and 199 ps, respectively [19]. The structure of TbMnO3 is identical with that of GdMnO3, and Gd and Tb ions are adjoining members of the lanthanum series. Therefore, the positron lifetimes for Gd-site and Mn-site monovacancy defects in GdMnO3 should be close to 259 and 199 ps, respectively. The experimental value of τ2 for the GdMn1−xO3 ceramics was in the range of 230–239 ps. Therefore, the type of cationic vacancies in the GdMn1−xO3 material was Mn vacancies. The long lifetime τ2 increased with increasing x, as indicated in Fig. 6a. Because higher τ2 values in solid materials reflected the larger size of vacancy defects in the samples, the Mn-deficiency caused an increase in vacancies size of GdMn1−xO3 ceramics. As shown in Fig. 6b, both I2 and τav increased with Mn-deficiency content from 0.00 to 0.10. It meant that the concentration vacancies increased with Mn-deficiency content, which might be explained by the deficiency of cations and the lattice shrinkage in the GdMn1−xO3 samples. According to formula (2), the variation of τav would cause the change of ne, that is, Mn-deficiency modulated the electron density of the GdMn1−xO3 samples.
To investigate the impact of Mn-deficiency on the dielectric properties of GdMn1−xO3, the variation of relative dielectric permittivity (εr) and dielectric loss (tanδ) with frequency in the range of 100 to 1.0 × 106 Hz were measured at room temperature and showed in Fig. 7. The curves in Fig. 7a revealed the strong frequency dispersion of relative dielectric permittivity for all samples. As the measurement frequency increased, the relative dielectric permittivity rapidly decreased by several orders of magnitude. This is mostly owing to many types of polarization (electronic and ionic displacement polarization, space-charge polarization, interfacial polarization etc.) presented in the ceramics, and only part of the polarizations responded to frequency in the high frequency range. As the Mn-deficiency content increased, the relative dielectric permittivity of GdMn1−xO3 samples showed an increasing trend, the relative dielectric permittivity of x = 0.10 sample (1288.2) was 16.1 times that of x = 0.00 sample (79.9) when the test frequency was 106 Hz. This was likely to result from the polar arrangement of electrons on the mixed valence structure of Mn3+/Mn4+, displacement polarization induced by structural distortion, and change of electron density [20]. Figure 7b showed the frequency variation of the dielectric loss (tanδ) for GdMn1−xO3 samples. With the increase of frequency, the variation trend of tanδ for GdMn1−xO3 samples was similar to that of relative dielectric permittivity. tanδ of Mn-deficiency samples was higher that of the stoichiometric GdMnO3, which should be mainly attributed to the leakage current induced by the mixed valence structure of Mn3+/Mn4+. In conclusion, Mn-deficiency could be used to effectively regulate the dielectric properties of GdMnO3 samples; the relative dielectric permittivity of GdMn0.90O3 sample could reach 1288.2.
Figure 8 depicted the temperature dependence of the magnetization for GdMn1−xO3 samples in zero-field-cooled (ZFC) and field-cooled (FC) conditions under magnetic field of 1 kOe between 5 and 300 K. The illustration was the partial enlargement. Previously, it has revealed that GdMnO3 with paramagnetic characteristics at room temperature underwent intricate magnetic transitions at lower temperature due to the change in the spin structure of Gd3+ and Mn3+ and the interaction between Gd3+ and Mn3+ sublattices. The ZFC and FC curves of GdMn1−xO3 samples showed in Fig. 8 coincided at high temperatures, while a distinct bifurcation could be observed at lower temperatures, indicating that the GdMn1−xO3 system has both antiferromagnetism and ferromagnetism at low temperature. According to literature [3, 4, 21], the first anomaly at ~ 44 K in the ZFC curve of GdMnO3 was caused by the transition from a paramagnetic to an incommensurate antiferromagnetic due to the appearance of Mn3+ spin ordering. However, such anomaly could not be detected in the ZFC curves of all samples, which was mainly due to the masking effect of great paramagnetic signal generated by Gd3+ moment. At ~ 23 K, the second anomaly occurred due to the canted spin ordering of Mn3+, which arose from canted antiferromagnetic ordering associated with weak ferromagnetism. The third anomaly at ~ 8 K in the ZFC curve was ascribed to the onset of Gd3+ ordering in the GdMnO3. As shown in the insets of Fig. 8, the temperature of the second and third anomalies did not change significantly for all GdMn1−xO3 samples, but the magnetization of Mn-deficiency samples was higher than that of the stoichiometric GdMnO3. It indicated that Mn-deficiency did not affect the ordering temperature of Gd and Mn moments, but it improved the magnetization. In the FC curves, the anomaly at a temperature of ~ 23 K could only be observed, which might arise from the increased local magnetic field at the Gd site due to the ferromagnetic ordering of the Mn3+ moment. To further investigate the magnetic interaction, the ZFC curve was fitted using the Curie–Weiss law, the calculated Curie–Weiss temperatures of the GdMn1−xO3 ceramics were negative, indicating that the antiferromagnetic interaction was dominant in GdMn1−xO3 ceramics [21].
Figure 9 showed the hysteresis loops of GdMn1−xO3 samples at T = 5 K and 30 K. At 30 K, the magnetization of all GdMn1−xO3 samples showed a linear change, indicating that all the samples had anti-ferromagnetic characteristics. At 5 K, all of the samples showed obvious hysteresis loops, indicating ferromagnetic characteristics at this temperature. The maximum magnetization of x = 0.00, 0.02, 0.05, 0.08, and 0.10 samples were 28.1, 30.2, 32.2, 40.6, and 30.5 emu/g, respectively. The remanent magnetizations were 7.4, 9.1, 9.7, 11.8, and 8.7 emu/g, respectively. It was obvious that the magnetization showed a trend of first increasing and then decreasing. On one hand, GdMn1−xO3 samples showed ferrimagnetic properties at 5 K, and the insertion of cation vacancies in ferrimagnetic orderings enhanced the magnetization. Moreover, GdMnO3 exhibited spiral spin magnetic configurations at low temperatures, and the structural distortion induced by Mn-deficiency could suppress the spiral spin magnetic configurations and led to an improvement of magnetization. In addition, the interaction between Mn3+ and Mn4+ led to ferromagnetic configuration, improving the magnetization. Therefore, the magnetization was improved with increasing Mn-deficiency content x from 0.00 to 0.08. However, Mn-deficiency would induce dilution effect and decrease the magnetization of GdMn1−xO3 samples. Therefore, the magnetization was decreased for the GdMn0.10O3 sample.
4 Conclusions
In this paper, the structure, vacancy defects, magnetic and dielectric properties of GdMn1−xO3 polycrystalline samples prepared by solid phase reaction were studied. Microstructure tests showed that Mn-deficiency could affect the lattice structure and cause lattice distortion. The XPS results showed that the Mn-deficiency introduced more Mn4+ in GdMn1−xO3 polycrystalline samples. The results of the positron annihilation lifetime spectroscopy showed that the vacancy concentration of Mn-deficiency samples increases greatly. The performances test showed that the Mn-deficiency samples had higher relative dielectric permittivity and dielectric loss. The magnetic measurements showed that the antiferromagnetic interaction was dominant in GdMn1–−xO3 compounds; Mn-deficiency improved the magnetization of the GdMnO3 system due to the vacancy defects, lattice distortion, and interaction between Mn3+ and Mn4+.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
A. Bagri, D.M. Phase, R.J. Choudhary, Acta Mater. 255, 119091 (2023)
V. Nagendar, N. Raju, S. Shravan Kumar Reddy, M. Sreenath Reddy, C. Gopal Reddy, P. Yadagiri Reddy, J. Mater. Sci. Mater. Electron. 34, 1535 (2023)
A. Pal, W. Prellier, P. Murugavel, J. Phys. Condens. Matter 30, 125801 (2018)
A. Pal, P. Murugavel, J. Appl. Phys. 123, 234102 (2018)
P. Tiwari, D. Gangwar, C. Rath, New J. Chem. 45, 13608 (2021)
P. Tiwari, S. Kumar, C. Rath, RSC Adv. 9, 39871 (2019)
A. Pal, C. Dhana Sekhar, A. Venimadhav, P. Murugavel, J. Phys. Condens. Matter 29, 405803 (2017)
T. Li, H.Z. Liu, J. Chen, D.W. Liu, H.Y. Dai, J. Mater. Sci. Mater. Electron. 33, 25920 (2022)
D.J. Keeble, S. Singh, R.A. Mackie, M. Morozov, S. McGuire, D. Damjanovic, Phys. Rev. B 76, 144109 (2007)
A. Rasras, R. Hamdi, S. Mansour, A. Samara, Y. Haik, J. Phys. Chem. Solids 149, 109798 (2021)
J.A. Dean, Lange’s Handbook of Chemistry, 15th edn. (McGraw-Hill, New York, 1999), pp. 4.30–4.43.
D. Singh, R. Gupta, K.K. Bamzai, J. Mater. Sci. Mater. Electron. 28, 5295 (2017)
N.D. Todorov, M.V. Abrashev, V.G. Ivanov, G.G. Tsutsumanova, V. Marinova, Y.Q. Wang, M.N. Iliev, Phys. Rev. B 83, 224303 (2011)
R. Vilarinho, E.C. Queiros, A. Almeida, P.B. Tavares, M. Guennou, J. Kreisel, J. Agostinho Moreira, J. Solid State Chem. 228, 76 (2015)
P. Pant, H. Agarwal, S. Bharadwaj, M.A. Shaz, Mater. Chem. Phys. 290, 126518 (2022)
T.D. Zhang, W.F. Pan, S.T. Ning, N. Qi, Z.Q. Chen, X.L. Su, X.F. Tang, Adv. Funct. Mater. 33, 2213761 (2023)
V.J. Ghosh, B. Nielsen, T. Friessnegg, Phys. Rev. B 61, 207 (2000)
K. Siemek, A. Olejniczak, L.N. Korotkov, P. Konieczny, A.V. Belushkin, Appl. Surf. Sci. 578, 151807 (2022)
D.J. Keeble, S. Wicklein, R. Dittmann, L. Ravelli, R.A. Mackie, W. Egger, Phys. Rev. Lett. 105, 226102 (2010)
N. Ikeda, H. Ohsumi, K. Ohwada, K. Ishii, T. Inami, K. Kakurai, Y. Murakami, K. Yoshii, S. Mori, Y. Horibe, H. Kito, Nature 436, 1136 (2005)
A. Nandy, A. Roychowdhury, T. Kar, D. Das, S.K. Pradhan, RSC Adv. 6, 20609 (2016)
Acknowledgements
This work is supported by the National Natural Science Foundation of China (12275242), and the Natural Science Foundation of Henan Province (212300410092).
Author information
Authors and Affiliations
Contributions
JW contributed to material preparation, investigation, and writing-original draft; HL contributed to material preparation, data collection, and validation; QS contributed to validation and analysis; and HD contributed to resources, conceptualization, investigation, and writing—original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Liu, H., Shen, Q. et al. Impacts of Mn-deficiency on the structure and physical properties of GdMnO3 ceramics. J Mater Sci: Mater Electron 34, 1918 (2023). https://doi.org/10.1007/s10854-023-11330-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11330-9